Preparation method of tibolone
A technology for tibolone and intermediates, applied in the field of preparation of tibolone, can solve the problems of high pollution in the production process, high cost of raw materials, long reaction route, etc., and achieve high production cost, few reaction steps, and low environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
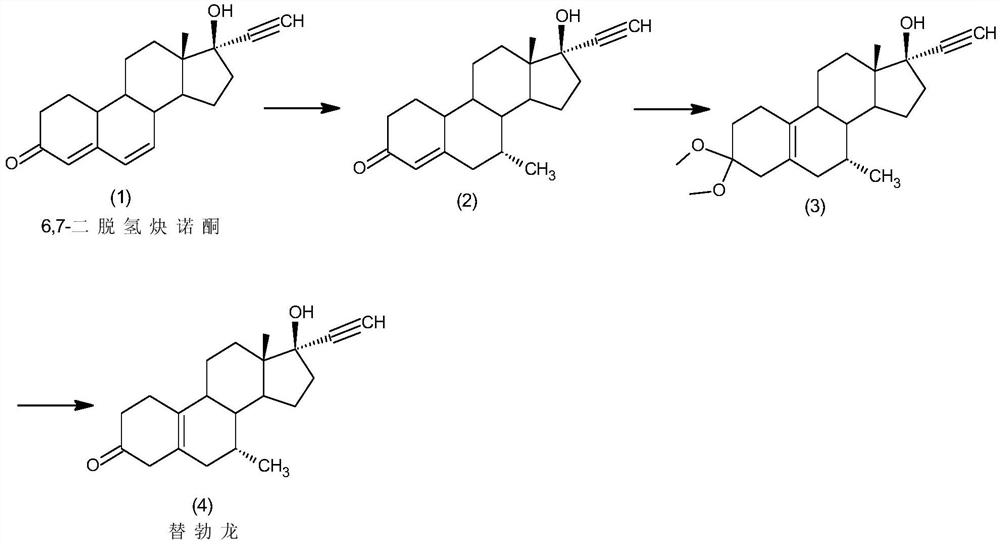
[0034] The preparation of embodiment 1 tibolone
[0035] Step 1: Under nitrogen protection, add 2.5g of cuprous chloride and 1M methylmagnesium iodide to 500ml of tetrahydrofuran solution in 500ml of tetrahydrofuran, add 50g of 6,7-didehydronorethindrone (1) under low temperature stirring at -20°C Carry out the reaction, after thin-layer chromatographic analysis shows that the conversion of the raw materials is complete, the reaction solution is added to 750ml of 5% hydrochloric acid aqueous solution, 250ml of dichloromethane is added for extraction, the organic layer is washed with water until neutral, concentrated, filtered, and dried to obtain 42.5g of the intermediate (2);
[0036] Step 2: Under nitrogen protection, add 42.5g of the above-mentioned intermediate (2) to 600ml of methanol, add 0.5g of pyridinium hydrobromide and 42.5g of boron trifluoride diethyl ether, and stir at 40°C for reaction. TLC analysis shows that the raw material After the conversion was complete,...
Embodiment 2
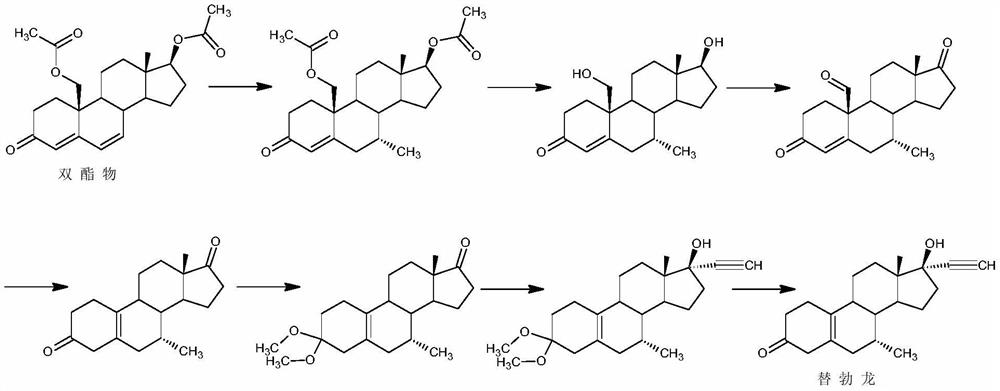
[0038] The preparation of embodiment 2 tibolone
[0039]Step 1: Under the protection of argon, add 5g of copper chloride and 340ml of ether solution of 3M methylmagnesium bromide to 1500ml of ether, add 50g of 6,7-didehydronorethindrone (1) under low temperature stirring at -30°C to carry out Reaction, after thin-layer chromatographic analysis shows that raw material conversion is complete, reaction solution is joined in 1500ml10% sulfuric acid aqueous solution, adds 1000ml ethyl acetate to extract, washes organic layer to neutrality, concentrates, filters, oven dry, obtains 43.1g intermediate ( 2);
[0040] Step 2: Under nitrogen protection, add 43.1g of the above intermediate (2) to 400ml of methanol, add 8g of malonic acid and 25g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt Acetate (EDCI), stirred and reacted at 20°C, and after thin-layer chromatography analysis showed that the conversion of the raw materials was complete, 4.5ml of pyridine was added, cooled to -...
Embodiment 3
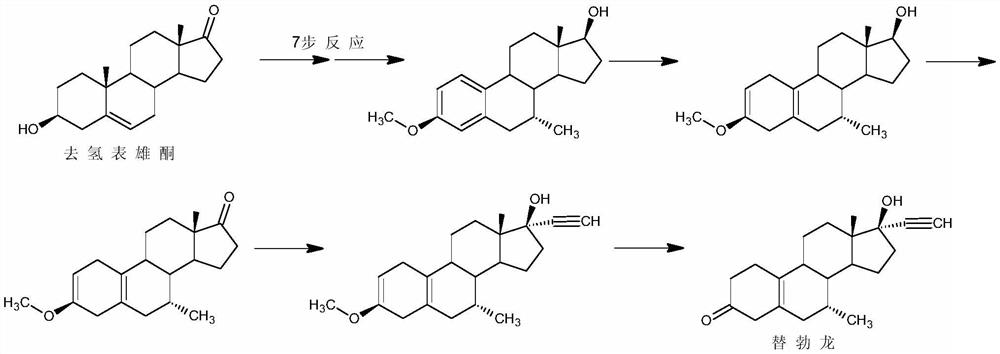
[0042] The preparation of embodiment 3 tibolone
[0043] Step 1: Under the protection of nitrogen, add 25g of copper acetate and 330ml of tetrahydrofuran solution of 5M methylmagnesium chloride to 2500ml of methyl tetrahydrofuran, and add 50g of 6,7-didehydronorethindrone (1) under low-temperature stirring at -40°C for reaction. Thin-layer chromatography analysis showed that the conversion of the raw materials was complete, the reaction solution was added to 1200ml of 30% acetic acid aqueous solution, 1500ml of toluene was added for extraction, the organic layer was washed with water until neutral, concentrated, filtered, and dried to obtain 42.7g of intermediate (2);
[0044] Step 2: Under nitrogen protection, add 42.7g of the above intermediate (2) to 50ml of methanol and 600ml of cyclohexane, add 1.3g of benzenesulfonic acid, reflux dehydration and stir the reaction, after thin-layer chromatography analysis shows that the conversion of the raw materials is complete, add 2ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com