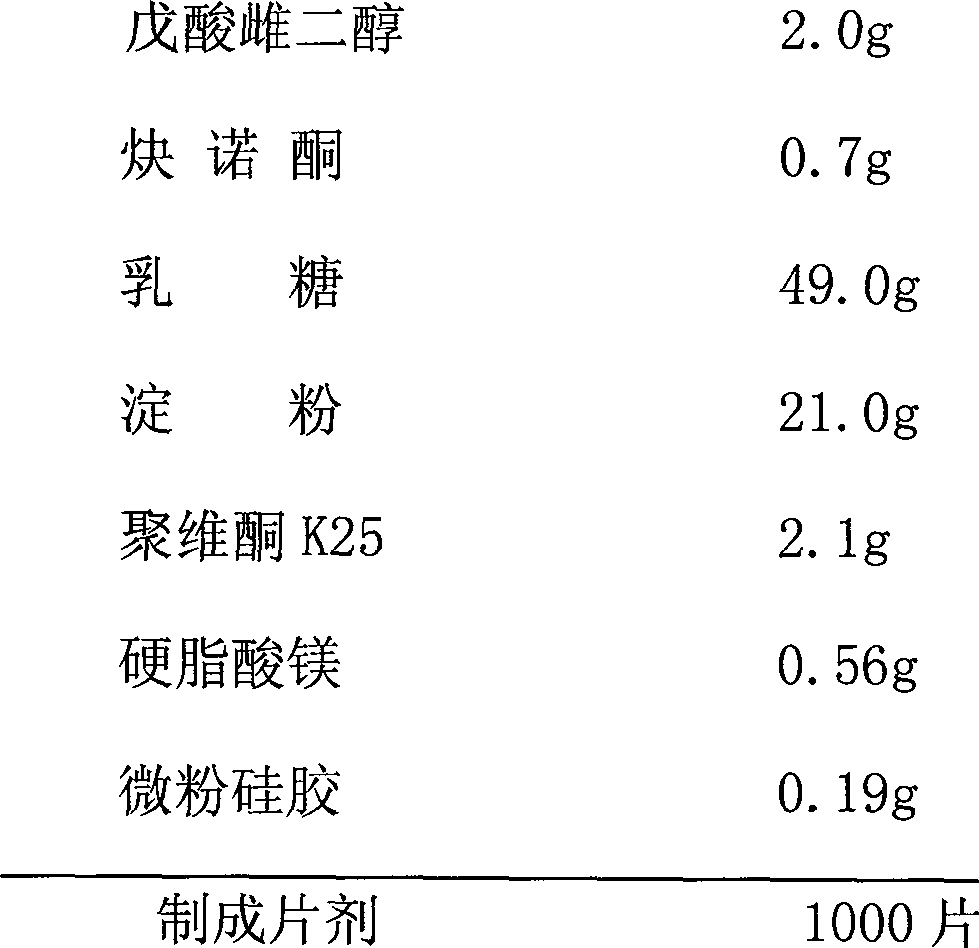
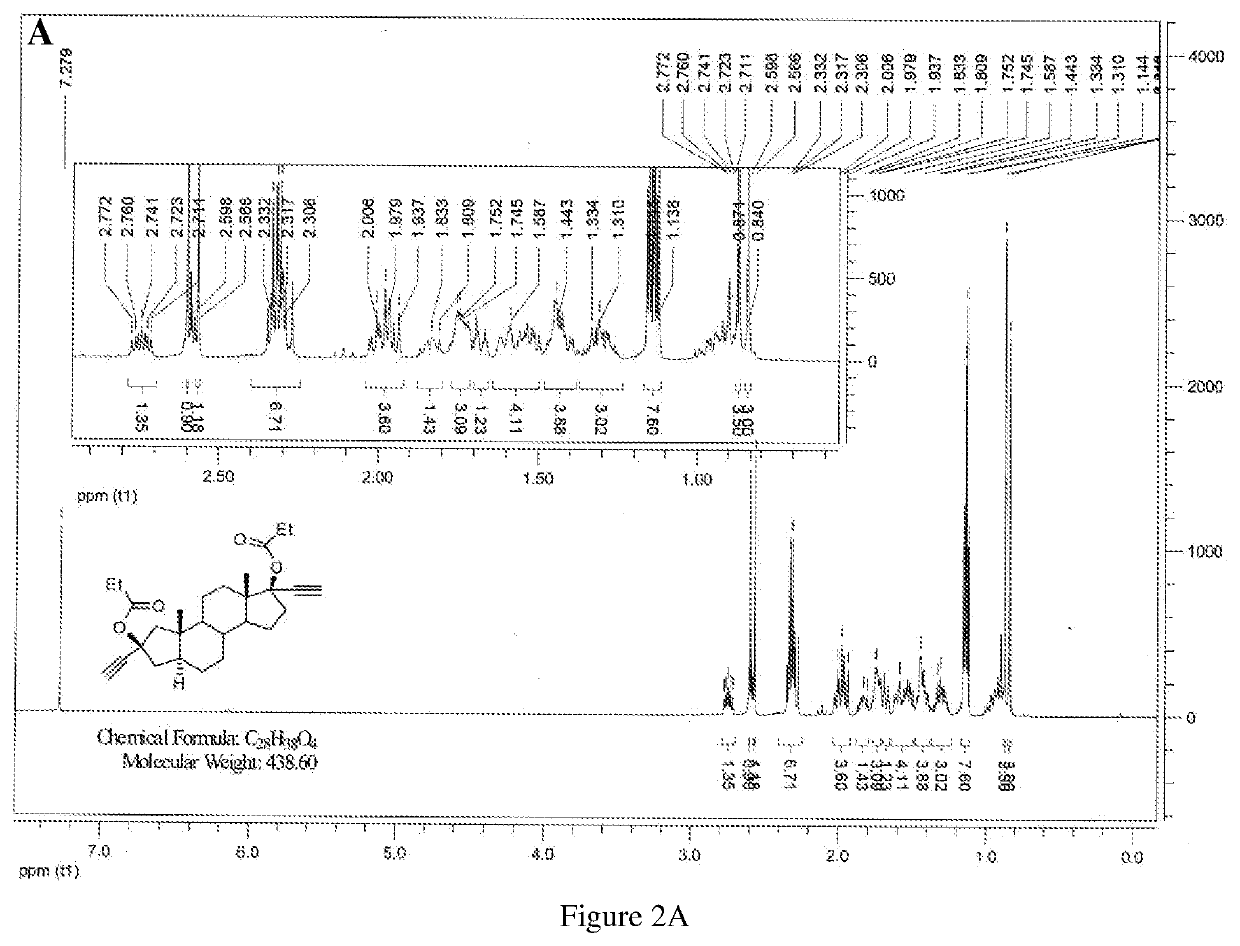
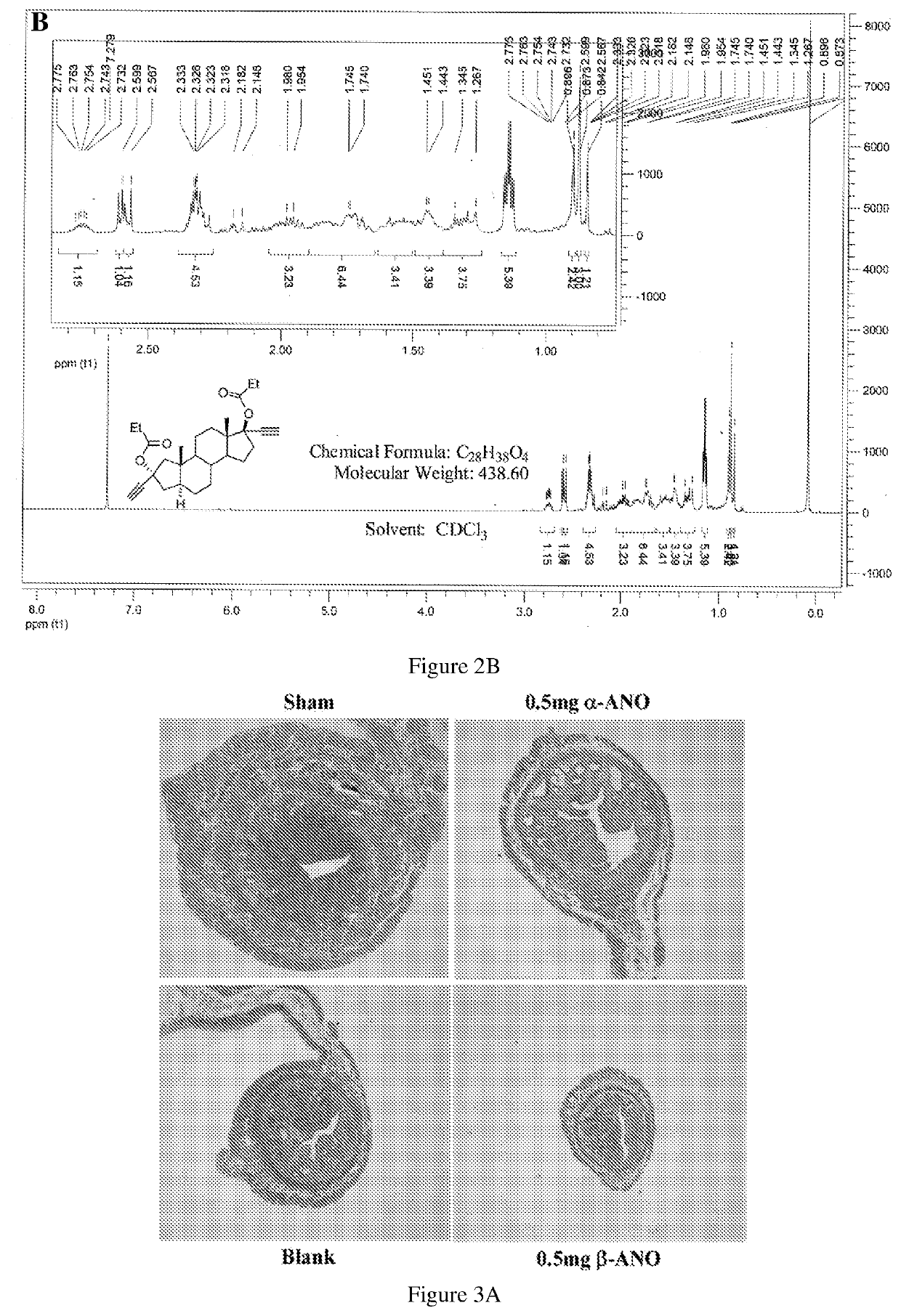
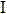Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Norethisterone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat women with abnormal bleeding from the uterus. It is also used to treat women who have stopped having menstrual periods for several months (amenorrhea) but who are not pregnant or going through menopause. In addition, this medication is used to treat a condition (endometriosis) in which tissue that normally lines the inside of the uterus is found outside the uterus in the abdomen/pelvic area, causing painful/irregular periods.
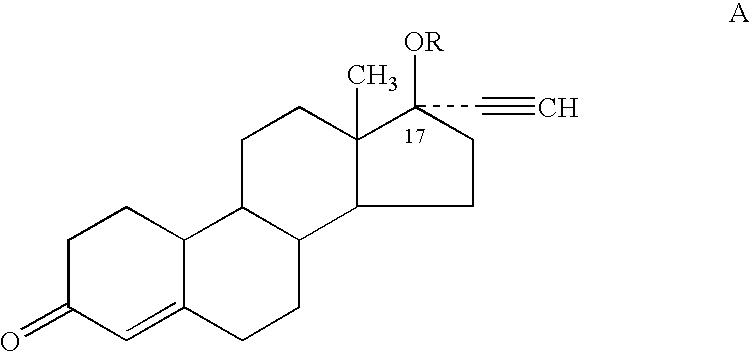
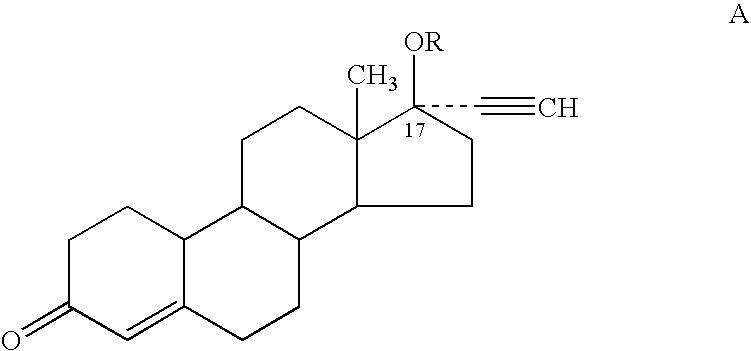
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:NIESCHLAG EBERHARD +5
Extended cycle multiphasic oral contraceptive method
ActiveUS8063030B2Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:BAYER SCHERING PHARMA AG
Extended cycle multiphasic oral contraceptive method
ActiveUS20070207945A1Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Compositions for preventing hormone induced adverse effects
InactiveUS20030004146A1Reduce adverse effectsInhibit cell proliferationHeavy metal active ingredientsBiocideSide effectEstrone
Owner:LYCORED NATURAL PRODS INDS
Extended estrogen dosing contraceptive regimen
A method of contraception that provides for sequentially administering to a female of child bearing age: (a) a first composition containing a progestin in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol for about 22 to about 26 days; (b) a second composition containing an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol for about 2 to about 3 days and an optional third composition that is a placebo provided that (i) if estrogen administration is continuous then the first composition is administered for 25 to 26 days, the second composition is administered for 2 to 3 days and no third composition is administered and (ii) if estrogen administration is not continuous then the first composition is administered for 22 to 24 days, the second composition is administered for 2 to 3 days and the third composition is administered for 1 to 4 days. The total cycle length is 28 days, with the first composition administered on day 1 of the menstrual cycle, defined as the first day of menstrual bleeding, or on the first Sunday after the first day of the menstrual cycle.
Owner:APTALIS PHARMA
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:BAYER SCHERING PHARMA AG
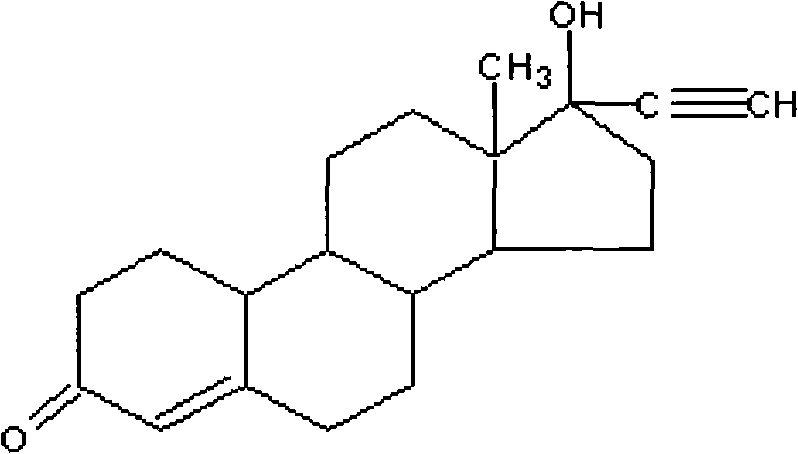
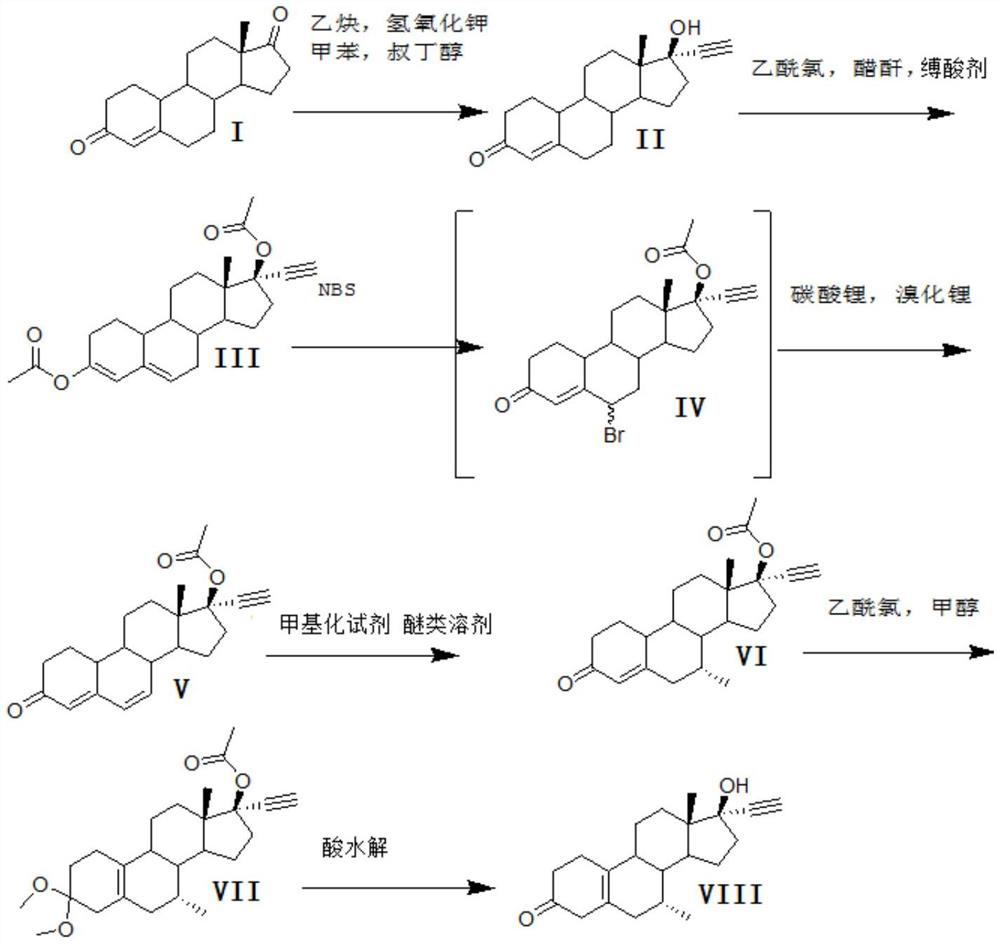
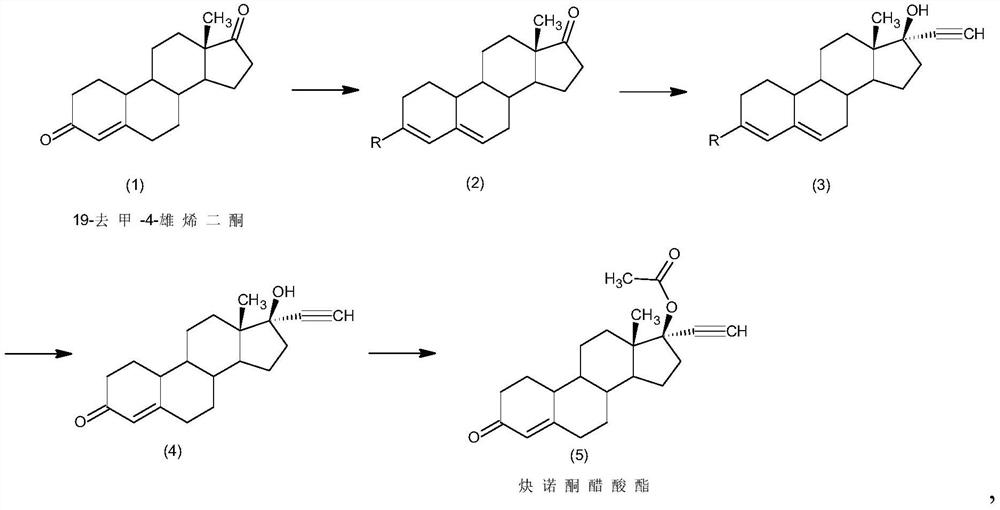
Method for synthesizing 6-methyl-17alpha- hydroxyl-19-nor-pregnene-4,6-diene-3,20-diketone
ActiveCN105017365AReduces the possibility of metal residueThe reaction is easy to operateSteroidsCyclohexeneKetone
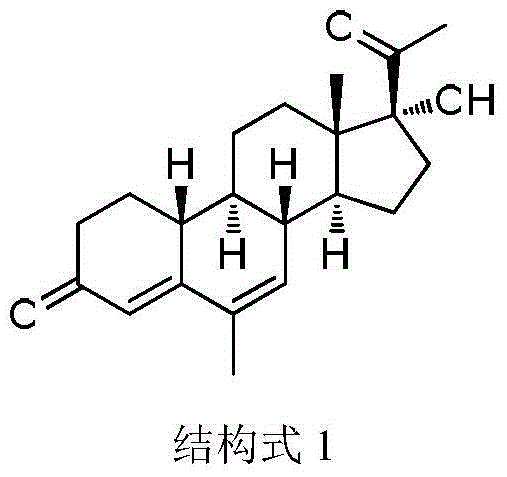
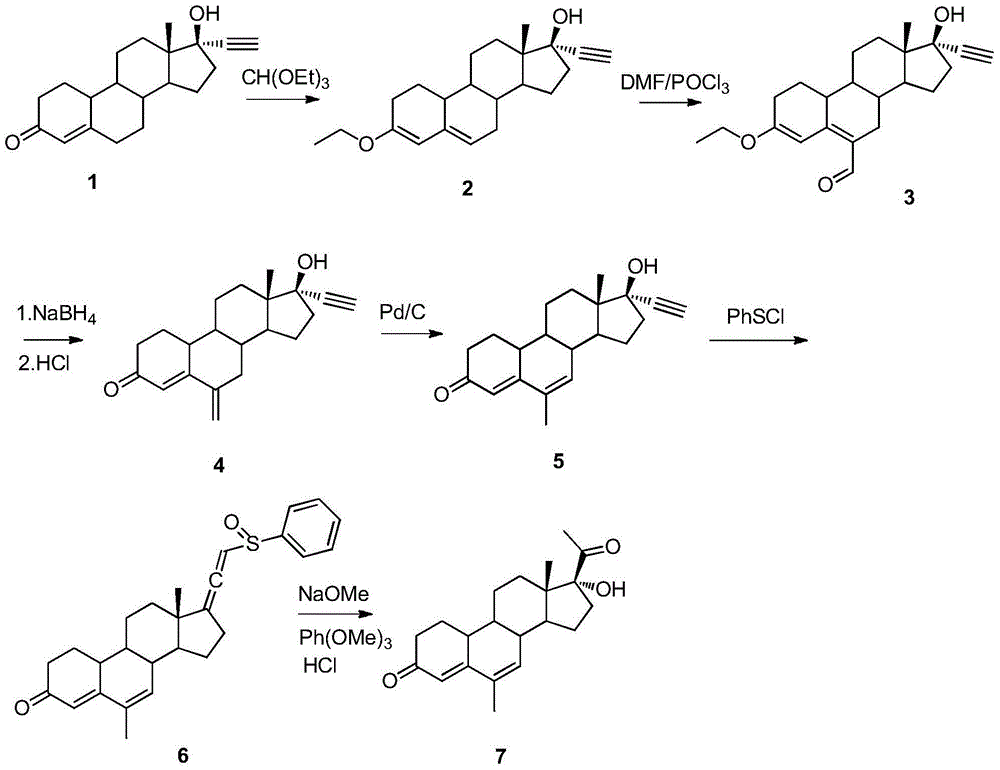
The invention relates to the field of pharmaceutical synthesis, in particular to a method for synthesizing 6-methyl-17alpha- hydroxyl-19-nor-pregnene-4,6-diene-3,20-diketone. The method comprises the steps of reacting norethisterone with ethylorthoformate to obtain 3-ethoxy-17alpha-acetenyl-19-demethyl-pregnene-3,5-diene-17beta-alcohol (2); causing the compound (2) to undergo Vilsmeier reaction to obtain 3-ethoxy-6-formoxyl-17alpha-acetenyl-19- demethyl-pregnene-3,5-diene-17beta-alcohol (3); reacting the compound (3) with NaBH4 and obtaining 6-methine-17alpha-acetenyl-17beta-hydroxyl-19-demethyl-pregnene-4-en-3-ketone (4) through acidification and dehydration; causing the compound (4) to undergo double bond shift under the effect of Pd-C / cyclohexene to obtain a product 6-methyl-17alpha-acetenyl-17beta-hydroxyl-19-demethyl-pregnene-4,6-diene-3-ketone (5); and reacting the compound (5) with benzene sulfenyl chloride to obtain 6-methyl-19-demethyl-21-phenylsulfinyl-pregnene-4,6,17(20)20-tetraene-3-ketone.
Owner:丽江华映激素药物科技开发有限公司
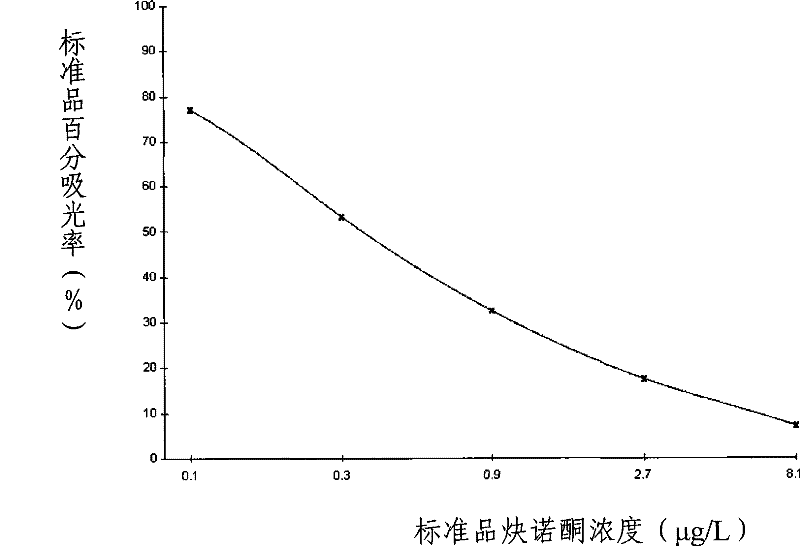
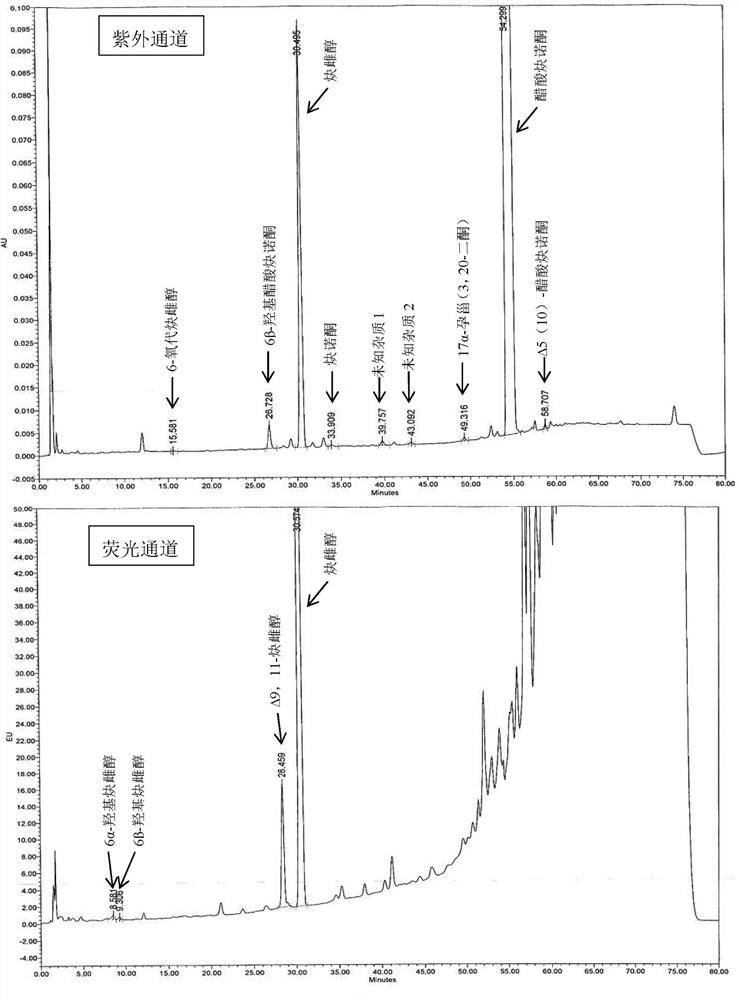
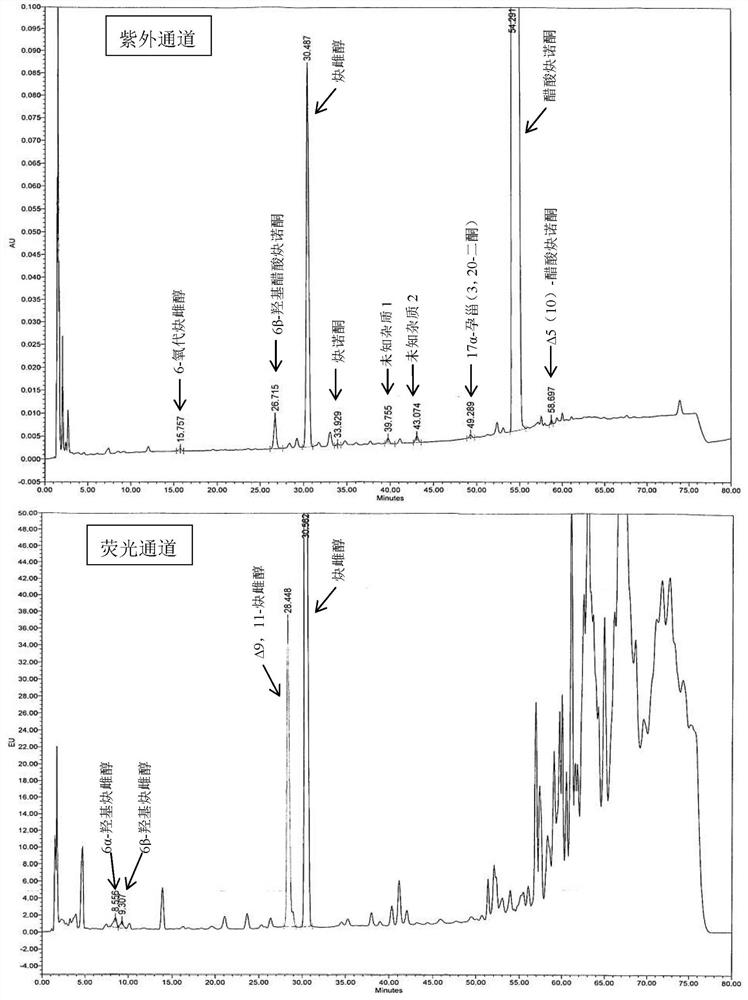
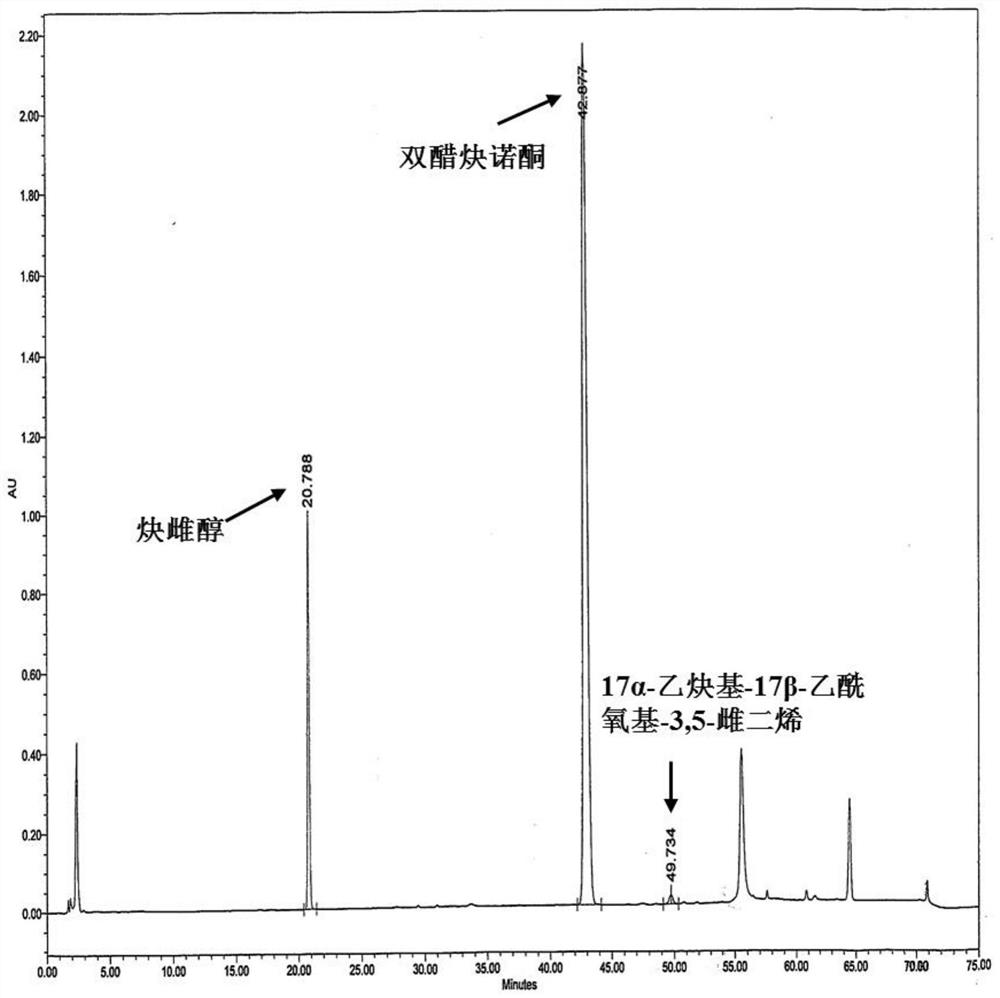
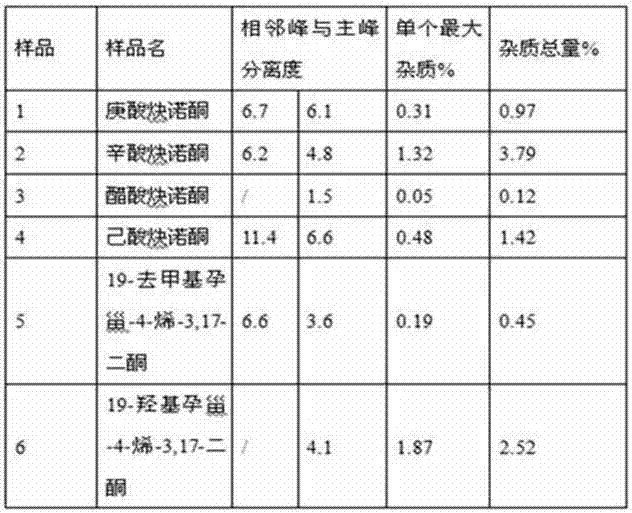
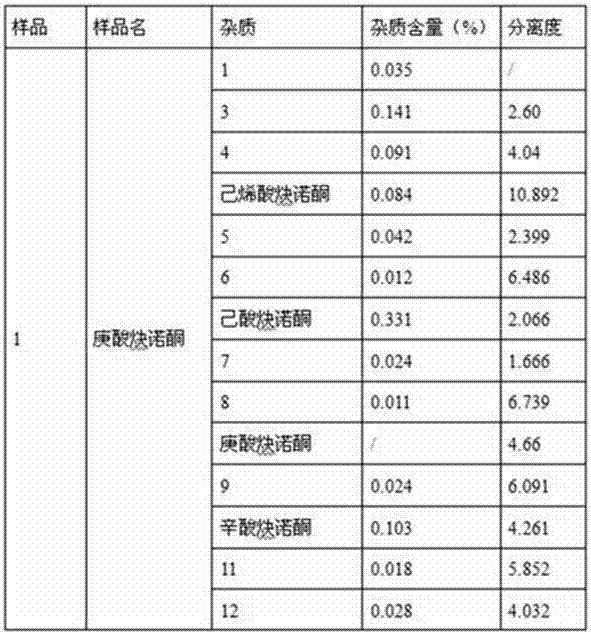
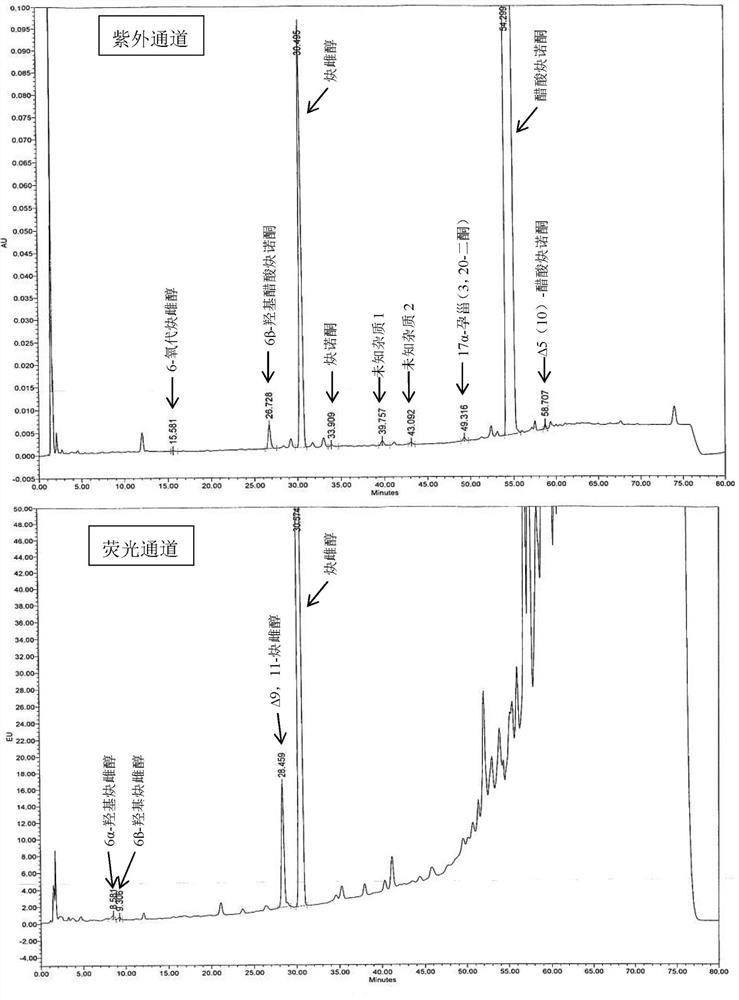
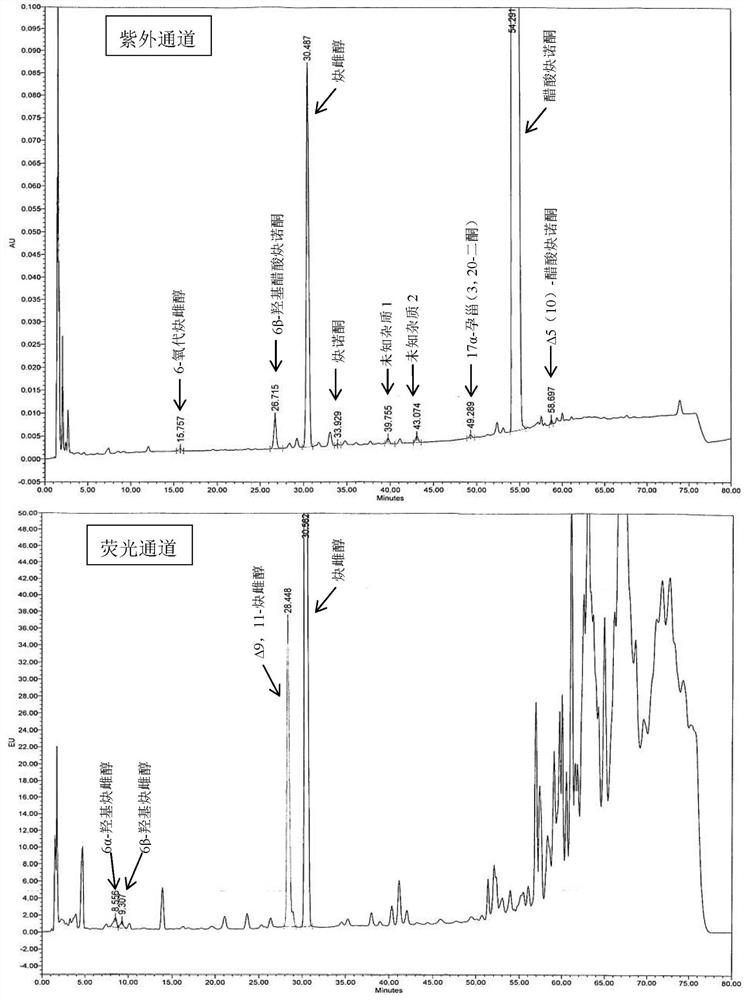
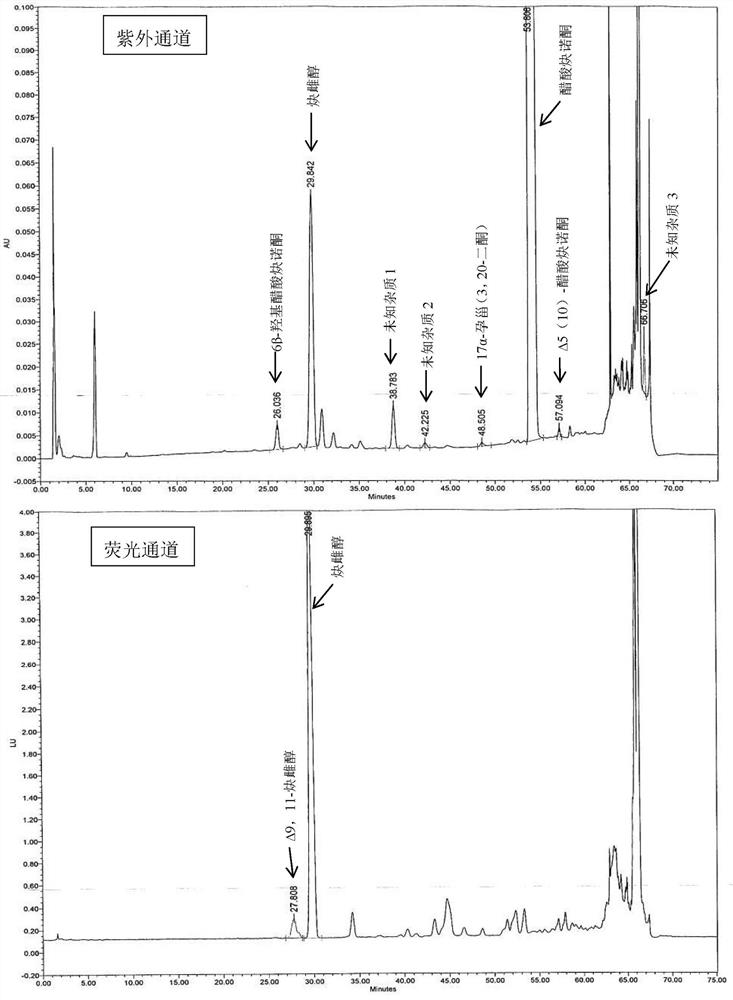
Impurity detection analysis method of norethisterone derivatives and intermediates thereof
ActiveCN105277633AEasy to operateEfficient separationComponent separationWater methanolGradient elution
The invention relates to an impurity analysis method of a steroid compound, and especially relates to an impurity detection analysis method of norethisterone derivatives and intermediates thereof. The method comprises the following steps: (1) a chromatographic column with octadecylsilane chemically bonded silica as a filler is selected; a mobile phase gradient elution is carried out, and the mobile phase contains water, methanol and acetonitrile; the detection wavelength is 230-254nm; and (2) a proper amount of the test sample norethisterone derivatives or intermediates thereof are selected, methanol is precisely weighed for dissolving and diluting the test sample to form a sample solution of a certain concentration, the solution is uniformly shaken, and a high performance liquid chromatography analysis is carried out at a proper flow velocity and a proper column temperature, in order to record a chromatogram. The method can be used for rapidly and accurately realizing impurity analysis of norethisterone derivatives, and is especially suitable for the impurity analysis of norethisterone enanthate, and simultaneously can be used for impurity analysis of norethisterone derivative intermediates, and can be used for effectively tracing impurities generated in the synthesis process.
Owner:ZHEJIANG XIANJU PHARMA
Application of ER and Ki-67 expression ratio in EC and AH retention fertility function treatment prognosis
PendingCN114441777ASignificant predictionMedical automated diagnosisDisease diagnosisComplete remissionStatistical analysis
The invention discloses an application of an ER and Ki-67 expression ratio in endometrial tissues in fertility function retention treatment prognosis of endometrial cancer (EC) and atypical endometrial hyperplasia (AH). According to the invention, 53 EC patients and 68 AH patients orally take MPA or MA, then gonadotropin is combined to release a hormone agonist and / or a levoynorethindrone intrauterine sustained release system, the patients are divided into a recurrence group and a non-recurrence group according to whether recurrence occurs after complete remission, then ER, PR, P16, P53, PTEN, Ki-67 and the like are subjected to immunohistochemical analysis, and statistical analysis is carried out. Results show that the expression rate of the Ki-67 is of great significance in predicting recurrence after the EC and AH retention fertility function treatment, and the optimal critical value (3.55) of ER / Ki-67 can predict recurrence after the EC and AH retention fertility function treatment better than that of a single immunohistochemical marker. The method has an important application value.
Owner:PEOPLES HOSPITAL PEKING UNIV
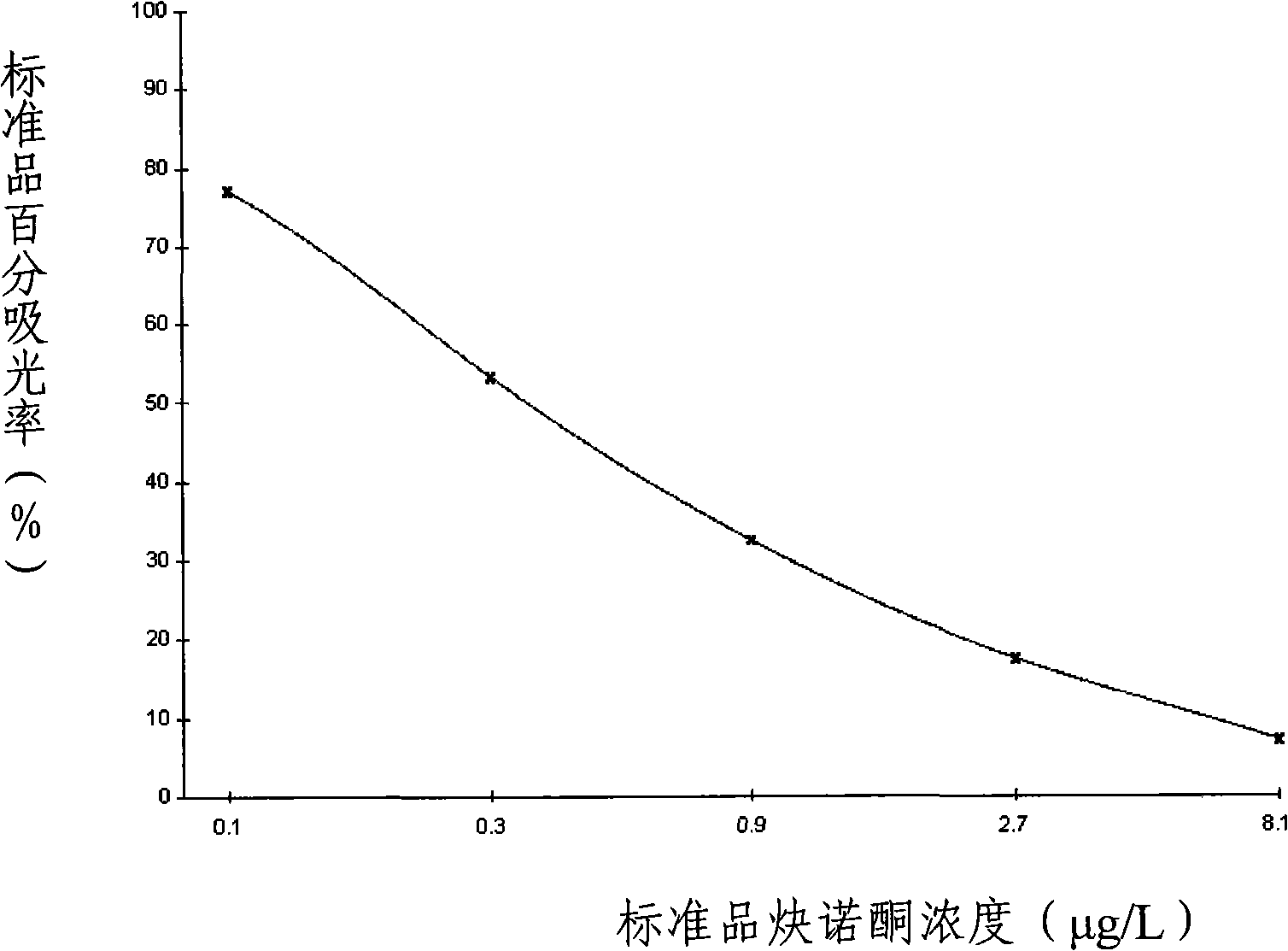
Method for detecting norethindrone and special ELISA kit thereof
InactiveCN101358965AStrong specificityHigh sensitivityFused cellsSteroidsElisa kitMonoclonal antibody
The present invention discloses a method of detecting norethisterone and a special enzyme linked immunosorbent kit thereof. The enzyme linked immunosorbent kit which is used for detecting the norethisterone comprises norethisterone haptens and specific antibodies of the norethisterone; and the specific antibodies are polyclonal antibodies or monoclonal antibodies of the norethisterone. The kit adopts the high specificity monoclonal antibodies of the norethisterone, which guarantees the reliability of detection results, and the experimental results show that the kit has the characteristics of high specificity, high sensitivity, high precision, high accuracy and the like; and the main reagents of the kit all adopt the form of working liquid, the operation is convenient, and the cost is low.
Owner:贵州勤邦食品安全科学技术有限公司
Method for removing norgestrel, norethisterone enanthate and norethindrone in waste water of pharmaceutical factory
InactiveCN105016449AStrong complexing abilityFast precipitationWater contaminantsNature of treatment waterArginineKetone
The invention relates to a method for removing norgestrel, norethisterone enanthate and norethindrone in waste water of a pharmaceutical factory. 1-vinyl-1-methyl-2,4-2(c-1-ene-2-base)cyclohexane, tetradecanoic acid-1-methyl ethyl ester, hydroxysuccinic acid, sodium pentahydyoxycaproate, argininyl-fructoyl-glucose, 5-allylguaiacol, 1 hydrogen-tetrazolium-1-acetic acid, (2R, 3R)-3,5,7-trihydroxy-2-(3,4,5-3 hydroxy phenyl) benzodihydropyran-4-ketone, alpha-naphthol-8-sulfonic acid, nitrilotrimethylene triphosphonic acid, 3-[10,10-dimethyl-9(10H)-anthracene subunits]-N, N-dimethyl propyl amine hydrochloride, beta-caryophyllene, alchlor, arginine and microcrystalline cellulose are sequentially added into the waste water in three steps. According to the method, the complexing ability with target materials is high, and the removing rate can reach 99.9 percent.
Owner:沈健龙
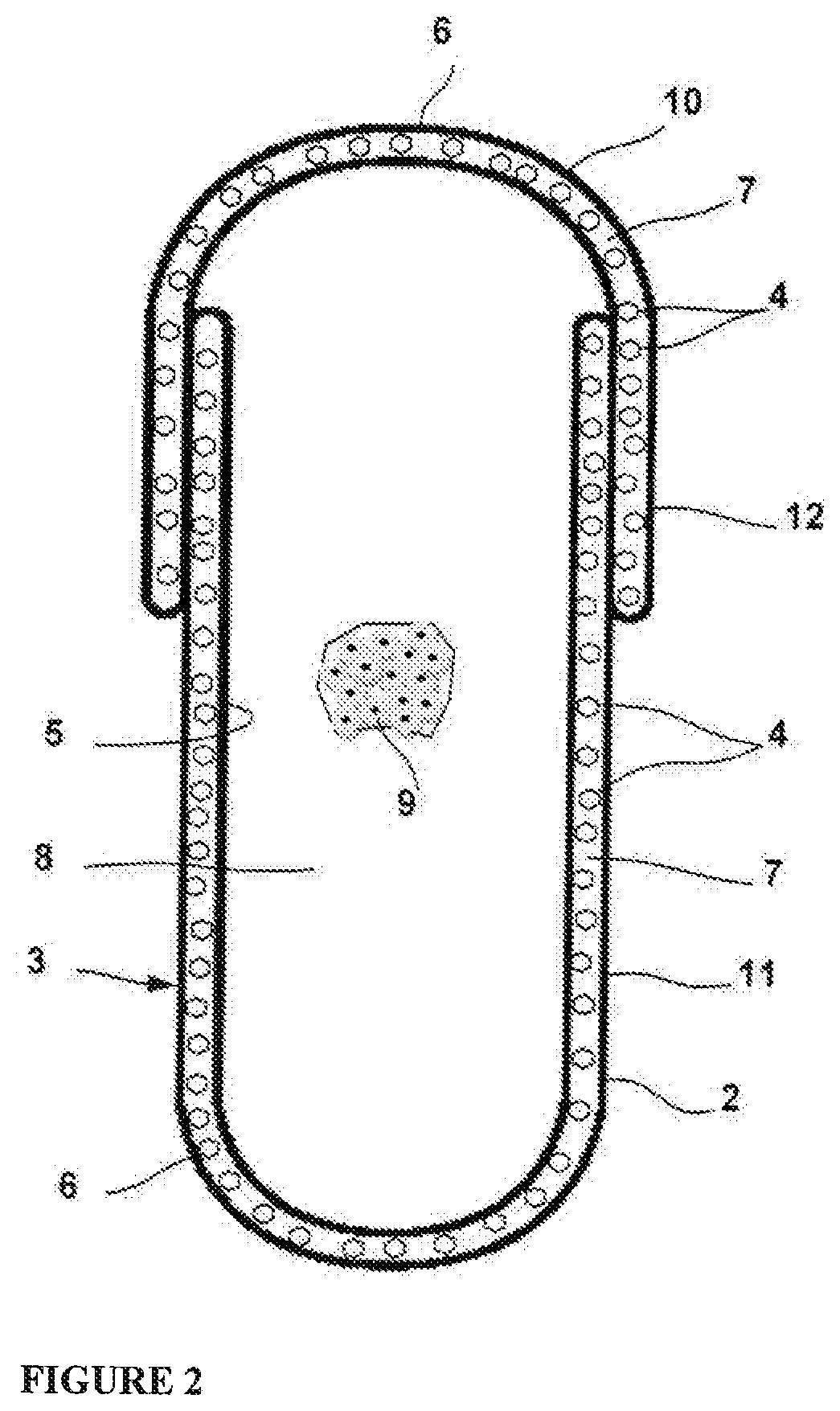
Hard Capsule Shell Compositions for the Oral Contraceptive Formulations
InactiveUS20190343770A1Organic active ingredientsInorganic non-active ingredientsDrospirenoneDietary supplement
A hard shell capsule includes a body and a cap cooperatively defining a hollow core hard shell capsule. Each of the body and the cap has a composition that includes a polymer forming a hard polymer structure of the body and of the cap and comprises a drug. The body further comprises a therapeutically effective amount of drug A loaded throughout the composition; the cap further comprises a composition comprising a therapeutically effective amount of drug B loaded throughout the composition. The body and cap compositions together containing a therapeutically effective amount of the drugs A and B; said drugs being oral contraceptive agents. The core of the capsule is filled with therapeutically effective amount of a second drug(s), a dietary supplement, minerals, a complexing agent and other excipients. With the help of FIG. 2, the invention can be very well understood easily. Drugs A and B are selected from the group of oral contraceptives, but are not limited to, Cyproterone acetate, Estradiol, Oestradiol, Norethindrone acetate, Ethinyl Estradiol, Levonorgestrel, Dienogest, Drospirenone, Desogestrel, Ethynodiol, Diacetate, Mestranol, Nomegestrol acetate, Norgestrel, Norgestimate, Dienogest, Norelgestromin, Norethisterone, Gestodene, Oestradiol valerate, and Ethynodiol diacetate.
Owner:JOSHI HEMANT N +1
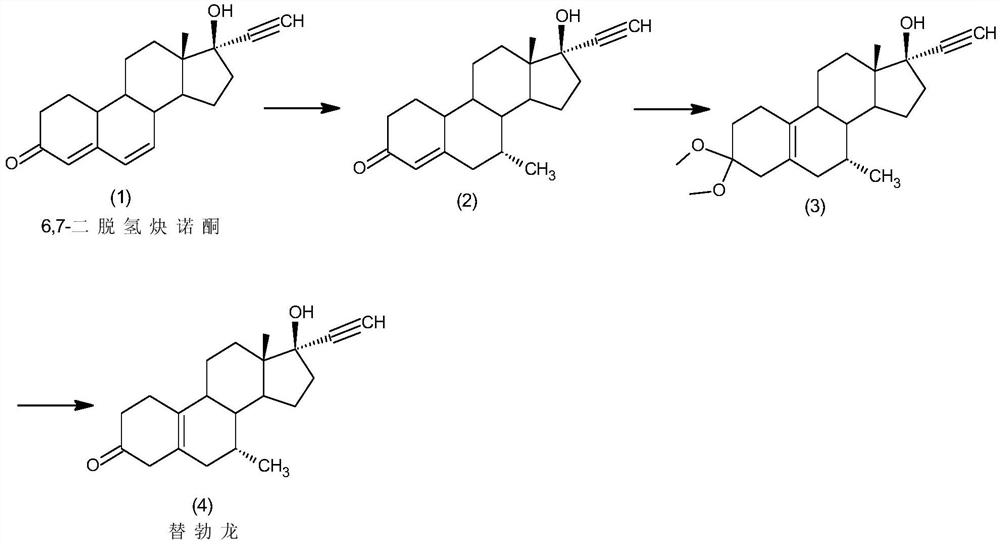
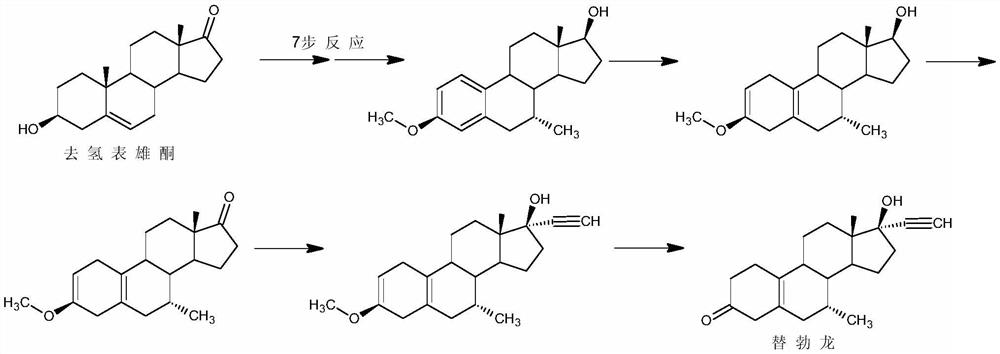
Preparation method of tibolone
InactiveCN111944001ASimple purification processLess amount of 7β-methyl isomerSteroidsChromatographic separationHormone drug
The invention discloses a preparation method of tibolone, and belongs to the technical field of preparation and processing of steroid hormone drugs. According to the method, 6,7-didehydronorethindroneis used as an initial raw material, and tibolone is prepared through Grignard reaction, dietherification reaction and hydrolysis reaction. The 7-methyl introduction step is included in the Grignard reaction step in the synthesis route, so that special protection on 17-site hydroxyl is avoided, and the ratio of the 7-alpha methyl intermediate to the 7-beta methyl isomer obtained through the reaction is larger than 20:1; therefore, the single 7-alpha methyl intermediate can be obtained through simple treatment without chromatographic separation, the purification process is simple, and the purity of the final product reaches 99.0% or above, and the yield is higher than 60%; the raw materials are cheap and easily available, the reaction steps are few, the reaction conditions are mild and safe, and the control operation is easy; in addition, reagents used in the reaction are low in environmental pollution, and good economic and social benefits are achieved.
Owner:ZHEJIANG SHENZHOU PHARMA
Compound estradiol valerate tablet and its preparation process
InactiveCN100462078CImprove bioavailabilityHigh dissolution rateOrganic active ingredientsPill deliveryOrally disintegrating tabletBioavailability
The invention relates to a compound estradiol valerate tablet and its preparation process, wherein the active constituents of the tablet include oestradiol valerate and norethisterone, and at least include a crumbling agent, a soluble deflocculating agent with binding property, a binding agent and a lubricating agent. The tablet has rather good bioavailability in vivo and increased dissolving degree externally.
Owner:HAINAN PULIN PHARMA +2
Asymmetric synthesis and uses of compounds in disease treatments
PendingUS20200179323A1Preventing and reducing estrogen-deficiency symptomEliminate side effectsSkeletal disorderEster active ingredientsDiseaseSide effect
The present application discloses, among other things, asymmetric synthesis a diastereomeric compound of formula (I) (e.g., α-anordrin) or salt thereof. Also provided are methods and compositions for treatment of estrogen deficiency as well as preventing or reducing an estrogen deficiency symptom using a diastereomeric compound of formula (I) (e.g., α-anordrin) or salt thereof alone or in combination with at least one additional agent. Further provided are methods and compositions for reducing a side effect of an additional agent in the context of combination therapy with a diastereomeric compound of formula (I) (e.g., α-anordrin) or salt thereof.
Owner:ZHEJIANG JIACHI DEV PHARMA LTD
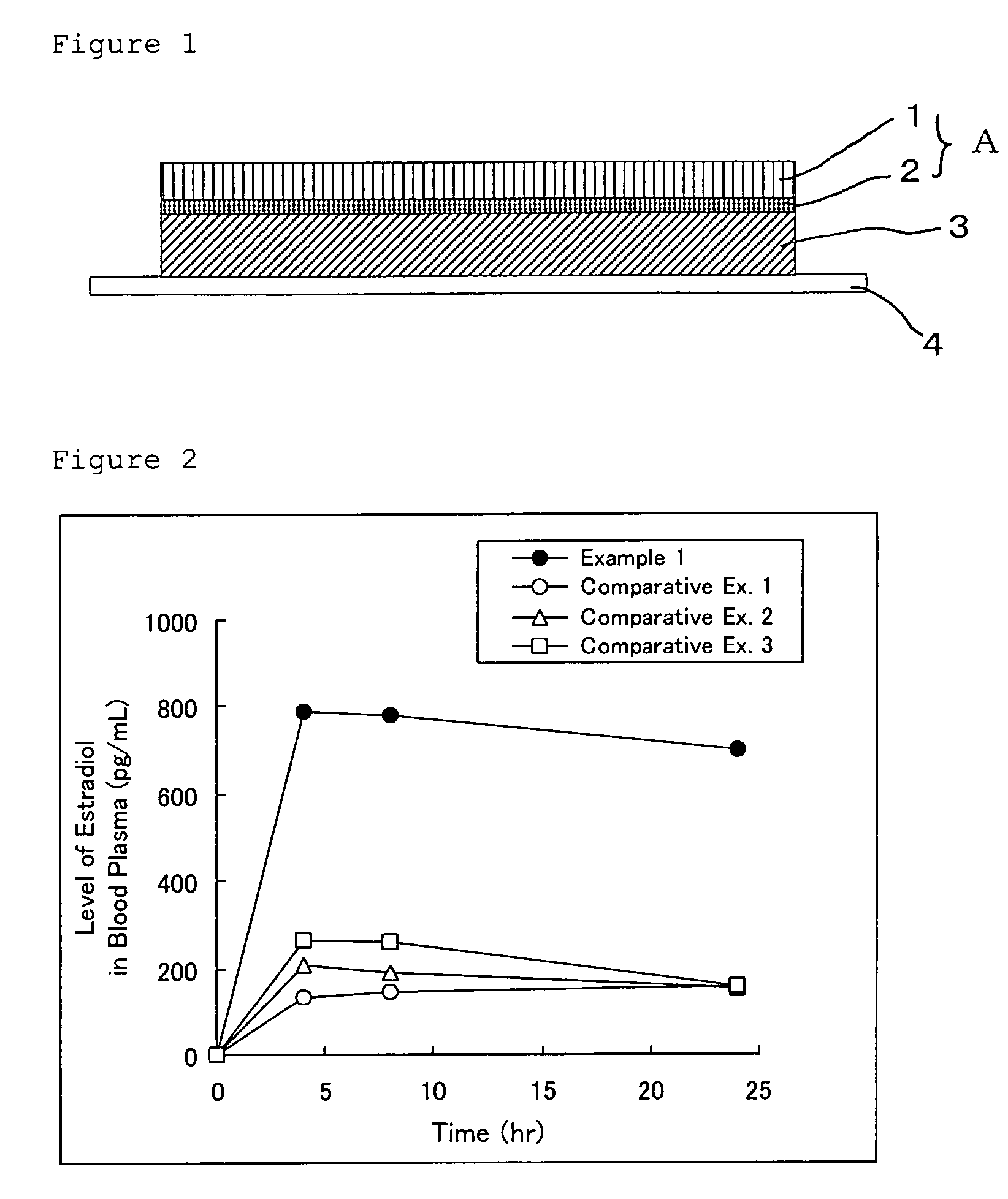
Norethisterone acetate-containing compound estradiol transdermal sustained release preparation and preparation method thereof
ActiveCN103054879AAvoid degradationAvoid first pass effectOrganic active ingredientsSkeletal disorderControlled releaseTransdermal patch
The invention discloses a norethisterone acetate-containing compound estradiol transdermal sustained release preparation and a preparation method thereof. The preparation comprises a backing layer, a protective film and a frame layer between the backing layer and the protective film, wherein the frame layer comprises an upper frame layer, a lower frame layer and a controlled release film between the upper frame layer and the lower frame layer; and the backing layer covers the surface of the lower frame layer. The upper and lower frame layers are respectively wrapped by estradiol and norethisterone acetate in a certain ratio, an intermediate frame layer is wrapped by a special transdermal patch of the controlled release film, estrogen estradiol effects in relieving or eliminating climacteric syndromes and preventing osteoporosis are fully played, and the preparation also has the characteristics of small stimulus to human bodies, small side effects, quality stability and long effective time.
Owner:ZHEJIANG YATAI PHARMA
Rapid-to-disintegrate vaginal soft capsule composition and preparation method thereof
InactiveCN112336698ADisintegrates quicklyStable traitsOrganic active ingredientsPharmaceutical non-active ingredientsCarrageenanEstriol
The invention relates to a rapid-to-disintegrate hormone vaginal soft capsule shell. The hormone vaginal soft capsule shell consists of a compound capsule shell capable of rapidly disintegrating in asmall amount of weakly acidic vaginal fluid and a functional content; the capsule shell consists of 6%-12% of carrageenan, 10%-40% of modified starch, 10%-25% of plasticizer, 3%-5% of disintegrating agent, 0%-0.2% of preservative and water; and the functional content can be hormone drugs including progesterone, estriol, norethindrone, megestrol and the like. The invention further discloses a method for preparing the soft capsule shell by using the composition and a soft capsule prepared from the soft capsule shell. The vagina soft capsule shell provided by the invention has the advantages of rapid disintegration, excellent aging resistance, stable components and the like.
Owner:南京科宁检测科技有限公司
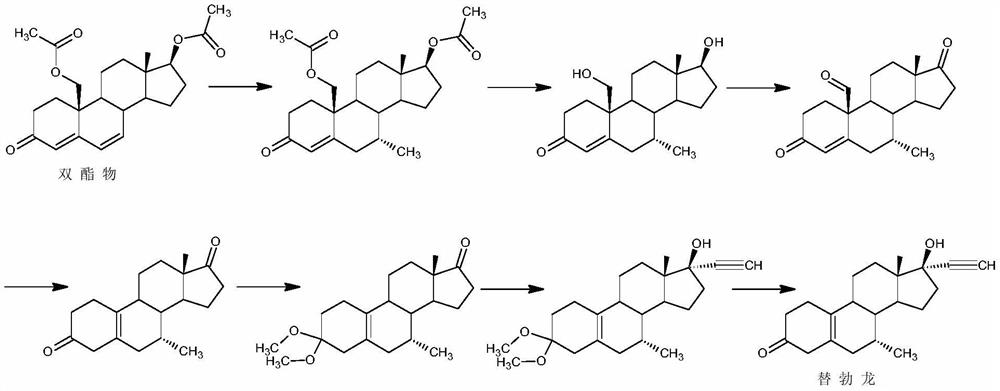
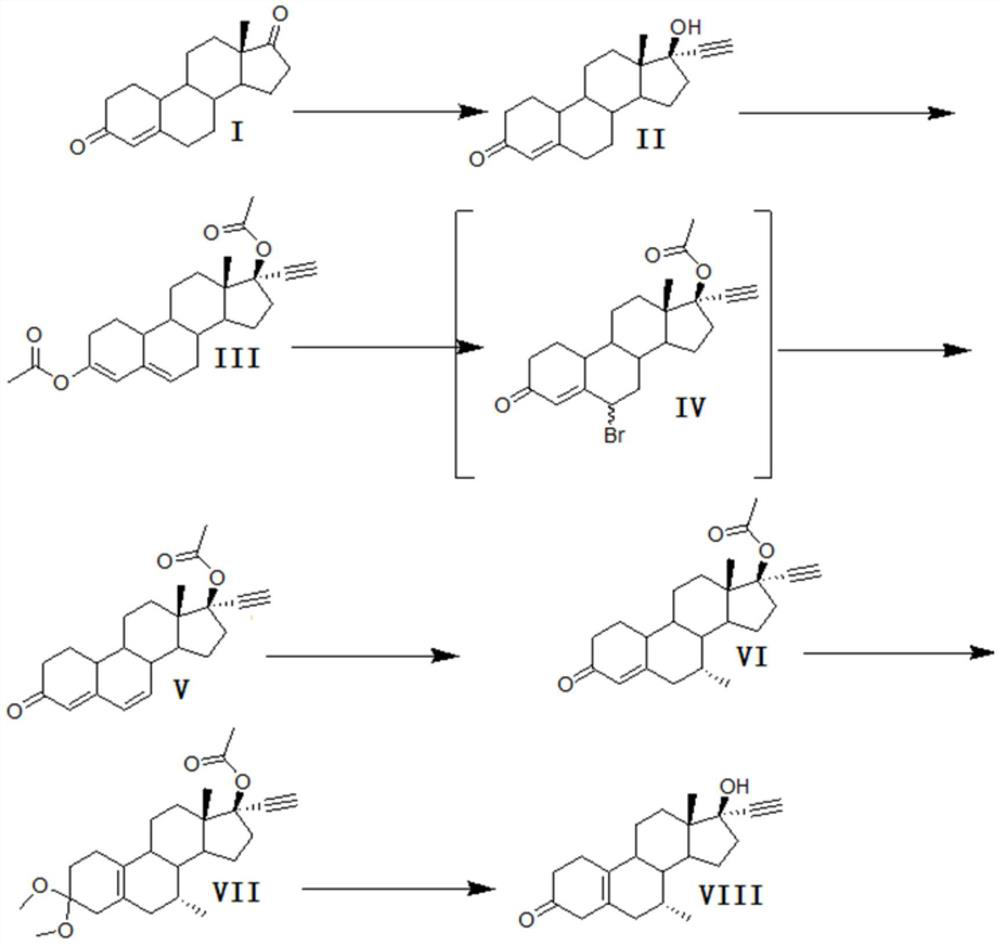
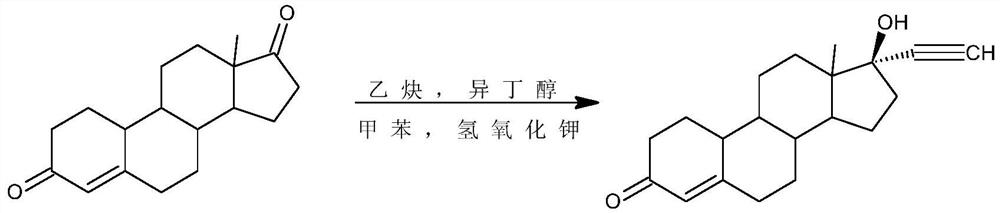
A kind of synthetic method of tibolone
A synthetic method for tibolone, specifically comprising the following steps: 1) acetylenylation reaction: acidic decarboxylation (I) toluene solution, feed acetylene gas to the raw material to react to obtain norethindrone (II); 2) acylation reaction, Step 1) Add acetic anhydride and acid-binding agent to the obtained norethindrone (II), add acetyl chloride dropwise under control at room temperature, and stir the reaction for 6 hours until the raw materials are completely reacted to obtain the acylate (III); 3) debromination reaction, the acyl compound reaction to obtain norethindrone 4,6-diene acetate (V); 4) methylation reaction, norethindrone 4,6-diene acetate (V), add ether solvent, and cool to -10~30°C , drop methylation reagent, control the temperature at 0-5°C and stir the reaction until the raw materials react completely to obtain 7α-norethindrone methyl acetate (VI); 5) transposition reaction, 7α-norethindrone methyl acetate (VI) React to get transposition (VII) wet product; 6) hydrolysis reaction, translocation (VII) reaction to get tibolone.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD +1
Method for detecting norethindrone and special ELISA kit thereof
InactiveCN101358965BStrong specificityHigh sensitivityFused cellsSteroidsElisa kitMonoclonal antibody
The present invention discloses a method of detecting norethisterone and a special enzyme linked immunosorbent kit thereof. The enzyme linked immunosorbent kit which is used for detecting the norethisterone comprises norethisterone haptens and specific antibodies of the norethisterone; and the specific antibodies are polyclonal antibodies or monoclonal antibodies of the norethisterone. The kit adopts the high specificity monoclonal antibodies of the norethisterone, which guarantees the reliability of detection results, and the experimental results show that the kit has the characteristics of high specificity, high sensitivity, high precision, high accuracy and the like; and the main reagents of the kit all adopt the form of working liquid, the operation is convenient, and the cost is low.
Owner:贵州勤邦食品安全科学技术有限公司
An analytical method for multiple active ingredients in oral contraceptive placebo tablets
ActiveCN110057939BThe testing process is simpleQuick testComponent separationOral contraceptive drugEthylic acid
The invention discloses an analysis method for various active ingredients in oral contraceptive pill placebo tablets. Firstly, put the placebo tablet to be tested into a measuring bottle, then add water to make it disintegrate, and then add an organic solvent (acetonitrile) for ultrasonic extraction. Then use acetonitrile to set the volume to scale, filter and get the filtrate as the test sample analysis; adopt liquid chromatography to analyze the test sample, start the chromatographic acquisition computer system to collect data; get norethindrone acetate, desogestrel and ethinyl estradiol contrast For the product, prepare the reference solution with a mixed solution of acetonitrile and water, and carry out liquid chromatography analysis simultaneously to check whether the placebo tablet contains multiple active ingredients. The invention has the advantages of strong specificity, rapid analysis, strong anti-interference and high sensitivity, and solves the problem that multiple active ingredients in oral contraceptive placebo tablets need to be repeatedly measured.
Owner:NOVAST LABORATORIES (CHINA) LTD
Stable pharmaceutical composition containing ethinylestradiol and norethindrone acetate
ActiveCN114129573AControl Oxidative DegradationImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsEthylic acidNorethisterone acetate
The invention discloses a stable pharmaceutical composition containing ethinylestradiol and norethindrone acetate. Vitamin E is added, so that the stability of ethinylestradiol is improved; and by using a specific amount of vitamin E, the production of related substances of ethinylestradiol and norethindrone acetate is controlled at the same time, and the overall stability of the compound preparation is improved.
Owner:北京联嘉医药科技开发有限公司
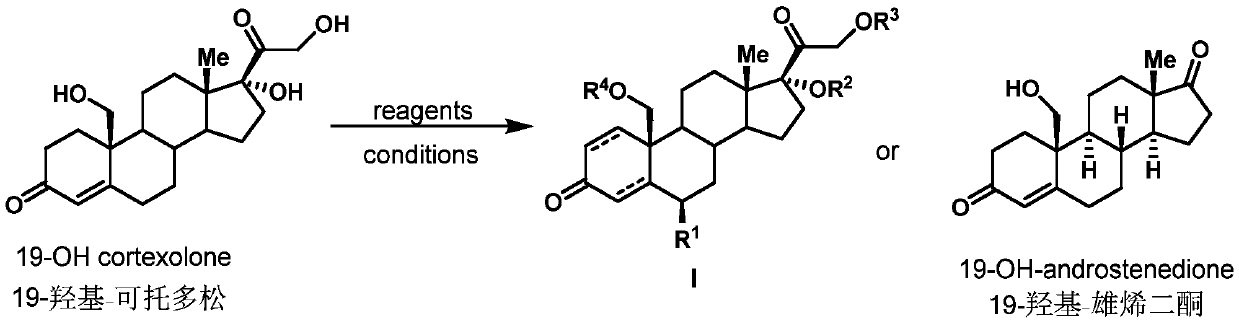
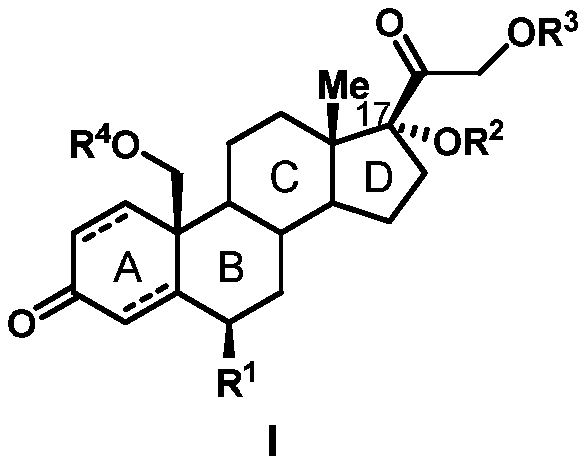
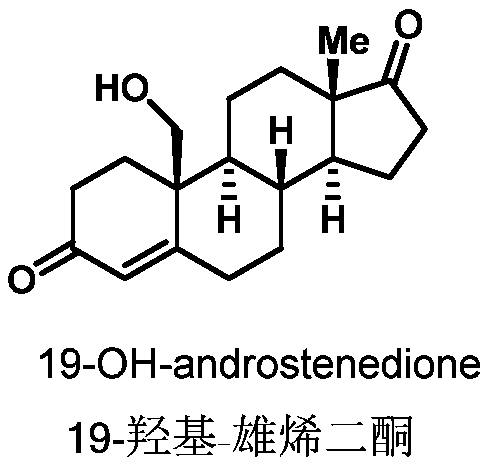
Preparation method of 19-hydroxylated cortodone derivatives and 19-hydroxyandrostenedione
Owner:WUHAN UNIV
Stable norethindrone ethinylestradiol compound tablet and preparation method thereof
ActiveCN114569567AMeet the requirements of rapid dissolutionPromote absorptionOrganic active ingredientsPill deliveryEngineeringEthinylestradiol
Owner:NOVAST LABORATORIES (CHINA) LTD
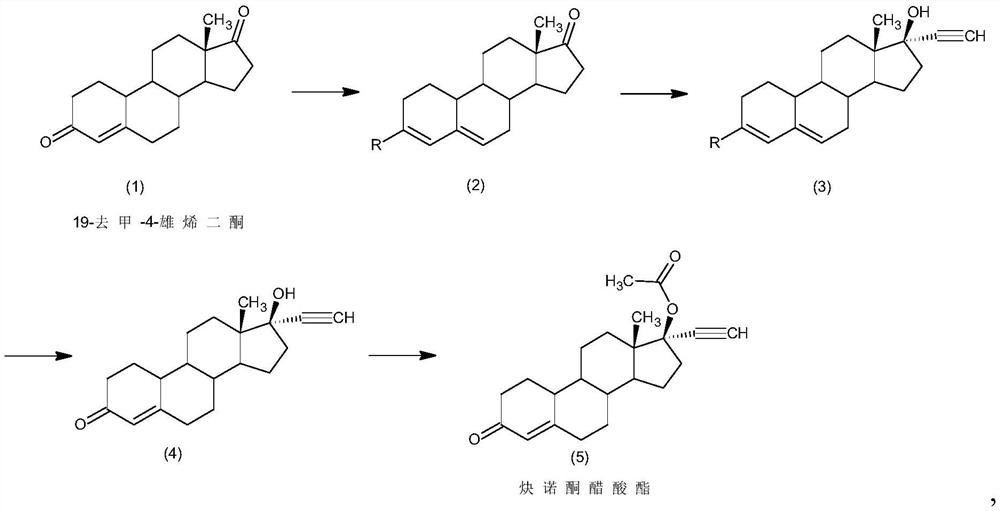
Preparation method of norethindrone acetate
InactiveCN111875656AAvoid it happening againMild responseSteroidsSexual disorderBiochemical engineeringEthylic acid
The invention discloses a preparation method of norethindrone acetate, and belongs to the technical field of preparation and processing of medicines. According to the method, 19-nor-4-rostenedione isused as an initial raw material, and the norethindrone acetate is prepared through four steps of protection, ethynylation, hydrolysis and esterification. According to the preparation method of norethindrone acetate, the defects of a traditional process are overcome, reaction conditions are mild, and formation of impurities is reduced; the method is high in overall conversion rate, simple and convenient to operate, suitable for industrial production and wide in market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Western medicine composition for treating tetanus and preparation method thereof
InactiveCN106620004AHeat-clearing and detoxifyingGreat tasteAntibacterial agentsOrganic active ingredientsSide effectVitamin C
The invention discloses a western medicine composition for treating tetanus and a preparation method thereof. The western medicine composition consists of the following raw materials in parts by weight: 3-7 parts of norethisterone tablets, 0.5-2 parts of ox horn, 8-15 parts of hydroxyethyl cellulose powder, 2-5 parts of cocoon shells, 2-5 parts of cut tobacco, 10-50 parts of normal saline, and 0.01-0.1 part of vitamin C. The western medicine composition achieves a synergistic effect by virtue of raw material compounding, has the effects of clearing away heat and toxic materials, diminishing inflammation and swelling, and drawing out toxin, and can inhibit and kill activity of haemophellolus influenza; complications are not discovered in a treatment process, and obvious toxic or side effects do not appear; the curative effect is short, the effect is achieved quickly, the preparation method is simple, the production cost is low, the economic pressure of a patient is reduced, pains are avoided in the treatment process, and the taste is good; and clinical tests prove that the western medicine composition is safe and effective for treating tetanus.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
Western medicine combination for treatment of tetanus and preparation method thereof
InactiveCN106511607AGreat tasteInhibition of activityAntibacterial agentsAnthropod material medical ingredientsSide effectVitamin C
The invention discloses a western medicine combination for treatment of tetanus and a preparation method thereof. The western medicine combination is composed of, by weight, 3-7 parts of norethisterone tablets, 0.5-2 parts of ox horns, 8-15 parts of leflunomide powder, 2-5 parts of cocoon shells, 2-5 parts of tobacco shreds, 10-50 parts of normal saline and 0.01-0.1 part of vitamin C. Through the synergistic effect of raw material compounding, the western medicine combination has the effects of clearing away heat and toxic materials, diminishing inflammation and swelling and drawing out poison, and can restrain and kill the viability of clostridium tetani; in the therapeutic process, no complication is founded, and no obvious toxic and side effect occurs; the course of treatment is short, quick effect is achieved, the preparation method is simple, the production cost is low, the economic pressure of patients is lowered, the patients have no pains in the treatment process, and the taste is good; and clinical tests prove that the western medicine combination is safe and effective and can be used for treatment of tetanus.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
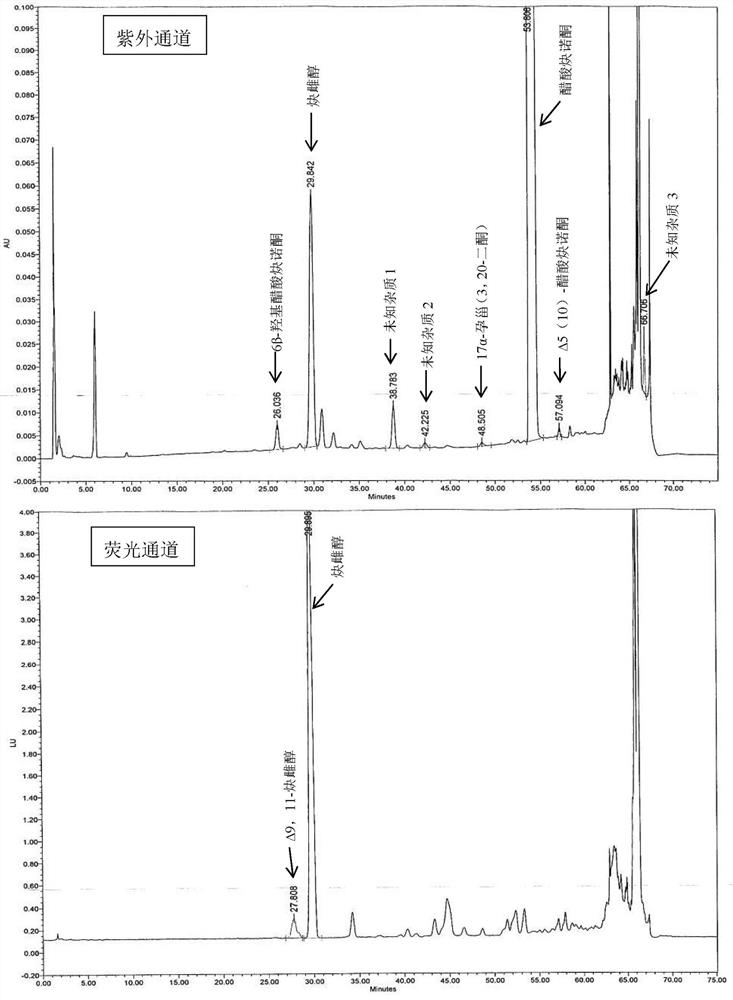
A kind of impurity detection and analysis method of norethindrone derivative and its intermediate
ActiveCN105277633BEasy to operateEfficient separationComponent separationSilica gelChromatography column
The present invention relates to a method for analyzing impurities of steroidal compounds, in particular to a method for detecting and analyzing impurities of norethindrone derivatives and their intermediates, comprising: (1) choosing octadecylsilane bonded silica gel as filler The chromatographic column; the gradient elution of the mobile phase, the composition of the mobile phase is water, methanol and acetonitrile; the detection wavelength is 230 ~ 254nm; (2) take the test sample norethindrone derivative or its intermediate amount, accurately weigh the methanol Dissolve and dilute to a certain concentration of the sample solution, shake it up, inject the sample, perform high-performance liquid chromatography analysis at an appropriate flow rate and column temperature, and record the chromatogram. The invention can quickly and accurately realize the impurity analysis of norethindrone derivatives, especially for the impurity analysis of norethindrone enanthate, and can be used for the impurity analysis of norethindrone derivative intermediates, and can effectively track the impurities produced in the synthesis process. Impurities.
Owner:ZHEJIANG XIANJU PHARMA
Female hormone-containing patch
ActiveUS8124122B2High bonding strengthAvoid failureOrganic active ingredientsNervous disorderPhysiologyAdditive ingredient
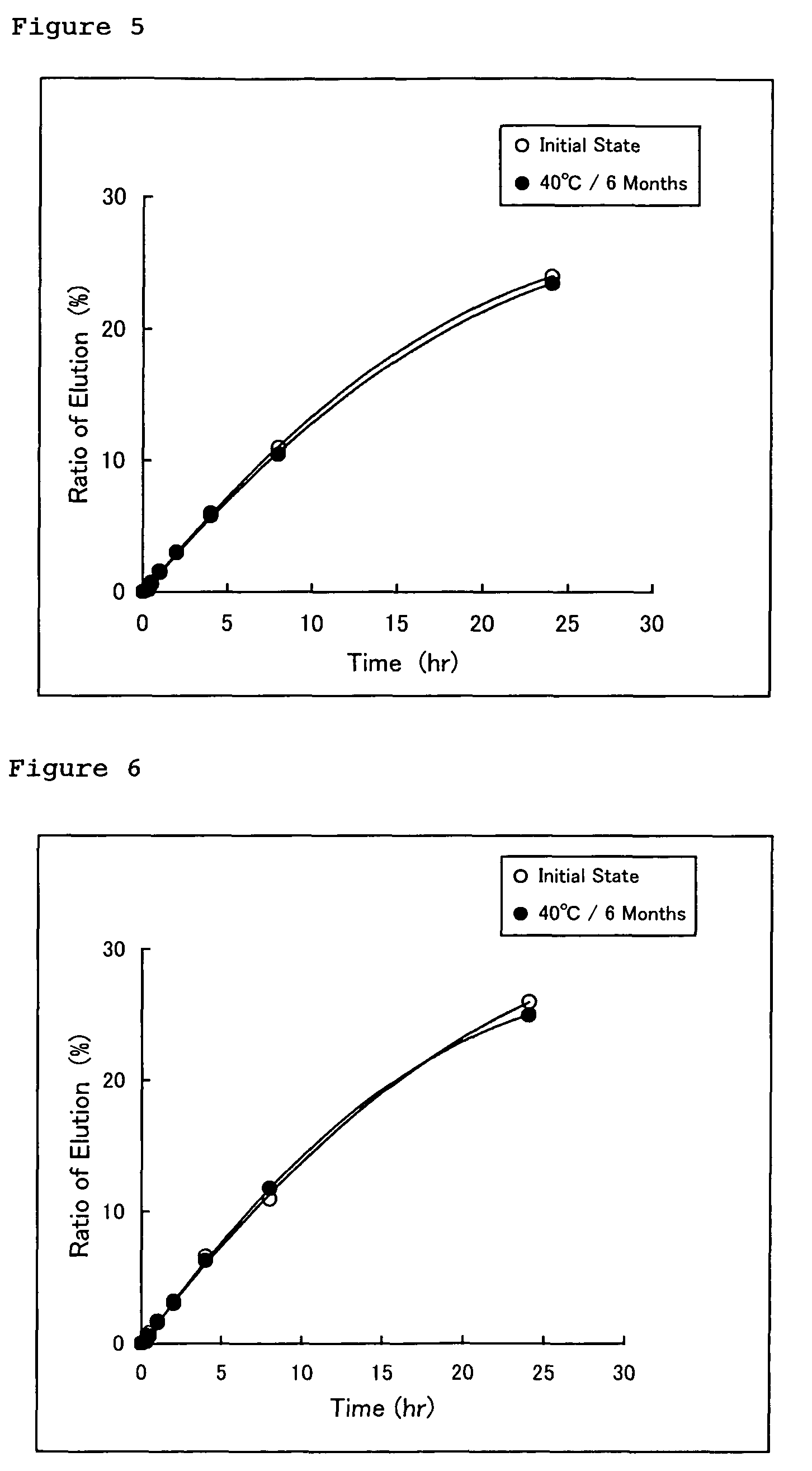
Provided herein is a female hormone-containing patch wherein an active ingredient is highly soluble in a pressure-sensitive adhesive layer and the active ingredient is not adsorbed to a backing, and the patch per se can follow the irregularities on the skin surface or body movements. The patch is an external patch containing, as a female hormone, a follicular hormone estradiol and / or its derivative or a progestational hormone norethisterone and / or its derivative. The external patch comprises an acrylic pressure-sensitive adhesive containing 0.01 to 1% by weight of an isocyanate-based crosslinking agent as an essential ingredient.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
A stable pharmaceutical composition comprising ethinyl estradiol and norethindrone acetate
ActiveCN114129573BControl Oxidative DegradationImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsEthylic acidNorethisterone acetate
A stable pharmaceutical composition comprising ethinyl estradiol and norethindrone acetate, the stability of ethinyl estradiol is improved by adding vitamin E; and by using specific amount of vitamin E, the stability of ethinyl estradiol and norethindrone acetate is controlled The production of related substances improves the overall stability of the compound preparation.
Owner:北京联嘉医药科技开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com