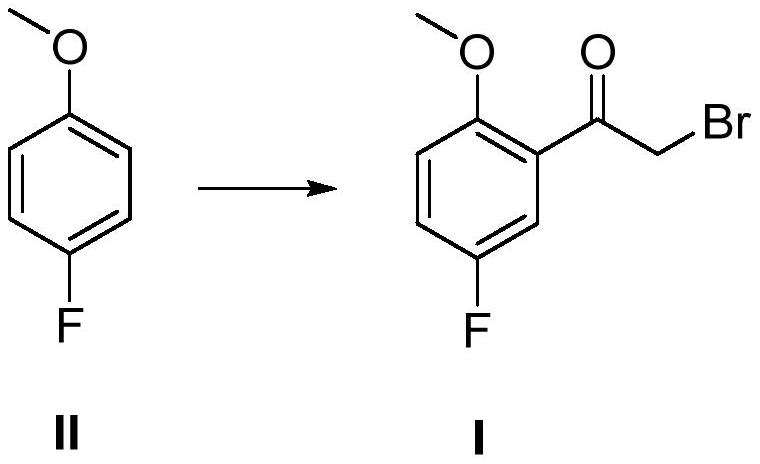
Preparation method of 2-methoxy-5-fluorobromoacetophenone
A fluorobromoacetophenone, methoxyl technology, applied in the field of preparation of 2-methoxy-5-fluorobromoacetophenone, can solve the problems of high cost, large pollution, low yield and the like, and achieves Low price, little pollution, easy to deal with
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of preparation method of 2-methoxy group-5-fluorobromoacetophenone, described preparation method comprises the steps:
[0024] Add 126g (1mol) p-fluoroanisole, 1000ml carbon disulfide, 146.3g (1.1mol) aluminum trichloride to the reaction flask, then add 222g (1.1mol) bromoacetyl bromide, and stir at 20°C for 12 hours after adding, HPLC Track the reaction until the reaction of p-fluoroanisole is complete; quench into 2000ml of ice water, adjust the pH=2, add 1000ml of dichloromethane to extract the layers, concentrate the organic layer to dryness, and recrystallize from n-hexane to obtain 192g of 2-methoxy -5-fluorobromoacetophenone, molar yield: 78%.
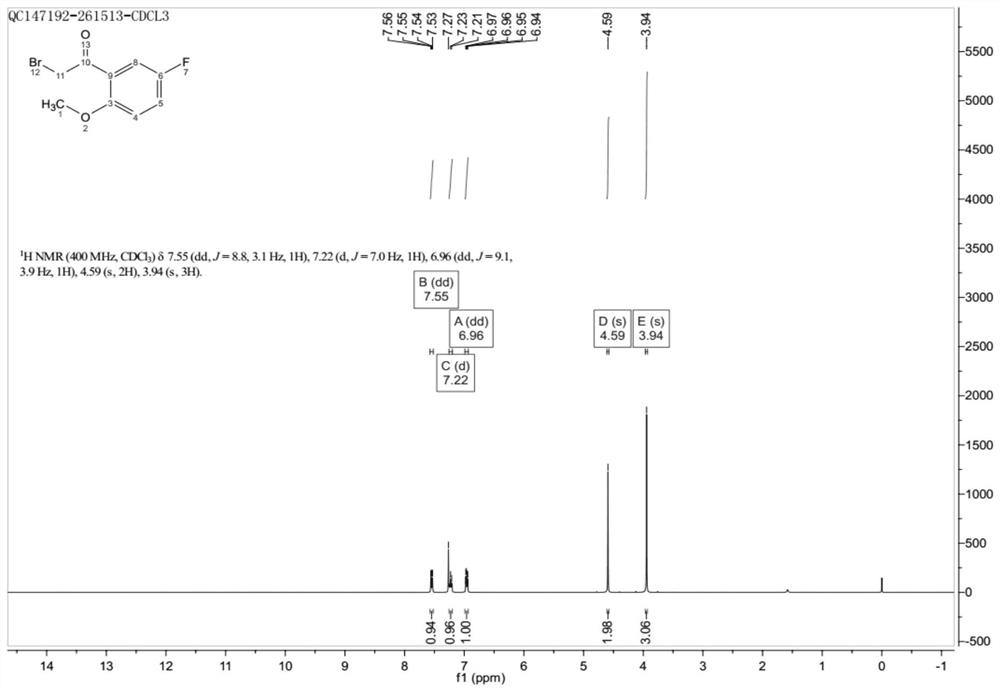
[0025] figure 1 It is the hydrogen spectrum of 2-methoxy-5-fluorobromoacetophenone, 1 H-NMR (400MHz, CDCl 3 )δ: 7.56~7.53(dd, 1H, Ph-H), 7.23~7.21(d, 1H, Ph-H), 6.97~6.94(dd, 1H, Ph-H), 4.59(s, 2H, CH 2 ), 3.94 (s, 3H, OCH 3 ).
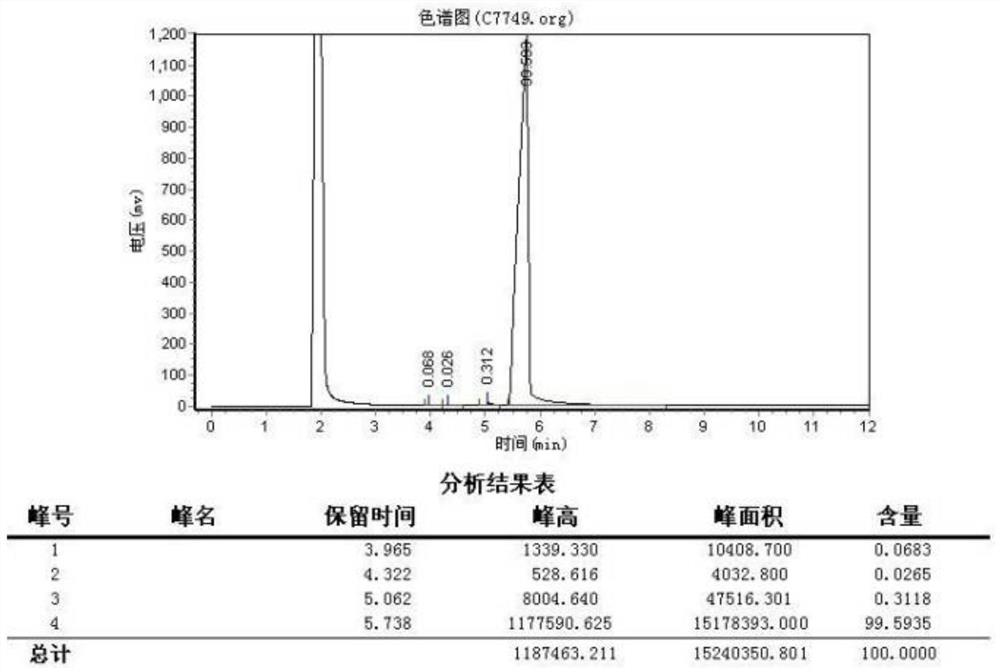
[0026] figure 2 It is a gas chromatogram of 2-methoxy-5-fluorobromoacetophenon...
Embodiment 2
[0028] A kind of preparation method of 2-methoxy group-5-fluorobromoacetophenone, described preparation method comprises the steps:
[0029] Add 126g (1mol) p-fluoroanisole, 1000ml carbon tetrachloride, 199.5g (1.5mol) aluminum trichloride to the reaction flask, then add 302.7g (1.5mol) bromoacetyl bromide, and stir at 40°C after adding After 2 hours, HPLC followed the reaction until the reaction of p-fluoroanisole was complete; quenched into 2000ml of ice water, adjusted to pH=2, added 1000ml of dichloromethane for extraction and separation, concentrated the organic layer to dryness, and recrystallized with n-hexane to obtain 177.8g 2-methoxy-5-fluorobromoacetophenone, molar yield: 72%.
Embodiment 3
[0031] A kind of preparation method of 2-methoxy group-5-fluorobromoacetophenone, described preparation method comprises the steps:
[0032] Add 126g (1mol) p-fluoroanisole, 1000ml dichloromethane, 195g (1.3mol) trifluoromethanesulfonic acid to the reaction flask, add 262.3g (1.3mol) bromoacetyl bromide in batches, and stir at 30°C after adding After 6 hours, HPLC followed the reaction until the reaction of p-fluoroanisole was complete; quenched into 2000ml of ice water, separated into layers, concentrated the organic layer to dryness, and recrystallized with n-hexane to obtain 167.3g of 2-methoxy-5-fluorobromide Substituted acetophenone, molar yield: 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com