A stable pharmaceutical composition comprising ethinyl estradiol and norethindrone acetate
A technology of norethindrone acetate and composition, which is applied in the field of pharmaceutical preparations, can solve problems such as oxidative degradation and drug effect decline, and achieve the effects of improving stability, ensuring stability, and improving overall stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0051] The compound tablet of ethinyl estradiol and norethindrone acetate is prepared by fluidized bed wet granulation tablet technology, and the processing steps are as follows:
[0052] Weighing: Weigh each component according to the prescription in Table 1.
[0053] Main drug solution preparation: dissolve ethinyl estradiol, norethindrone acetate, and vitamin E in a mixed solvent of ethanol and chloroform 1:1 (v / v);
[0054] Screening: pass lactose and starch through a sieve with a pore size of 20 mesh;
[0055] Granulation and drying: Put the sieved material into a fluidized bed granulation pot, spray the main drug solution in a fluidized state, and then fluidize and dry at 40°C;
[0056] Screening: pass the dried material through a sieve with an aperture of 20 mesh;
[0057] Blending and tableting: add the prescribed amount of magnesium stearate to the sieved granules, mix evenly, and then press into 5mm round tablets.
[0058] Table 1 Example 1-5 Tablet Prescription ...
Embodiment 6
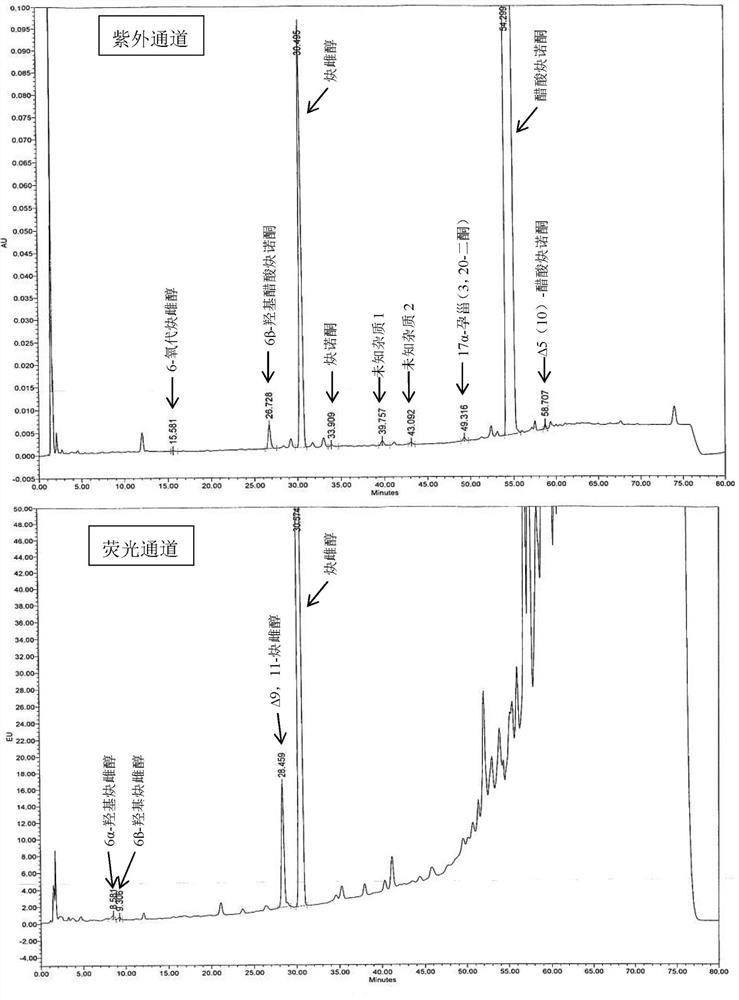
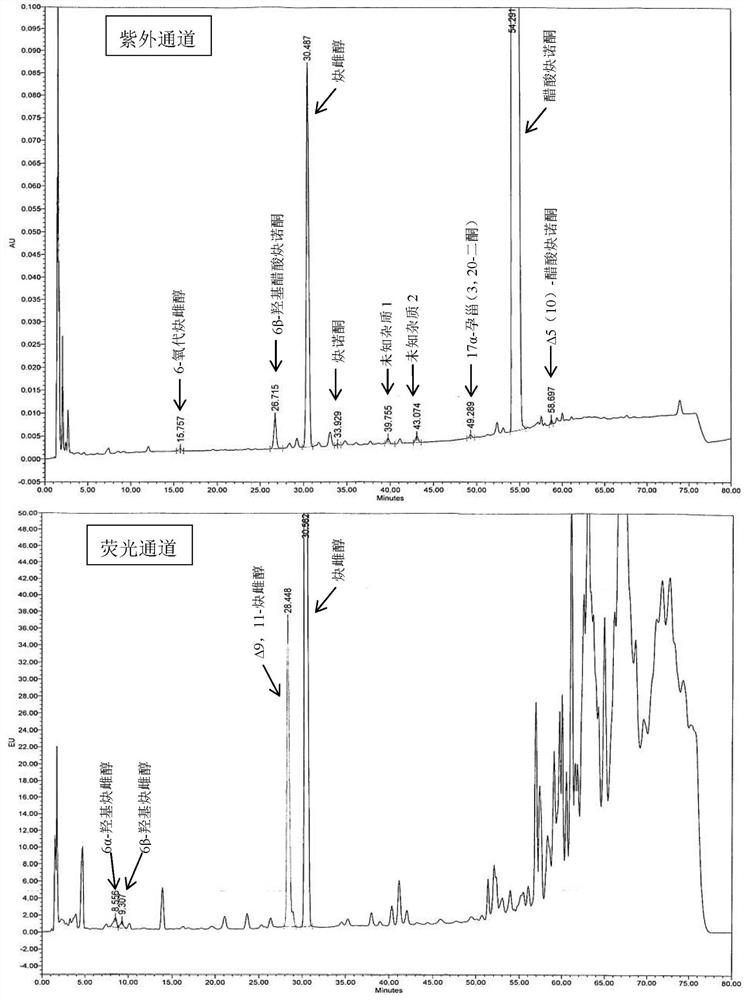
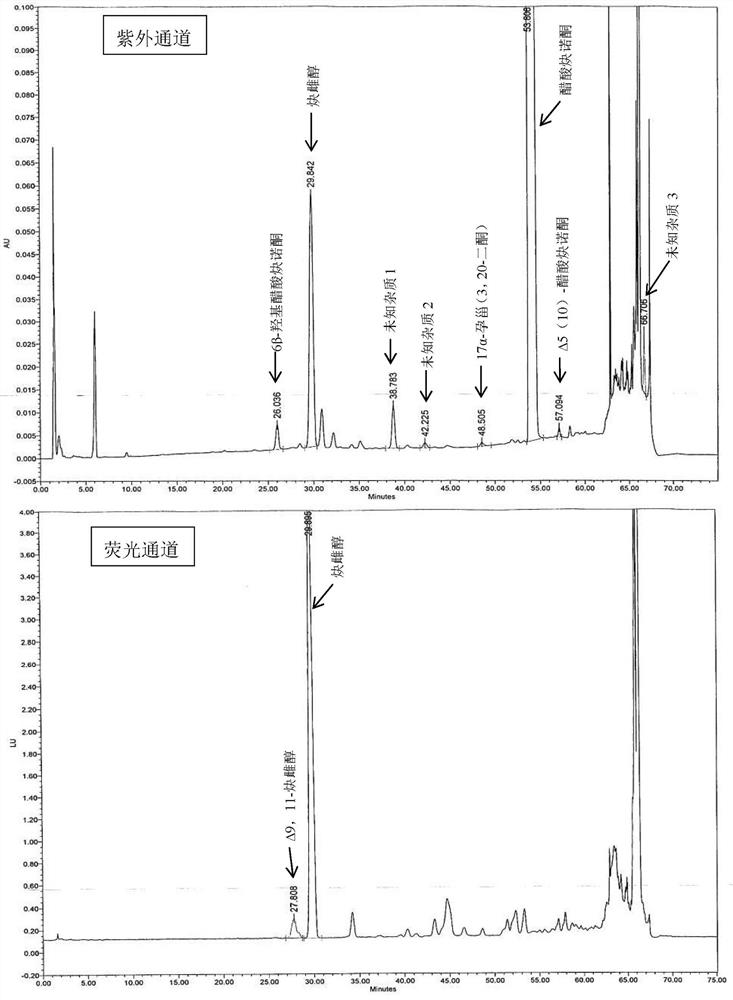
[0060] Embodiment 6 Determination of related substances
[0061] The tablet prepared by Example 1-5 was preserved for 3 months under 40°C / 75%RH condition, and the packaging condition was a heat-sealed high-density polyethylene bottle, and the high-performance liquid chromatography was used to measure 0 days and 3 tablets respectively. The amount of related substances in the monthly samples, the determination results are shown in Table 3.
[0062] The HPLC chromatographic conditions are: use octyl bonded silica gel as filler; use acetonitrile containing 0.5% phosphoric acid as mobile phase A, acetonitrile containing 0.5% phosphoric acid: water = 1:3 as mobile phase B, perform linearity according to Table 2 Gradient elution; flow rate is 1.6ml per minute; FLDex285nm, em350nm (6α-hydroxyethinylestradiol, 6β-hydroxyethinylestradiol and Δ9,11-ethinylestradiol), UV 210nm (other impurities); column temperature: 40ºC; Sample volume: 30µl.
[0063] Table 2 Gradient elution parameter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com