Impurity detection analysis method of norethisterone derivatives and intermediates thereof
An analysis method and intermediate technology, applied in the field of impurity analysis of steroid compounds, to achieve the effect of improving detection efficiency and short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] A method for detecting and analyzing impurities of norethindrone derivatives and intermediates thereof:
[0084] (1) Chromatographic conditions:
[0085] Chromatographic column: C18, 4.6mm×25cm, 5μm
[0086] Detection wavelength: 240nm
[0087] Injection volume: 10μL
[0088] Flow rate: 1.5ml / min
[0089] Column temperature: 30°C
[0090] Mobile phase: water, methanol and acetonitrile,
[0091] (2) Analyze according to the following operation method, and the program gradient elution is the same as in Table 1.
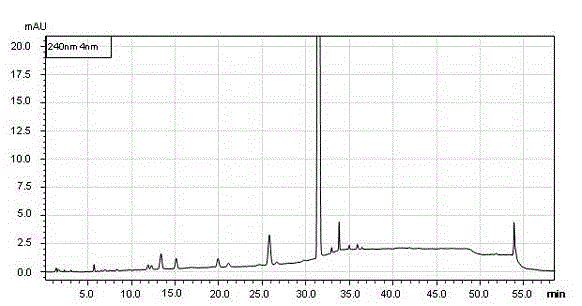
[0092] Operation: Accurately weigh an appropriate amount of samples 1-6 of norethindrone derivatives or intermediates involved in their synthesis, place them in a volumetric flask, add methanol to dissolve and dilute to the mark, and make a solution containing 1mg of samples per 1ml, shake well , Precisely measure 10 μL and inject it into the liquid chromatograph, and record the chromatogram. See attached chromatogram Figure 1~6 , the results are shown i...
Embodiment 2
[0099] A kind of impurity detection analysis method of norethindrone enanthate:
[0100] (1) Chromatographic conditions:
[0101] Chromatographic column: C18, 4.6mm×25cm, 5μm
[0102] Detection wavelength: 230nm
[0103] Injection volume: 10μL
[0104] Flow rate: 1.3ml / min
[0105] Column temperature: 40°C
[0106] Mobile phase: water, methanol and acetonitrile
[0107] (2) Analyze according to the following operation method, and the program gradient elution is the same as in Table 1.
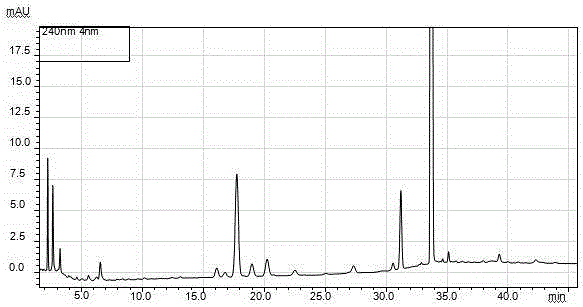
[0108] Operation: Accurately weigh an appropriate amount of norethindrone enanthate sample 7, put it in a volumetric flask, add methanol to dissolve and dilute to the mark, make a solution containing 1mg of sample per 1ml, shake well, accurately measure 10μL and inject it into the liquid chromatograph , record the chromatogram. See attached chromatogram Figure 7 , the results are shown in Table 4.
[0109] Table 4
[0110]
[0111] From the results in Table 4, it can be seen that ...
Embodiment 3
[0113] A kind of impurity detection analysis method of norethindrone enanthate:
[0114] (1) Chromatographic conditions:
[0115] Chromatographic column: C18, 4.6mm×25cm, 5μm
[0116] Detection wavelength: 254nm
[0117]Injection volume: 10μL
[0118] Flow rate: 1.0ml / min
[0119] Column temperature: 25°C
[0120] Mobile phase: water, methanol and acetonitrile,
[0121] (2) Program gradient elution according to Table 5
[0122] table 5
[0123]
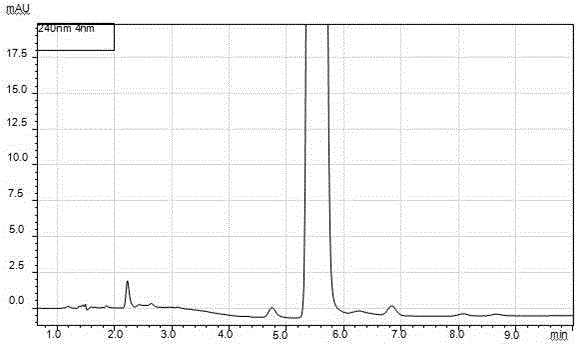
[0124] Operation: Accurately weigh an appropriate amount of norethindrone enanthate sample 8, put it in an appropriate volumetric flask, add methanol to dissolve and dilute to the mark, make a solution containing 1 mg of sample per 1 ml, shake well, accurately measure 10 μL and inject into the liquid chromatograph, Record the chromatogram. See attached chromatogram Figure 8 , the results are shown in Table 6.
[0125] Table 6
[0126]
[0127] From the results in Table 6, it can be seen that when the method is used f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com