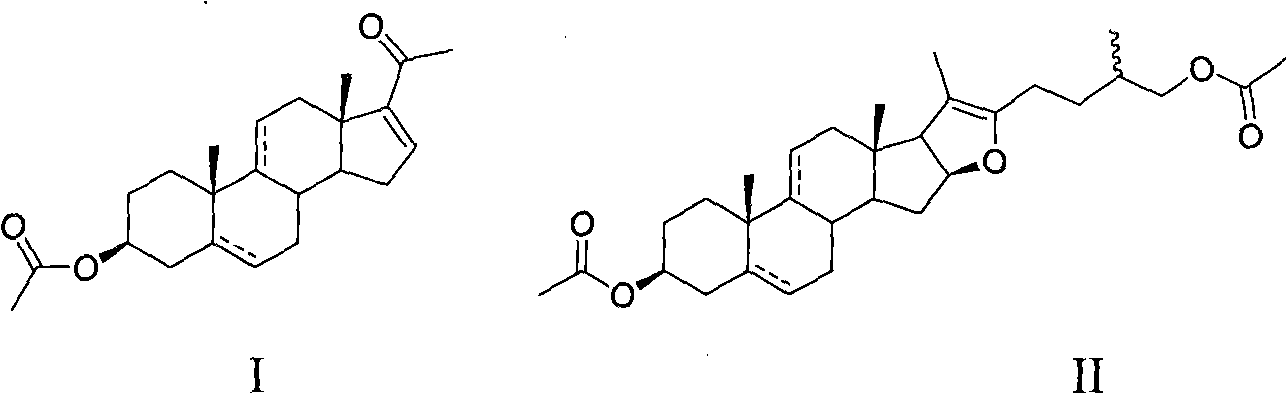
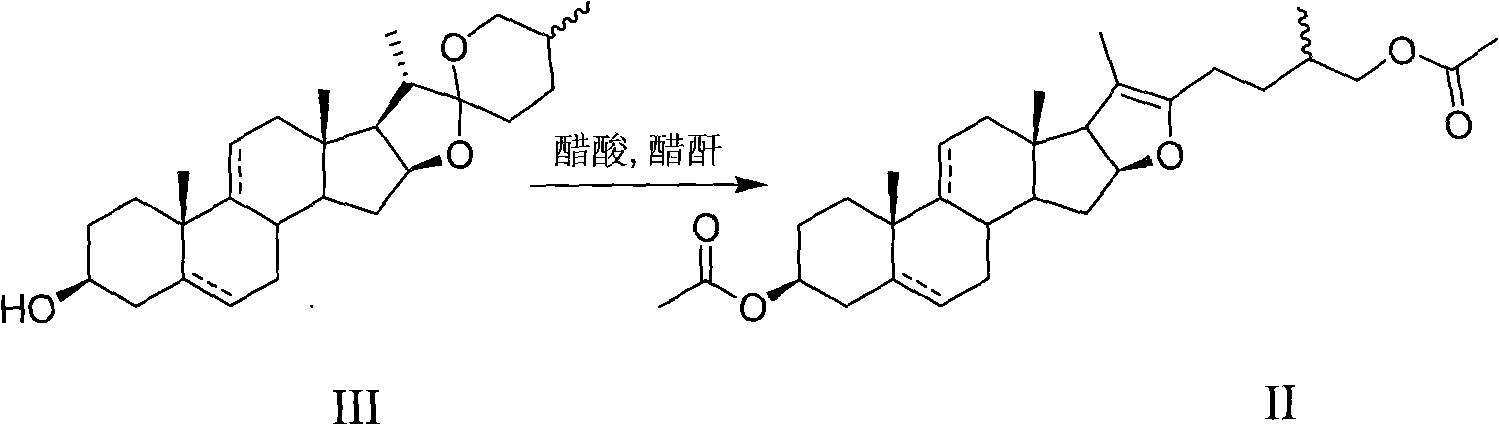
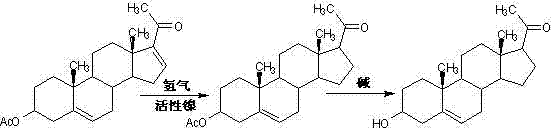
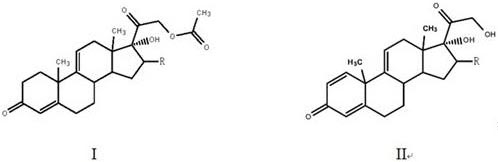
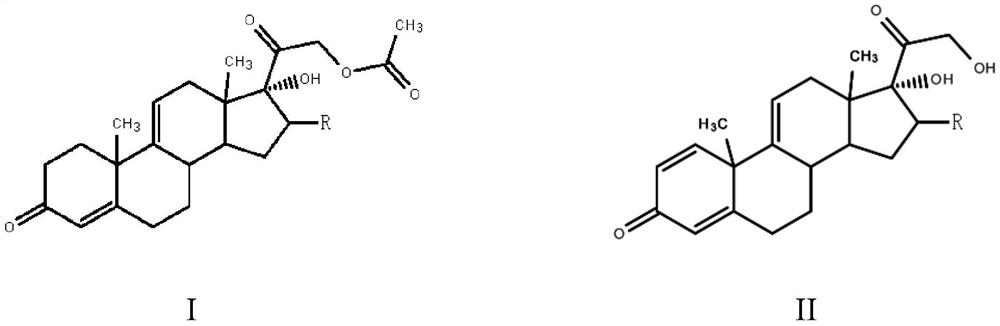
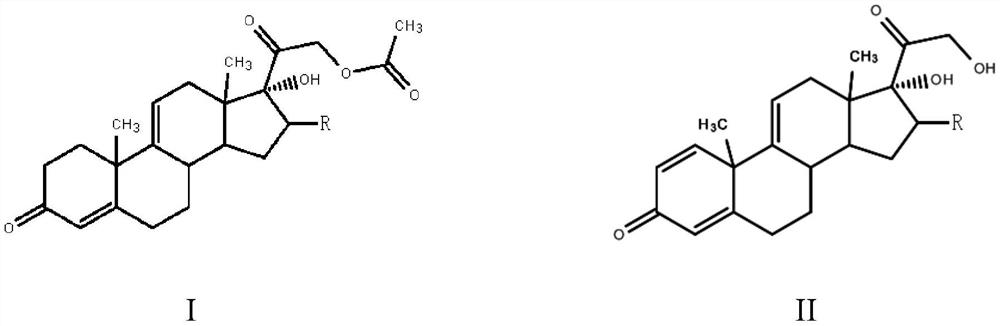
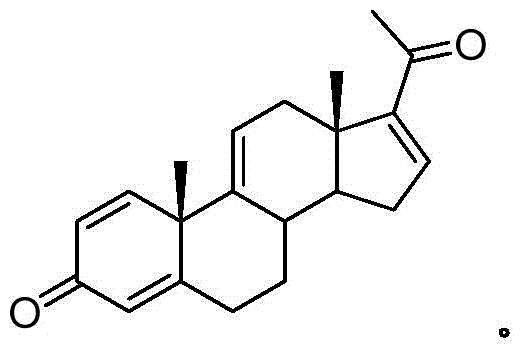
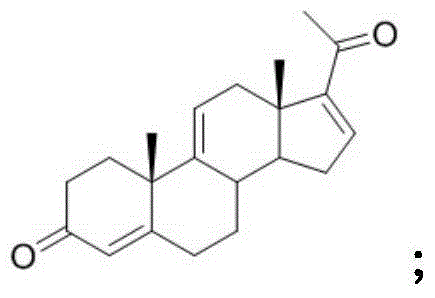
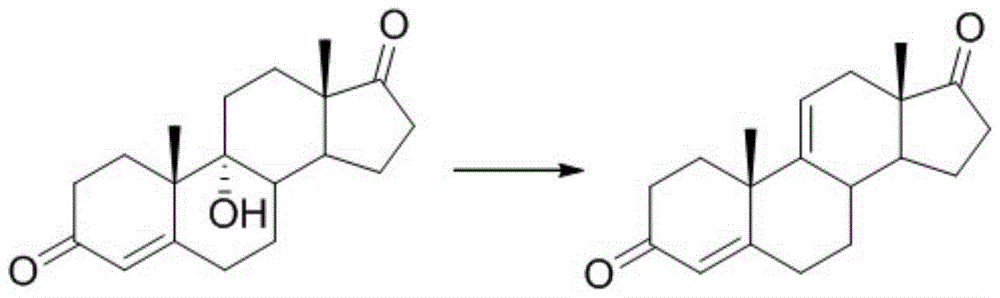
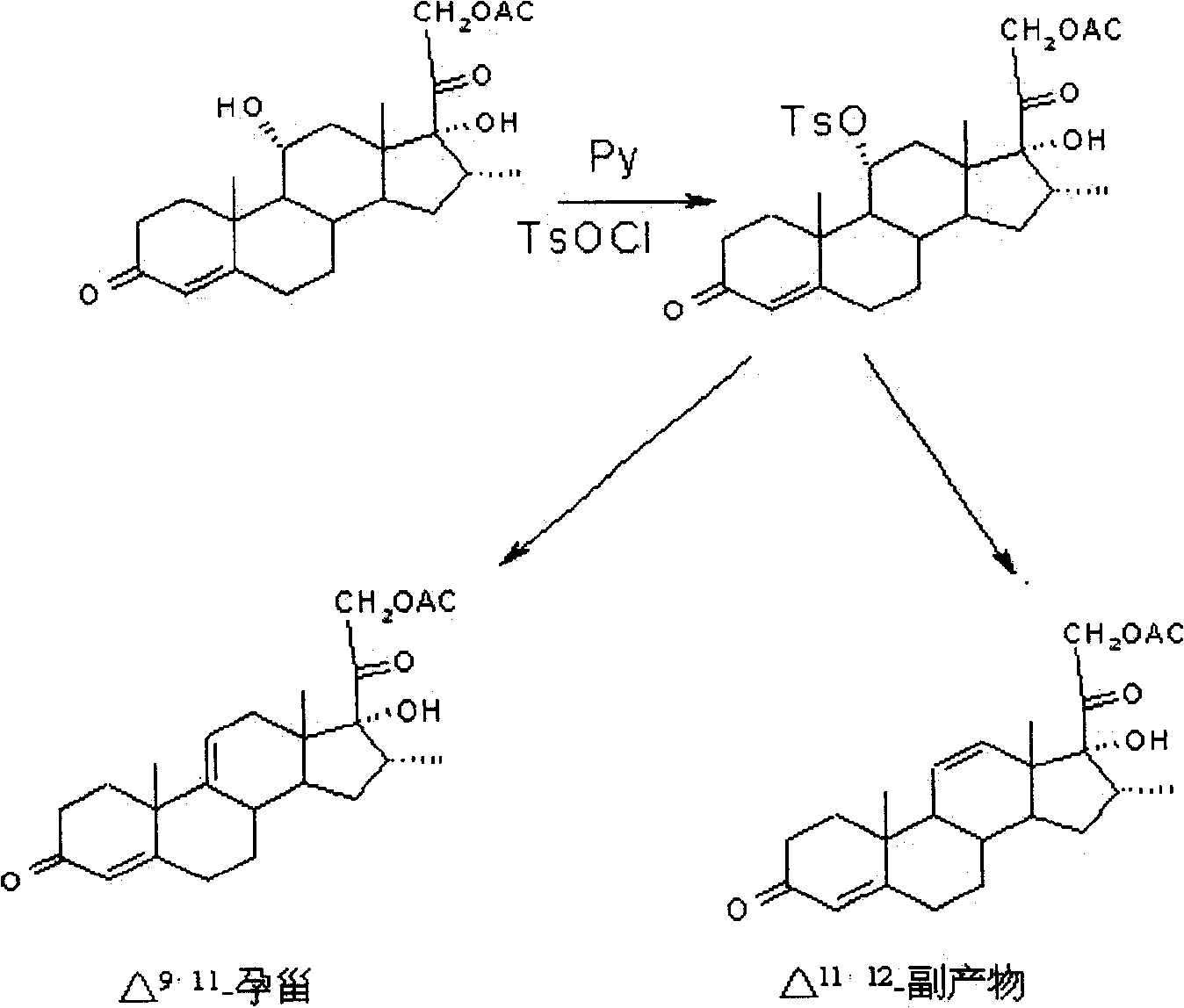
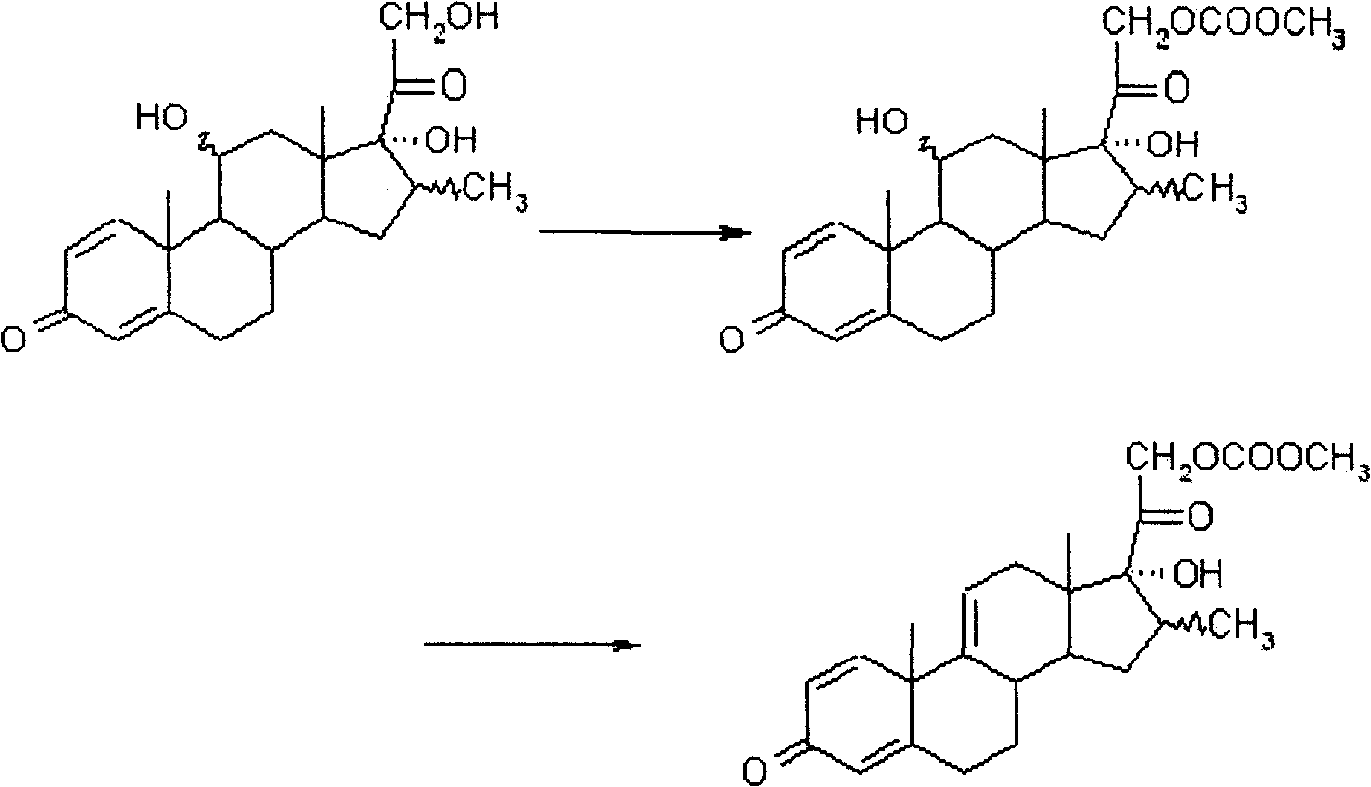
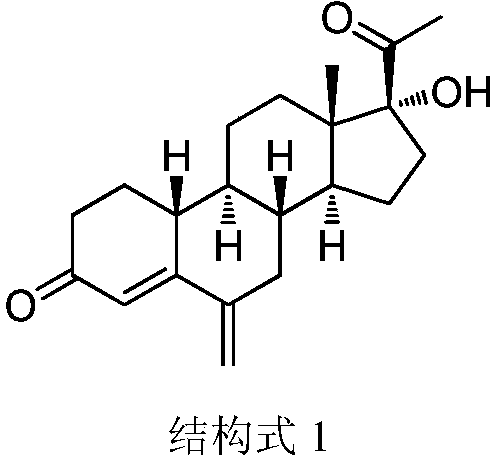
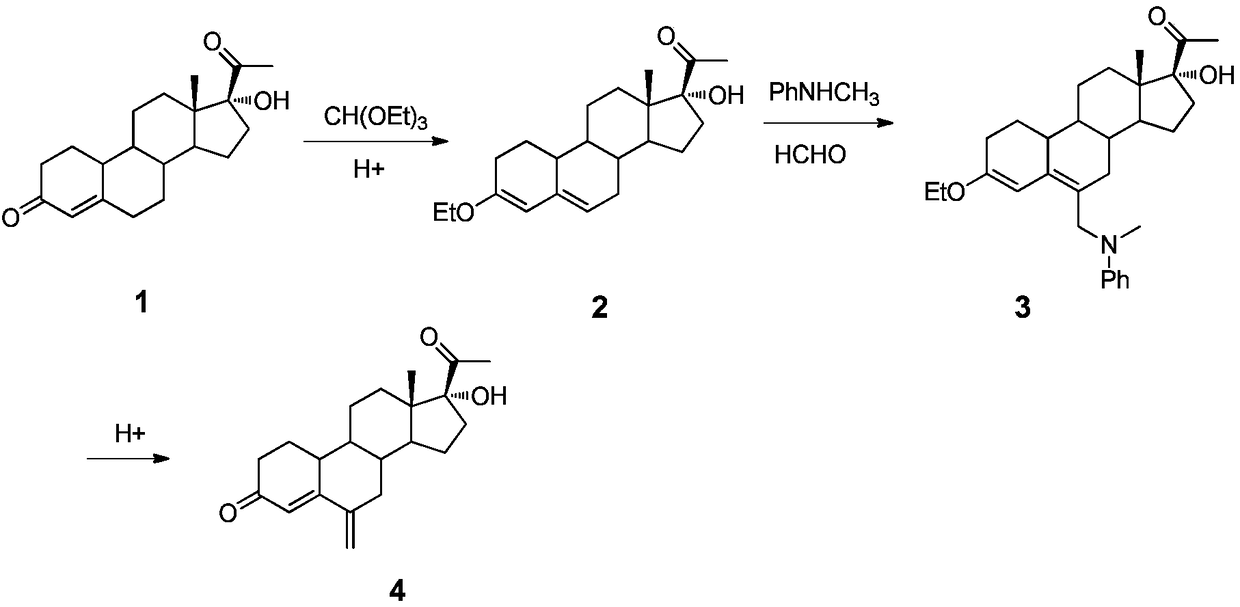
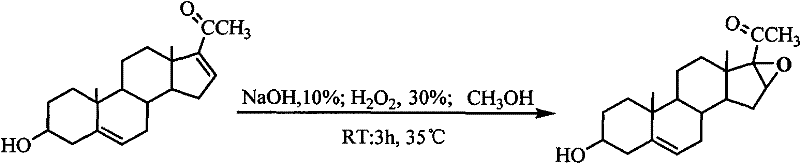Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Pregnene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A pregnene is an alkene derivative of a pregnane. An example is cortisone.
Method for synthesizing cholesterol by using stigmasterol degradation products as raw materials
The invention provides a method for synthesizing cholesterol by using stigmasterol degradation products as raw materials. The method comprises the following steps: 1) performing an etherification reaction on 3-carbonyl-4-pregnene-22-aldehyde and triethyl orthoformate to obtain 3-ethyoxyl-3,5-pregnadiene-22-aldehyde; 2) preparing a 3-methylbutyltriphenyl phosphonium chloride solution; 3) adding potassium tert-butoxide into the 3-methylbutyltriphenyl phosphonium chloride solution, performing a wittig reaction on the 3-methylbutyltriphenyl phosphonium chloride solution and the 3-ethyoxyl-3,5-pregnadiene-22-aldehyde to obtain 3-ethyoxyl-3,5,22-triene cholestane; 4) catalyzing the 3-ethyoxyl-3,5,22-triene cholestane to perform a selective hydrogenation reaction to obtain 3-ethyoxyl-5-ene cholestane; 5) performing a reaction on the 3-ethyoxyl-5-ene cholestane and acetic anhydride to obtain 3-acetyl-5-ene cholestane; 6) performing a hydrolysis reaction on the 3-acetyl-5-ene cholestane to obtain the cholesterol. The synthesizing method is simple in process, and the mole yield of the cholesterol exceeds 85 percent by using the stigmasterol degradation products which are cheap and easily obtained as the raw materials; the production cost is low, the process is environmentally friendly, and the method is economical and environmentally friendly, and facilitates industrial implementation.
Owner:HUNAN KEREY BIOTECH
Preparation method of hydrocortisone
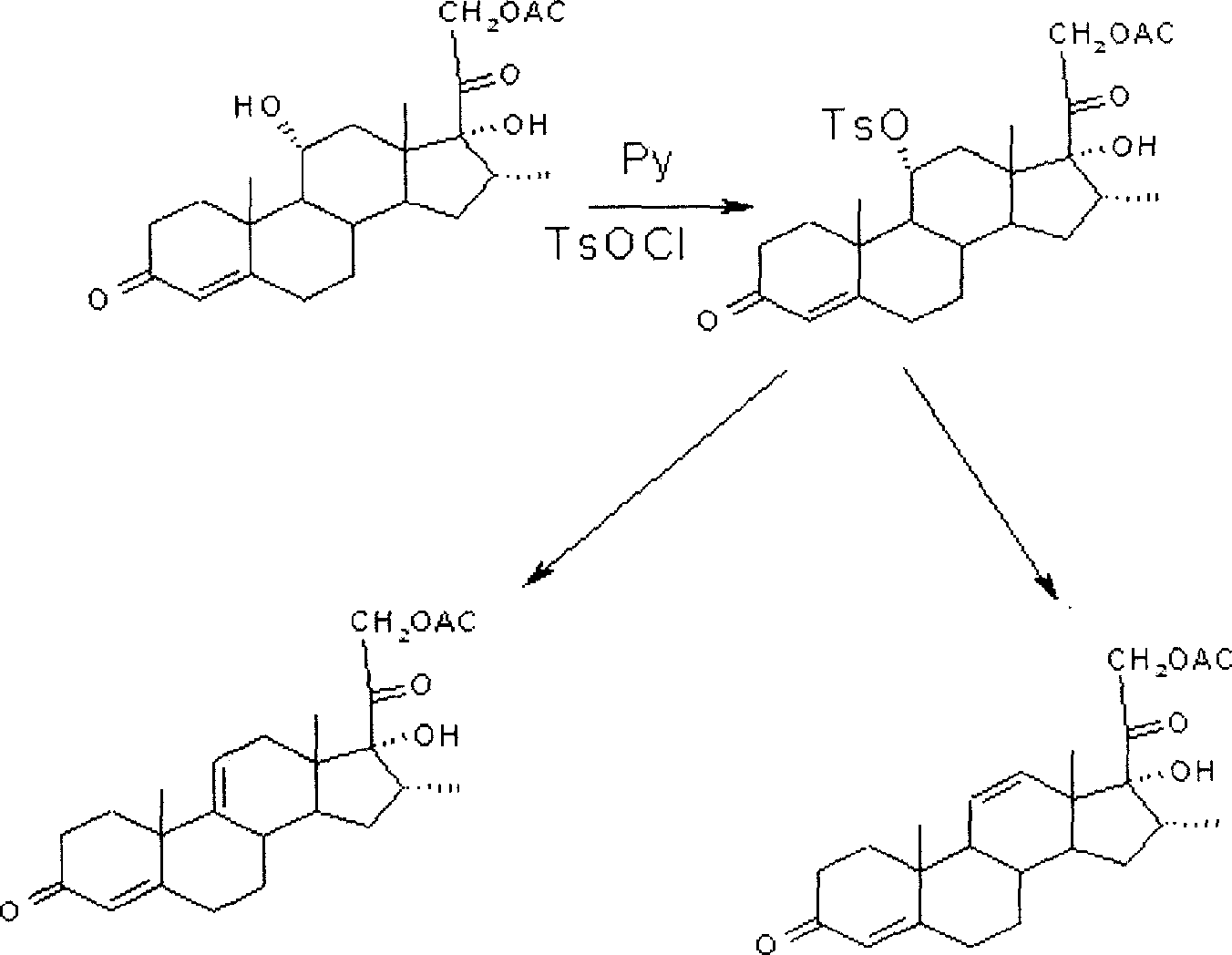
ActiveCN102367262AAvoid it happening againRaw materials are easy to getSteroidsDisplacement reactionsHydrocortisone acetate
The invention, relating to the field of pharmaceutical synthesis, particularly relates to a preparation method of hydrocortisone, comprising the following steps: using an intermediate 17 alfa-hydroxy-4-pregnene-3,11,20- triketone (II) in a Rhizopus nigricans method as raw material, successively carrying out ketal reaction, reduction reaction, hydrolysis reaction, iodine adding reaction, and displacement reaction to obtain hydrocortisone acetate (VII), and finally carrying out sodium hydroxide hydrolysis reaction to obtain the hydrocortisone (I). The invention has the advantages of easy obtainment of raw material, common auxiliary materials, and no need of using toxic, highly toxic, and carcinogenic reagents. The method is more efficient than the process in the prior art, and the products has good quality and yield.
Owner:ZHEJIANG XIANJU PHARMA
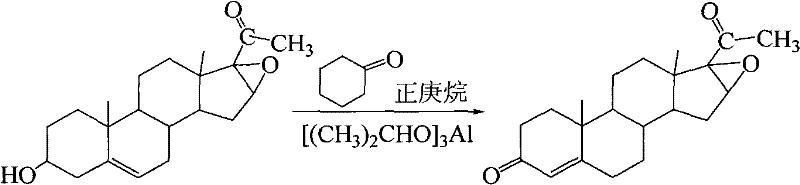
Synthesis of 16α,17α-epoxy-4-pregnene-3,20-dione
The invention discloses a synthesis method of 16α, 17α-epoxy-4-pregnene-3, 20-dione. The process steps are: 10.0 parts of oxygen bridge, 70-80 parts of n-heptane and 35-40 parts of Mix 6-7 parts of cyclohexanone, heat and stir with water; after cooling down, add aluminum isopropoxide catalyst, reflux vigorously for 4 hours, cool down; add 6-7 parts of 30% NaOH solution dropwise, reflux for 20 minutes, add 2 % NaOH solution 20-25 parts, reflux for 30min, and remove alkaline water; then add 1% NaOH solution 20-25 parts, distill until no oil drops, suction filter, wash, dry; then dissolve with absolute ethanol, Reflux, freezing, suction filtration, Dewas oxide. The consumption of solvent, oxidant and catalyst of the present invention is less, only needs twice alkali washing, has simplified technology, has reduced production cost, and the content of Wo Shi oxidation reactant can reach 98.2%, and productive rate is more than 80%; The use of toluene reduces environmental pollution.
Owner:SHAANXI UNIV OF TECH
Synthesis method and intermediates of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone
ActiveCN104628808AReduce usageRaw materials are cheap and easy to getSteroids preparationBulk chemical productionSynthesis methodsSide chain
The invention relates to a synthesis method and main intermediates of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. The synthesis method sequentially comprises the following steps of reacting a second intermediate and tosylmethyl isocyanide in an organic solvent at the temperature of lower than 35 DEG C below zero to generate a third intermediate; reacting the third intermediate and a methylated reagent in an organic solvent at the temperature of 70-90 DEG C, and then, removing methyl ether protecting groups and tosylmethyl isocyanide under the action of an acid to obtain a fourth intermediate; and reacting the fourth intermreidate under the action of 3-ketosteroid-1-dehydrogenase to generate pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. Raw materials of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone are cheap and available; the yield of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone is relatively high; a C17-position side chain is introduced by using tosylmethyl isocyanide, so that acetone cyanohydrin serving as a highly-toxic reagent is prevented from being used; and the synthesis method is safe, environment-friendly and suitable for industrial production.
Owner:山东国九堂制药集团股份有限公司
Method for synthesizing progesterone midbody 3beta-hydroxy-5-pregnene-20-ketone
ActiveCN102964415AAvoid it happening againReduce oxidation recovery processSteroidsPregneneProgesterones
The invention relates to a method for synthesizing progesterone midbody 3beta-hydroxy-5-pregnene-20-ketone. By the method, a refining procedure and a by-product oxidation recovery processing procedure of a traditional synthesizing process are saved. The obtained midbody does not need to be refined, the once quality yield is more than 86%, and the product purity is more than 99.0%.
Owner:HUAZHONG PHARMA
Method for synthesizing 16-dehydropregndiketonic alcohol acetic ester and its analogs
InactiveCN1884297AImprove utilizationEliminate pollutionSteroidsChemical synthesisTemperature control
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Novel technology for synthesizing pregnene 11-site beta-hydroxy
ActiveCN102372762AReduce consumptionReduce labor costsSteroids preparationBulk chemical productionPregneneOrganic solvent
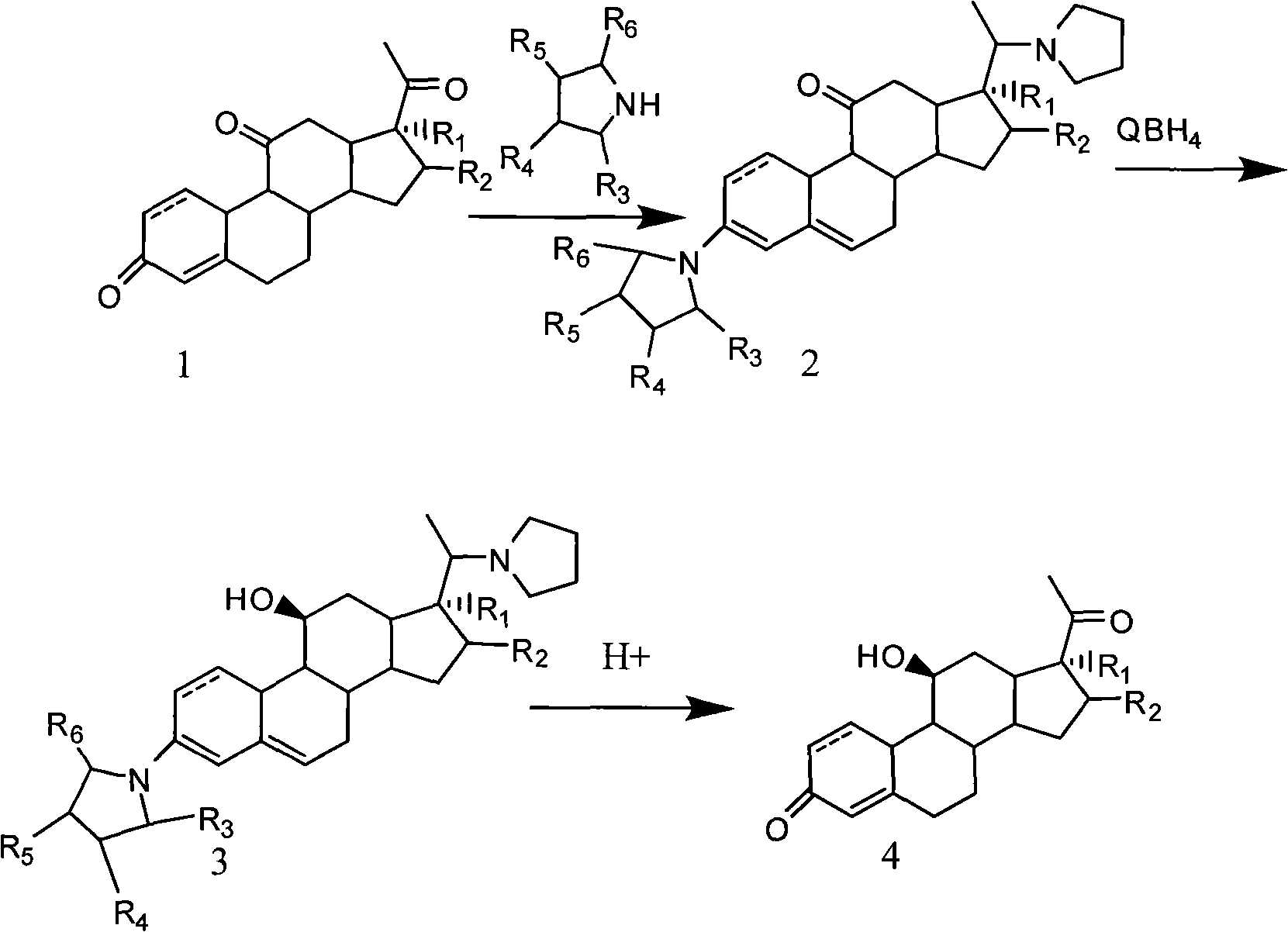
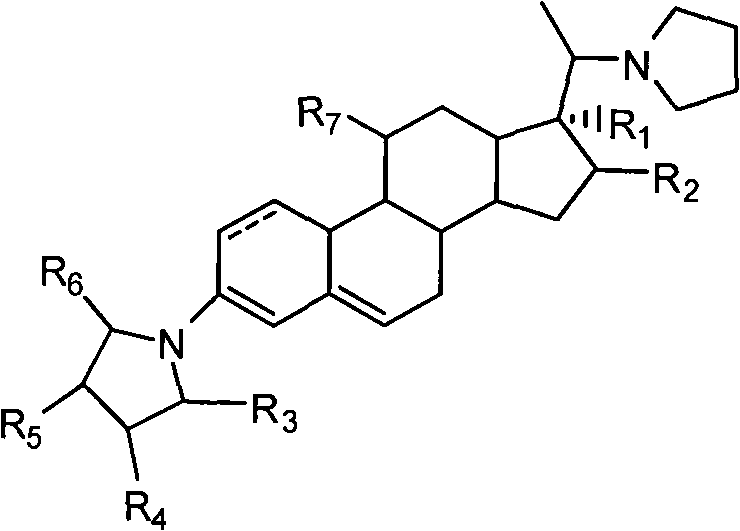
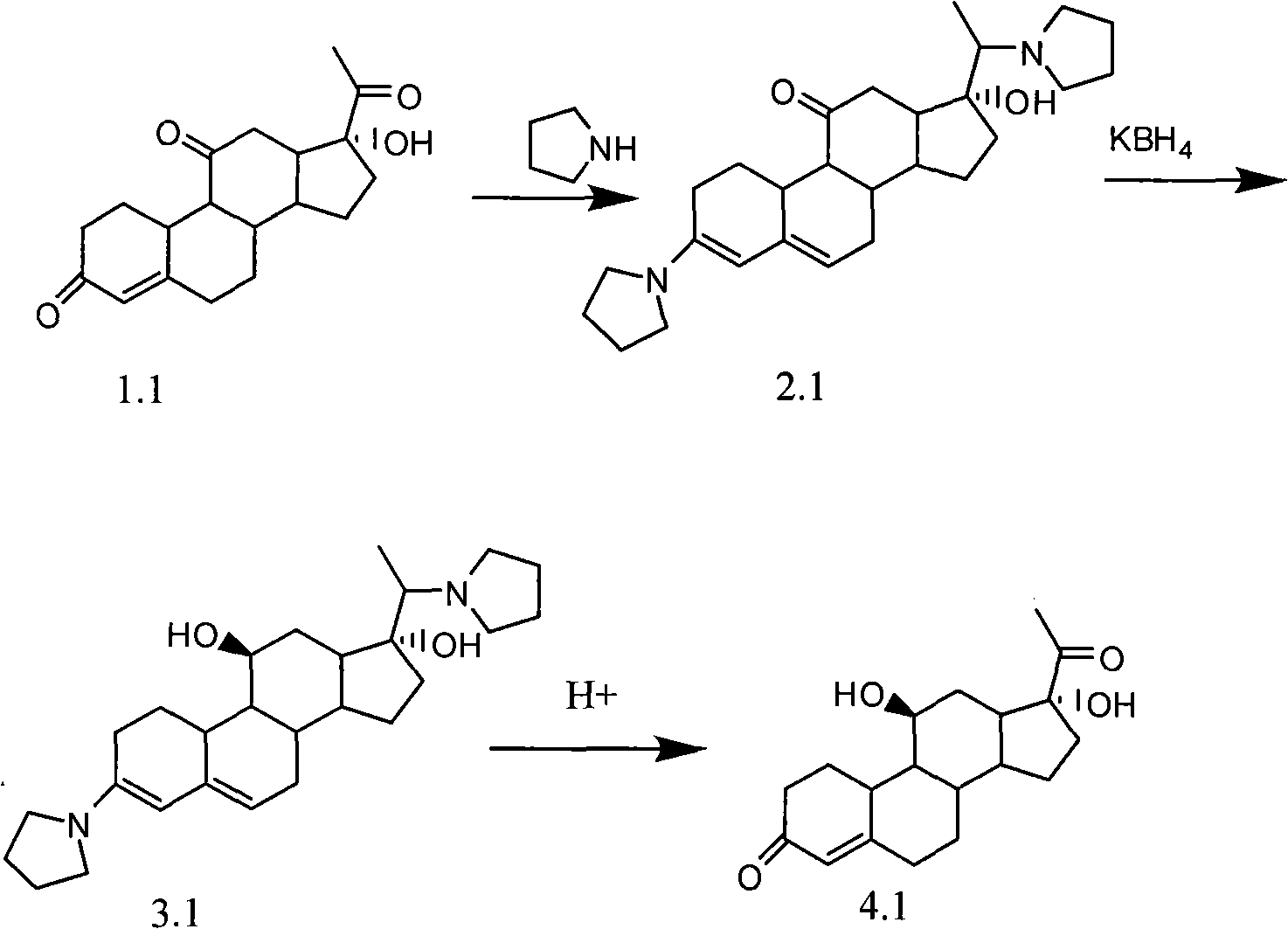
The invention discloses a novel technology for synthesizing pregnene 11-site beta-hydroxy. According to the invention, nafoxidine or a derivative thereof is used for forming a protecting group for pregnene 3, 20 ketone; borohydride is used for reducing pregnene 11 ketone; and nafoxidine is dissociated by using an acid. According to the invention, the three steps of reactions can be completed in a same reaction system; or the last two steps can be completed in a same reaction system. In the step c, the protecting group can be removed without the application of an organic solvent.
Owner:TIANJIN JINYAO GRP
Preparation method for prednisone acetate and intermediate of same
The invention relates to the field of preparation of steroid drugs and an intermediate of the same, and in particular relates to a preparation method for prednisone acetate. The method comprises the steps of taking 11 alpha, 17 alpha-dyhydroxy Pregnene-1,4-diene-3,20-dione as an initial material, oxidizing to obtain 17 alpha-hydroxy Pregnene-1,4-diene-3,11,20-trione, and carrying out iodization reacting to obtain the prednisone acetate. The invention provides a novel oxidation technology more suitable for production of the intermediate of the prednisone acetate, the synthesis path has the characteristics of low cost and simple operation, environment pollution pressure can be greatly reduce, and the yield and quality of the prednisone acetate can reach a satisfactory level.
Owner:JIANGSU YUANDA XIANLE PHARMA
Periploca sepium bunge neo-glycoside agricultural insecticidal compound
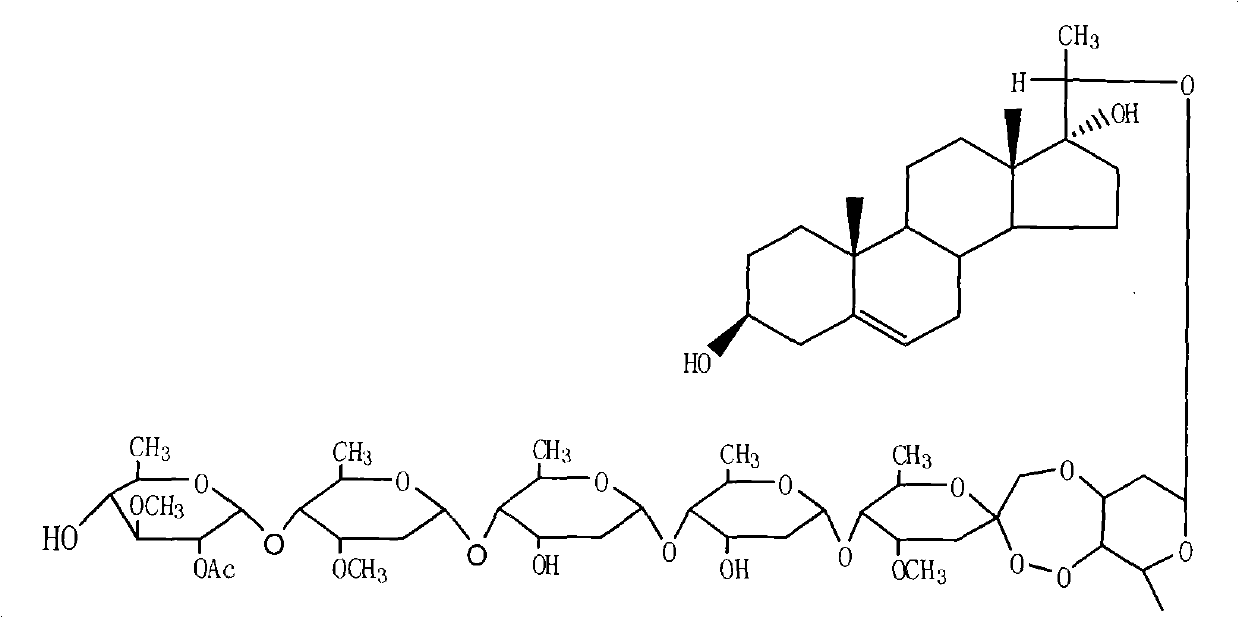
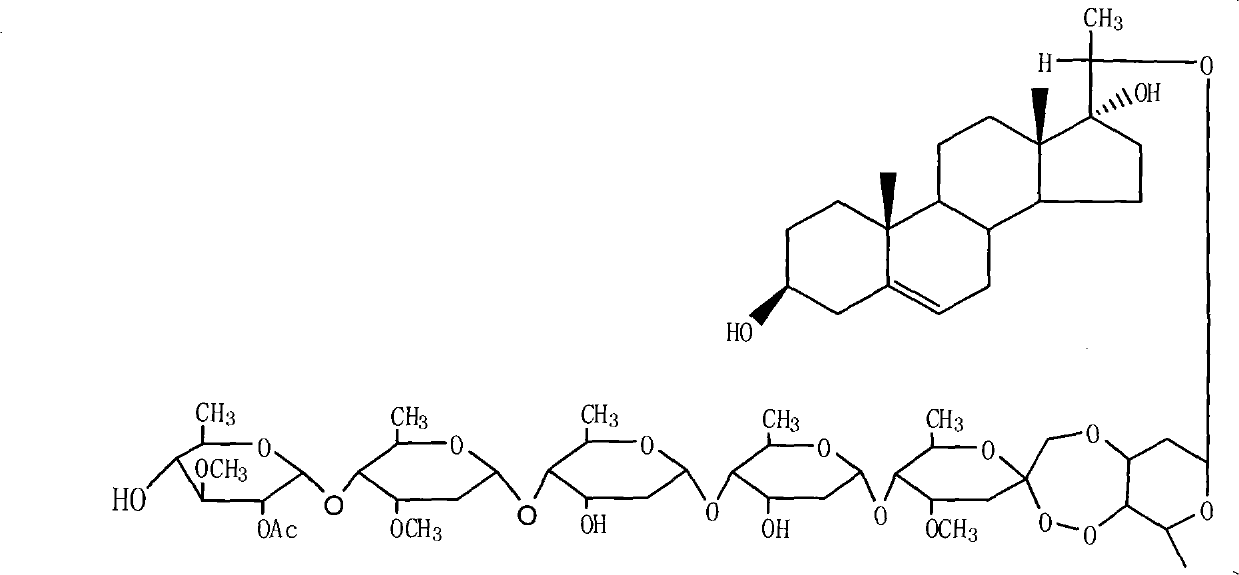
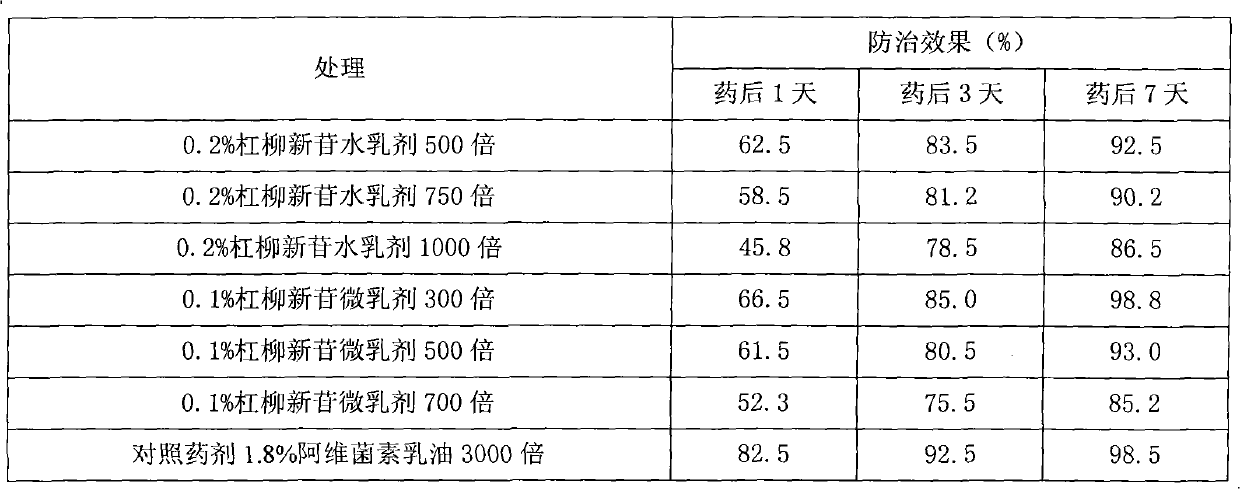
The invention provides a periploca sepium bunge neo-glycoside compound with agricultural insecticidal activity, belonging to the technical field of pesticides. The compound provided by the invention has the structural formula shown in the specifications, and the IUPAC (International Union of Pure and Applied Chemistry) chemical name thereof is delta<5>-pregnene-3beta,17alpha-dyhydroxyl-,20(S)-oxy-(2-O-acetyl-beta-D-digitalose(1->4)-beta-D-cymarose-(1->4)-beta-D-cymarose-(1->4)-beta-D-cymarose-(1->4)-((1->5)-3,7-dideoxy-4-O-methyl-alpha-D-glucose-2-ketoheptose(2,4)-dyhydroxyl-(1->3)-beta-D-cymarose). The compound provided by the invention has strong stomach toxicity insecticidal activity selectively on cabbage worm, plutella xylostella and beet armyworm, and can be processed into an agricultural insecticidal preparation.
Owner:PESTICIDE INST XIBEI AGRI & FORESTRY TECHUNIV
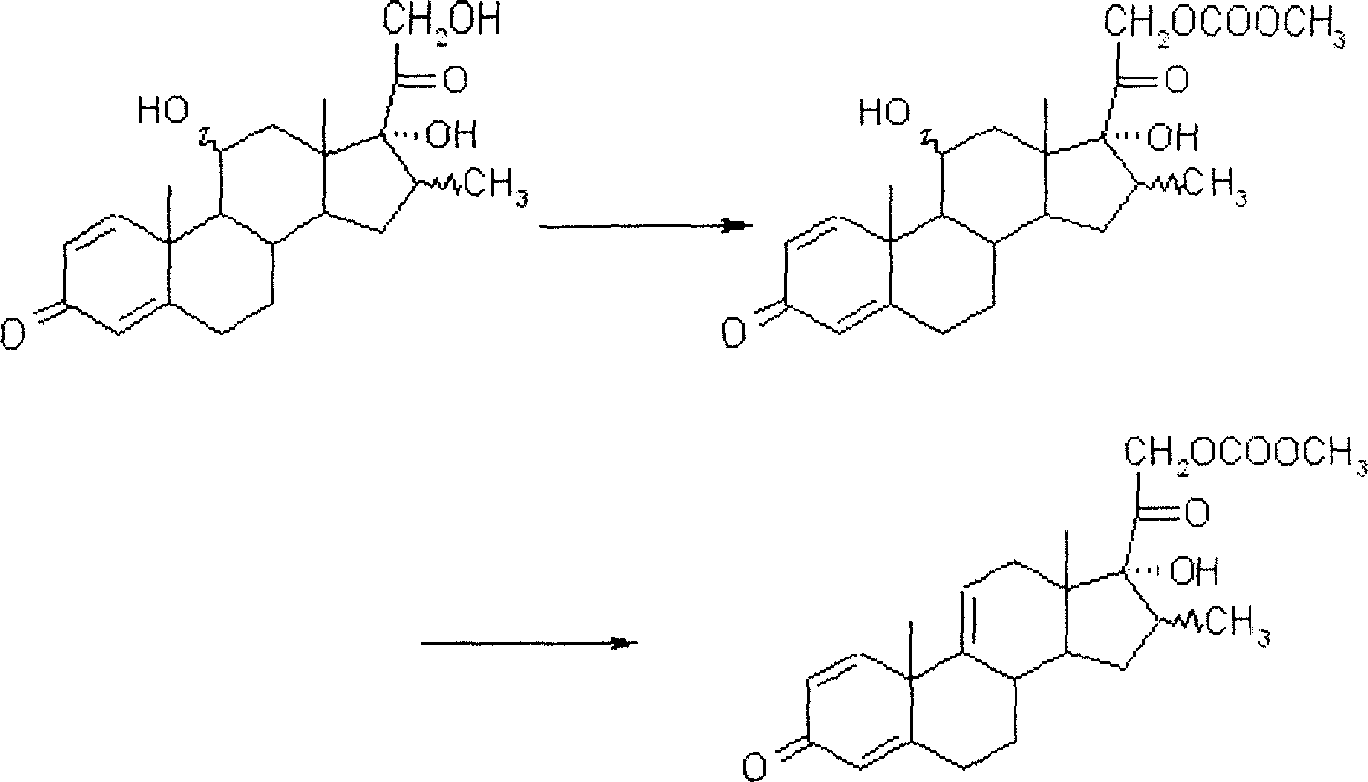
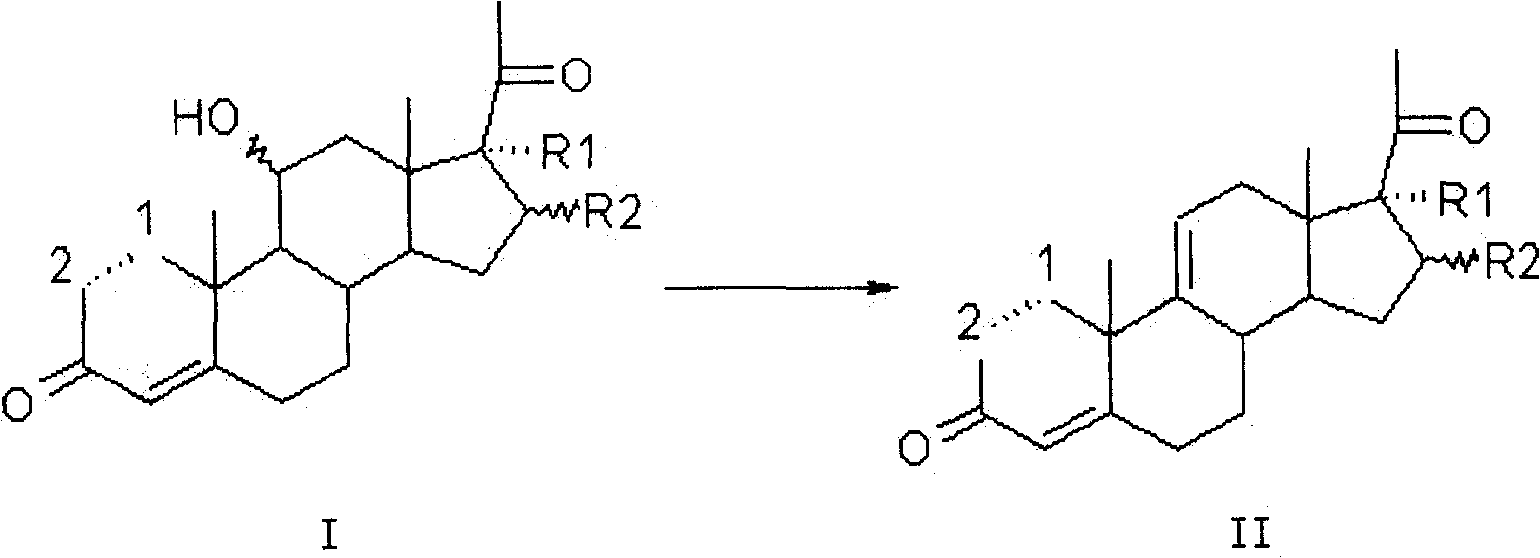
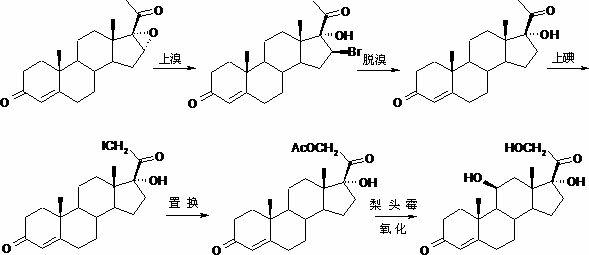
Method for preparing pregna-5-ene-3[beta], 21-diol by resting cell method
InactiveCN111778307ARaw materials are easy to getReduce manufacturing costBacteriaMutant preparationBiotechnologyPregnene
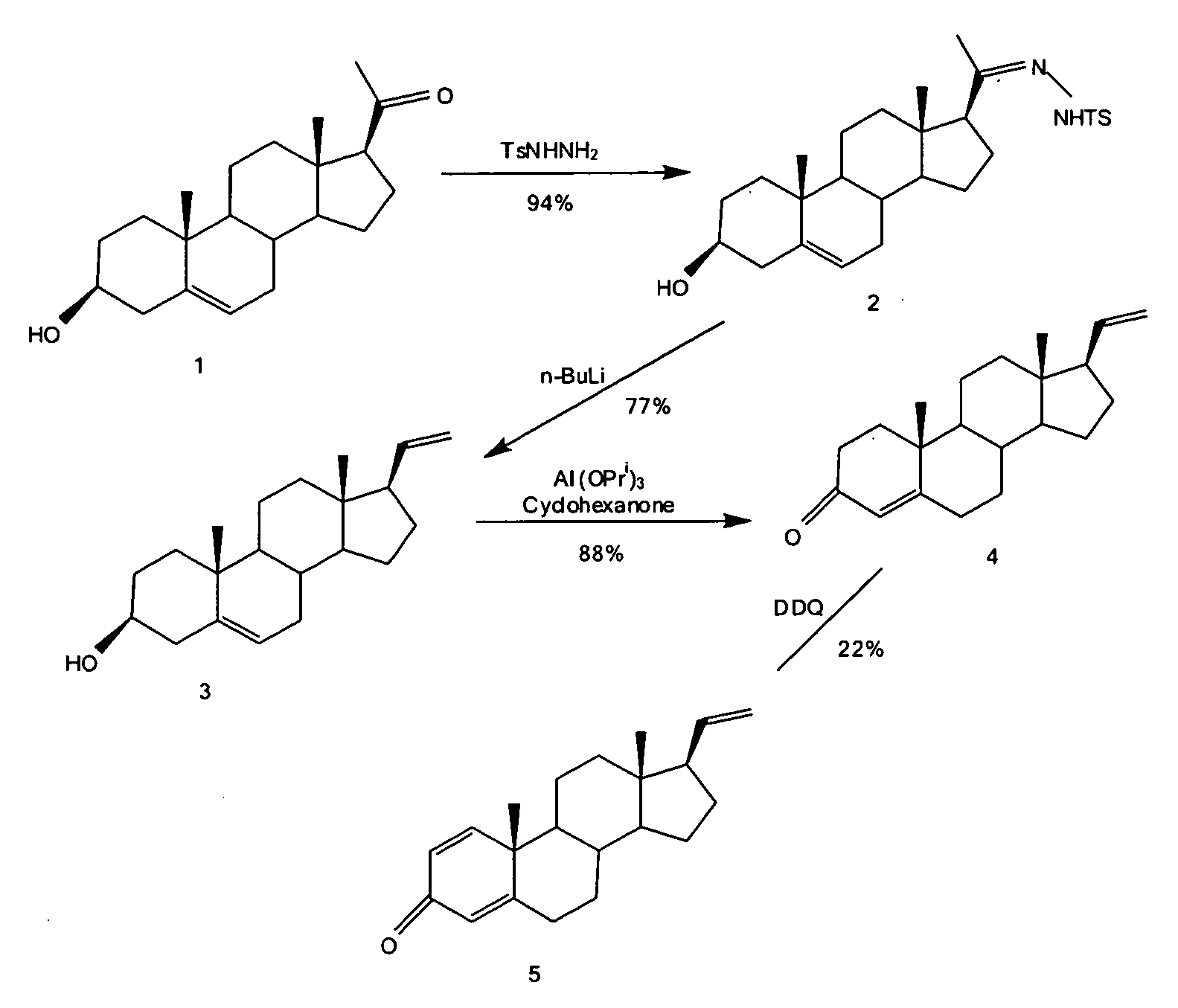
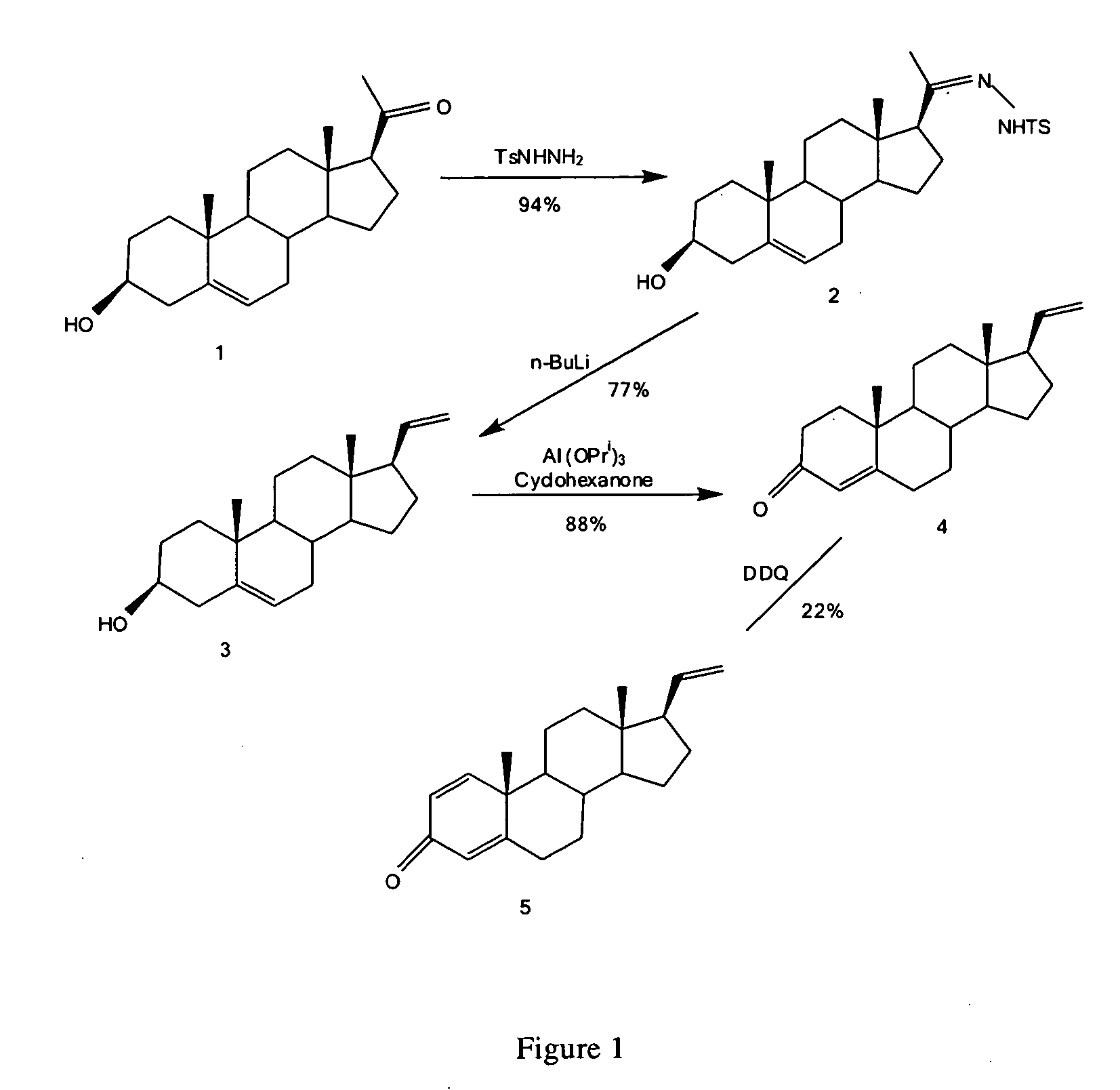
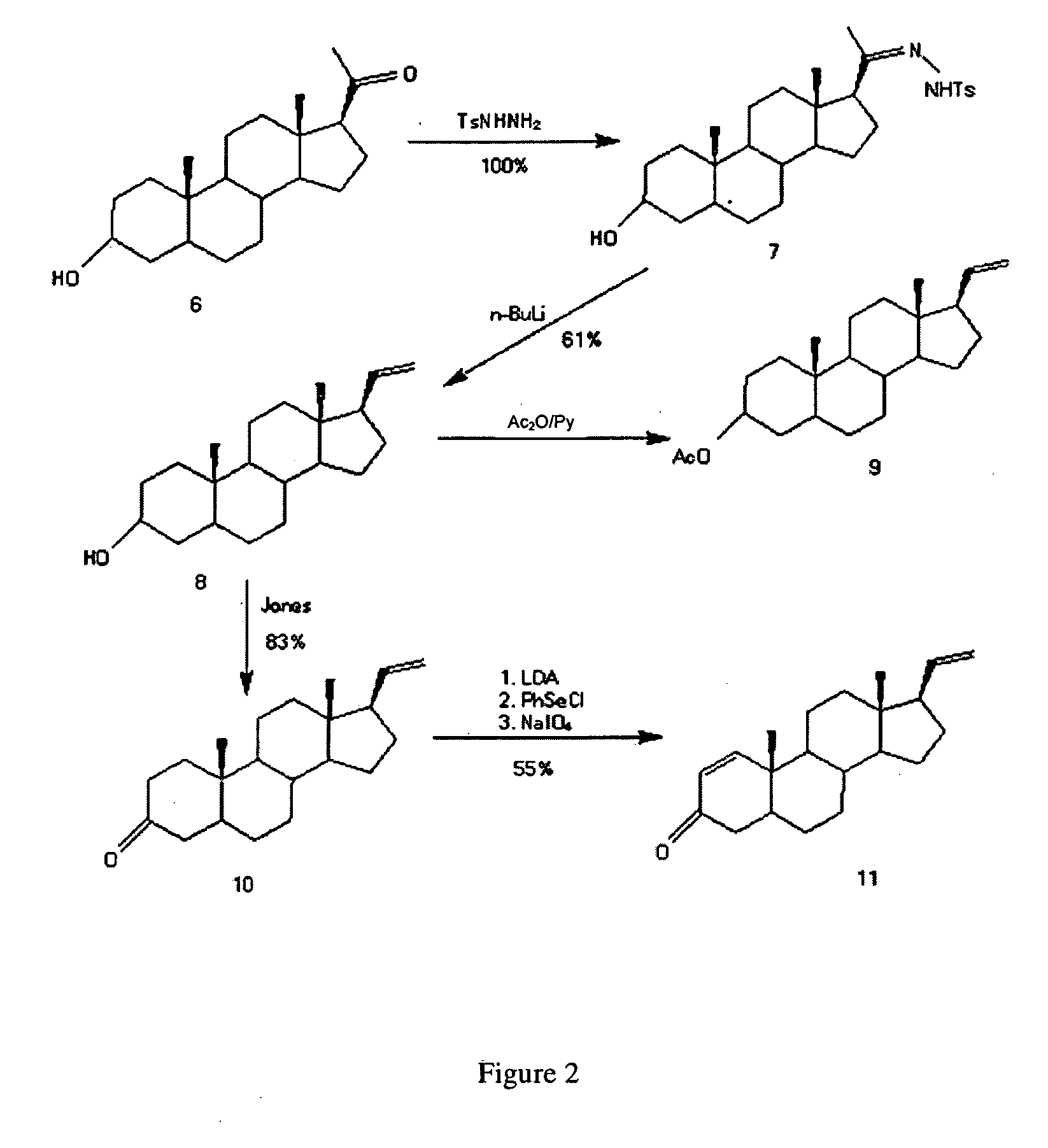
The invention relates to a production method of a steroid drug intermediate, in particular to a method for preparing pregna-5-ene-3[beta], 21-diol by a resting cell method. The method comprises the steps of (1) 3-site protection of phytosterol, (2) transformation of resting cells, (3) extraction, (4) hydrolysis and (5) refining. The phytosterol is used as a raw material to produce the pregnene-5-ene-3[beta], 21-diol, the raw material is easy to obtain, and the production cost is reduced; functional group protection is carried out on 3-hydroxyl of the phytosterol by adopting a protective agent,the pregn-5-ene-3[beta], 21-diol can be directly prepared through hydrolysis after fermentation, few byproducts are generated, the yield is higher, a reaction route is shorter, and many reaction steps and post-treatment steps required in a traditional preparation method are omitted.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
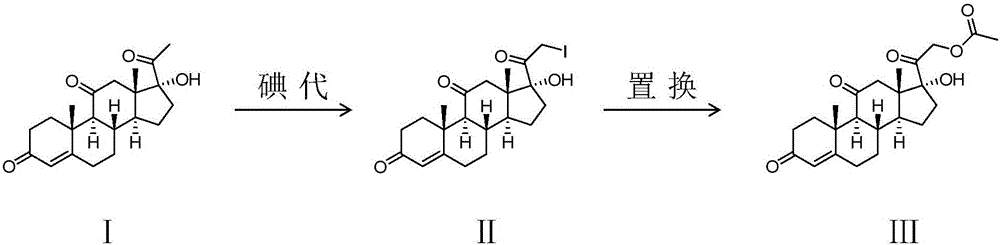
Preparing method of cortisone acetate
The invention provides a preparing method of cortisone acetate. The method comprises the three following steps of iodine solution preparation, the iodo reaction and the replacement reaction. In the step one, an idodine solution and bromine are mixed to prepare bromine and iodine liquid. In the step two of the iodo reaction, a reactant 17 alpha-hydroxyl-pregnene-4-alkene-3,11,20-triketone (I) and the bromine and iodine liquid are subjected to the iodo reaction to obtain an intermediate product iodine substitute (II). In the step three of the replacement reaction, the iodine substitute (II) and acetate are subjected to the replacement reaction to obtain a final product cortisone acetate (III). According to the preparing method, the bromine and iodine mixture iodo reaction replaces the traditional iodo reaction, the amount of adopted iodine is reduced greatly, cost is low, environmental pollution is reduced, and applicability is high.
Owner:河北远大九孚生物科技有限公司
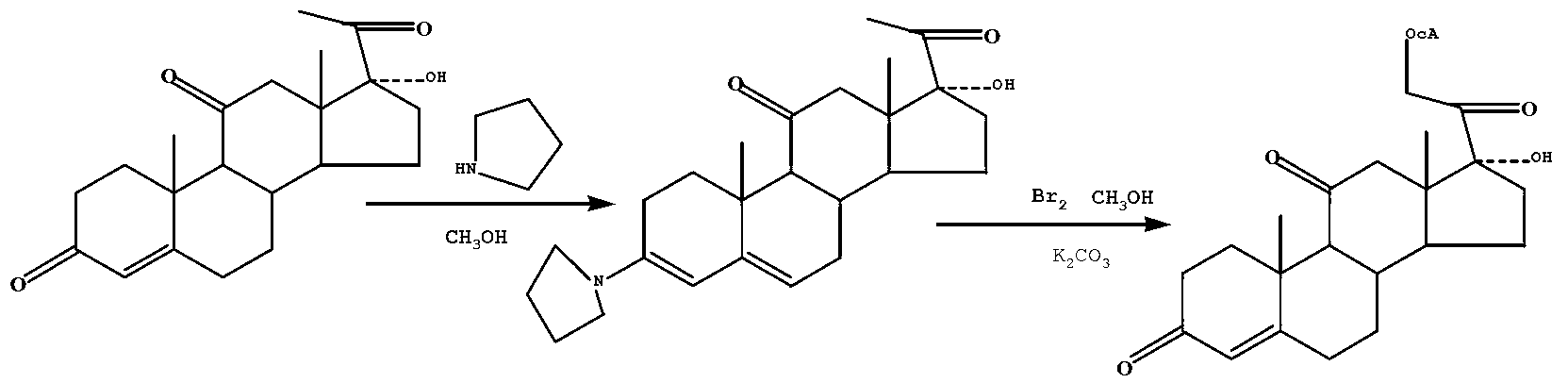
Preparation method of cortisone acetate
ActiveCN103232514AReduce manufacturing costImprove the production operating environmentSteroidsEpoxyKetone
The invention discloses a preparation method of cortisone acetate, which comprises the following steps: preparing the raw material 11-hydroxy-16,17-epoxy-4-ene-3,20-dione into 17-alpha-hydroxy-4-pregnene-3,11,20-trione; and carrying out substitution and replacement reaction on the 17-alpha-hydroxy-4-pregnene-3,11,20-trione with raw materials bromine, potassium acetate and the like to obtain the cortisone acetate. The method specifically comprises the following steps:(a) in an inert gas protective atmosphere, reacting 17-alpha-hydroxy-4-pregnene-3,11,20-trione with pyrrolidine by using alcohol as a solvent to obtain an intermediate compound A; and (b) in an inert gas protective atmosphere and in the presence of catalysts methylsulfonic acid and triethyl orthoformate, carrying out substitution reaction on the intermediate compound A and bromine by using alcohol as a solvent, and carrying out replacement reaction on the product and postassium acetate to obtain the cortisone acetate. The new process disclosed by the invention can greatly lower the production cost of cortisone acetate.
Owner:河北远大九孚生物科技有限公司
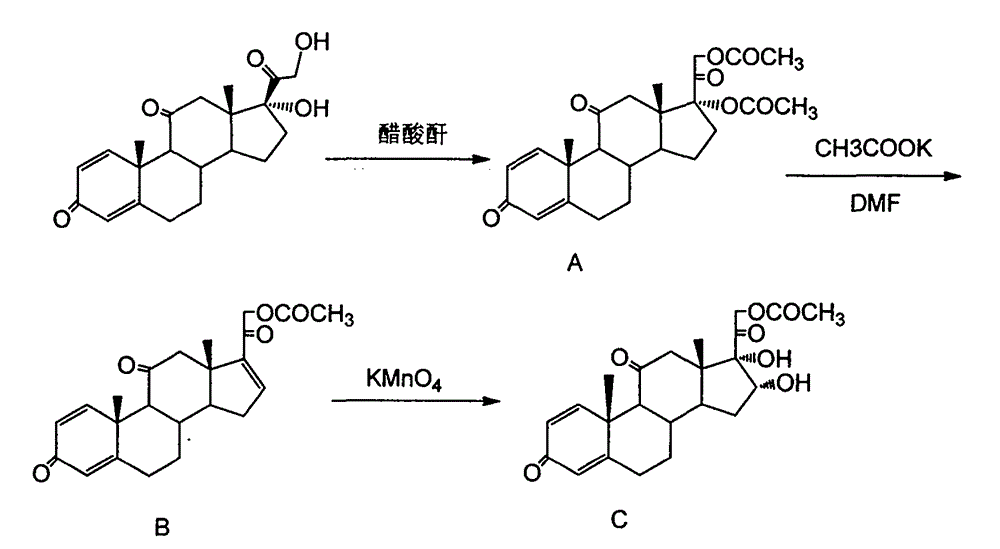
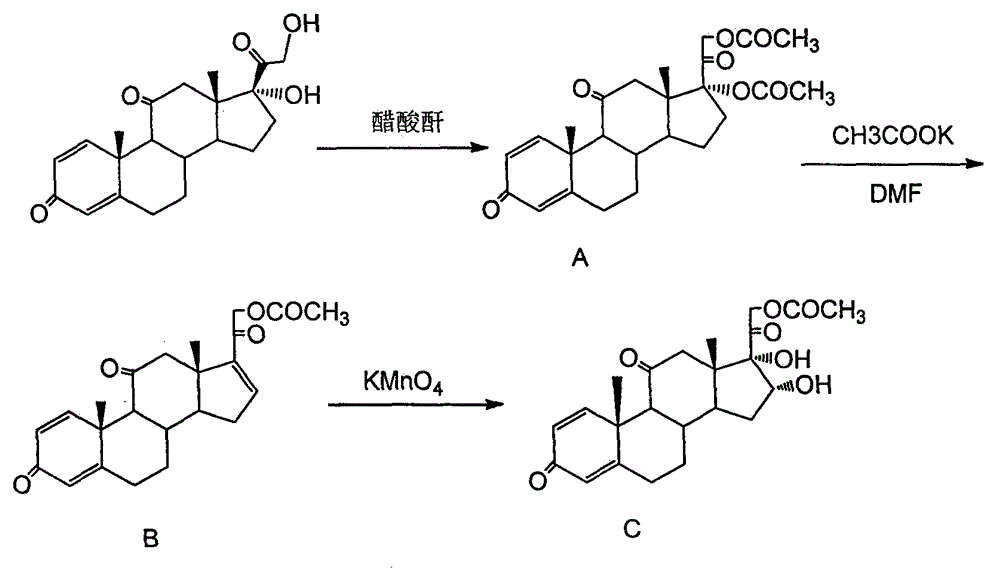
Preparation method of 16a,17a-dyhydroxyl-21-acetoxyl-1,4-pregnene diene-3,11,20-triketone
The invention provides a preparation method of 16a,17a-dyhydroxyl-21-acetoxyl-1,4-pregnene diene-3,11,20-triketone. The preparation method comprises the following steps: step 1, by taking prednisone as a raw material, allowing the prednisone to have an esterification reaction with acetic oxide; step 2, performing a degreasing reaction by use of alkali; and step 3, oxidizing by use of potassium permanganate under an acidic condition. The method has the advantages of adopting raw materials which are low in cost and easy to obtain, simplifying the production process route and being simple to operate and suitable for large-scale industrial production.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Method for synthesizing 3beta-hydroxy-16alpha,17alpha-epoxy-5-pregnene-20-ketone
The invention discloses a method for synthesizing 3beta-hydroxy-16alpha,17alpha-epoxy-5-pregnene-20-ketone, which comprises the following steps of: adding 348.8 weight parts of methanol into 7.0 weight parts of diene, and heating to the temperature of 40 DEG C with stirring; reducing the temperature to be between 26 and 27 DEG C, and adding 68.7 weight parts of 30 percent H2O2, 34.3 weight parts of 10 percent NaOH, 22.9 weight parts of 30 percent H2O2 and 11.4 weight parts of 10 percent NaOH slowly; controlling the temperature to be between 28 and 30 DEG C in the process of adding liquid, raising the temperature to be between 34 and 35 DEG C, keeping the temperature, reacting for 3 to 4 hours, and distilling under reduced pressure to obtain a white solid; and washing the white solid, performing suction filtration, leaching, and drying at the temperature of 105 DEG C to obtain a high-purity oxygen-bridged object. In the method, a synthetic process is short in time, oxygen-bridged reaction is complete, a melting point of the product is stabilized between 189.5 and 191.2 DEG C, the content of the product is 99.3 percent, and the yield of the product is between 98.5 and 99.1 percent. The method is suitable for industrial production.
Owner:SHAANXI UNIV OF TECH +1
Fragrance compositions and other compositions which contain naturally occurring substances found in corals
InactiveUS20090075964A1Improve friendlinessImprove the level ofCosmetic preparationsOrganic active ingredientsFlavorPregnene
This invention is generally related to the fields of fragrance compositions, personal care products, and home consumer products. This invention also relates to 20-pregnenes, in particular those found naturally occurring in corals and which affect mood in humans, to the incorporation of these 20-pregnene compounds into various compositions, and to methods of affecting the mood of individuals using such compounds.
Owner:HUMAN PHEROMONE SCI
Preparation method of 16-dehydropregnenolone acetate and 16-dehydropregnenolone acetate congeners
InactiveCN101974057ANo generationReduce manufacturing costSteroidsChemical recyclingAmmonium compoundsKetone
The invention discloses a preparation method of 16-dehydropregnenolone acetate and 16-dehydropregnenolone acetate congeners. The method comprises the following steps of: performing one-pot reaction on pseudo-steroid sapogenin acetate, a vanadium-containing compound catalyst, a quaternary ammonium compound catalyst and hydrogen peroxide in a molar ratio at the temperature of between 55 and 100 DEG C with mechanical stirring in a reactor for 3 to 24 hours so as to obtain a reaction solid-liquid mixture; and performing post-treatment on the reaction solid-liquid mixture so as to obtain the 16-dehydropregnenolone acetate and the 16-dehydropregnenolone acetate congeners, namely, 3 beta-acetoxyl group-5 alpha-pregnene-16(17)-alkene-20-ketone, 3 beta-acetoxyl group-5 beta-pregnene-16(17)-alkene-20-ketone or 3 beta-acetoxyl group-5 alpha-pregnene-9(11),16(17)-diene-20-ketone. The preparation method has the advantages that: oxidation and elimination are realized in one pot; an organic solvent is not used in the reaction; and the catalysts are used circularly, so that high yield, low cost and environmental protection are realized.
Owner:TIANJIN UNIV
Synthesis process of a pregnane compound
The invention discloses a synthesizing method of Delt9,11-pregnene compound, which is characterized by the following: adopting 21-non-hydroxylating pregnene compound as starting material; reacting with phosphorus pentachloride, phsophorus trichloride or phosphorus oxytrichloride in the organic solvent of tetrahydrofuran and acetonitrile; eliminating C11-hydroxy; obtaining the product with purity over 95% and the content of impurity isomer between 2 and 4%.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
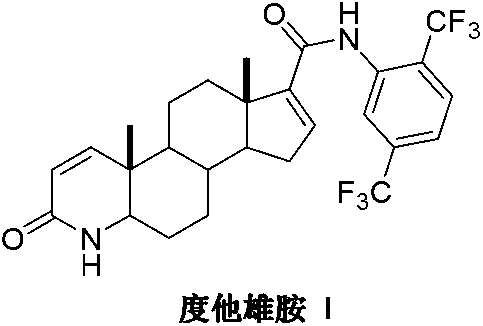
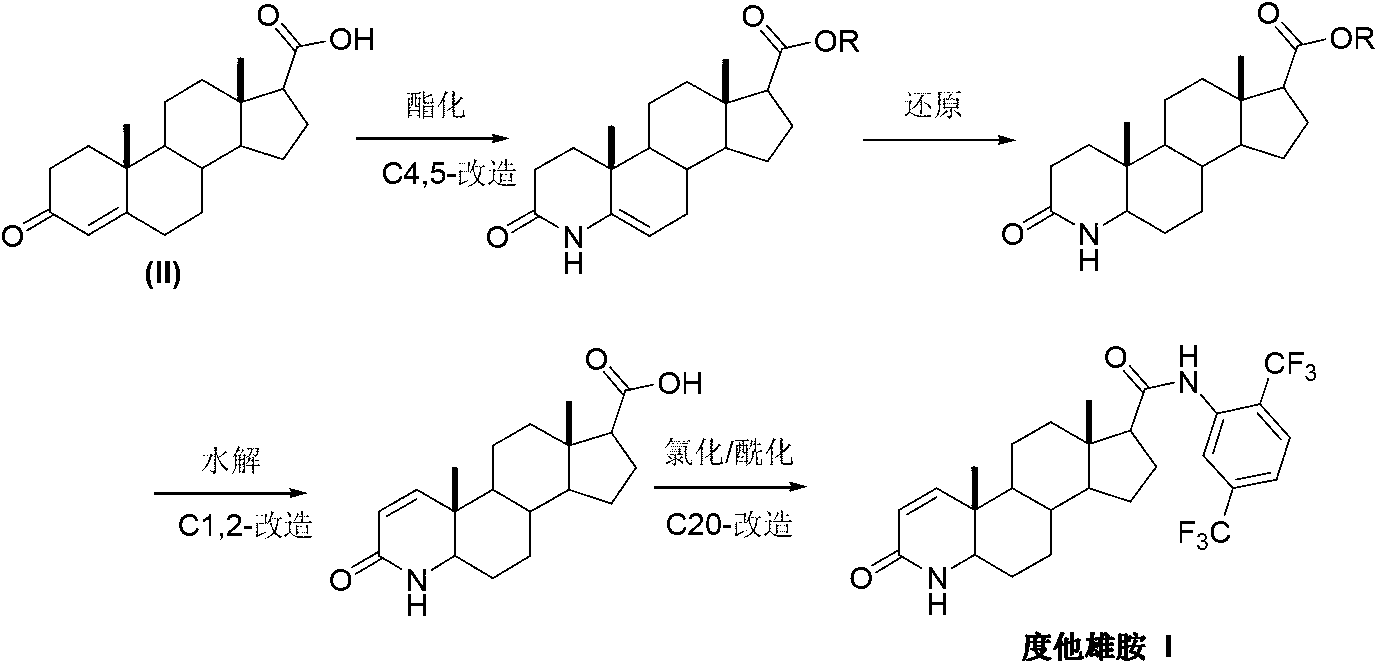
Preparation method of dutasteride
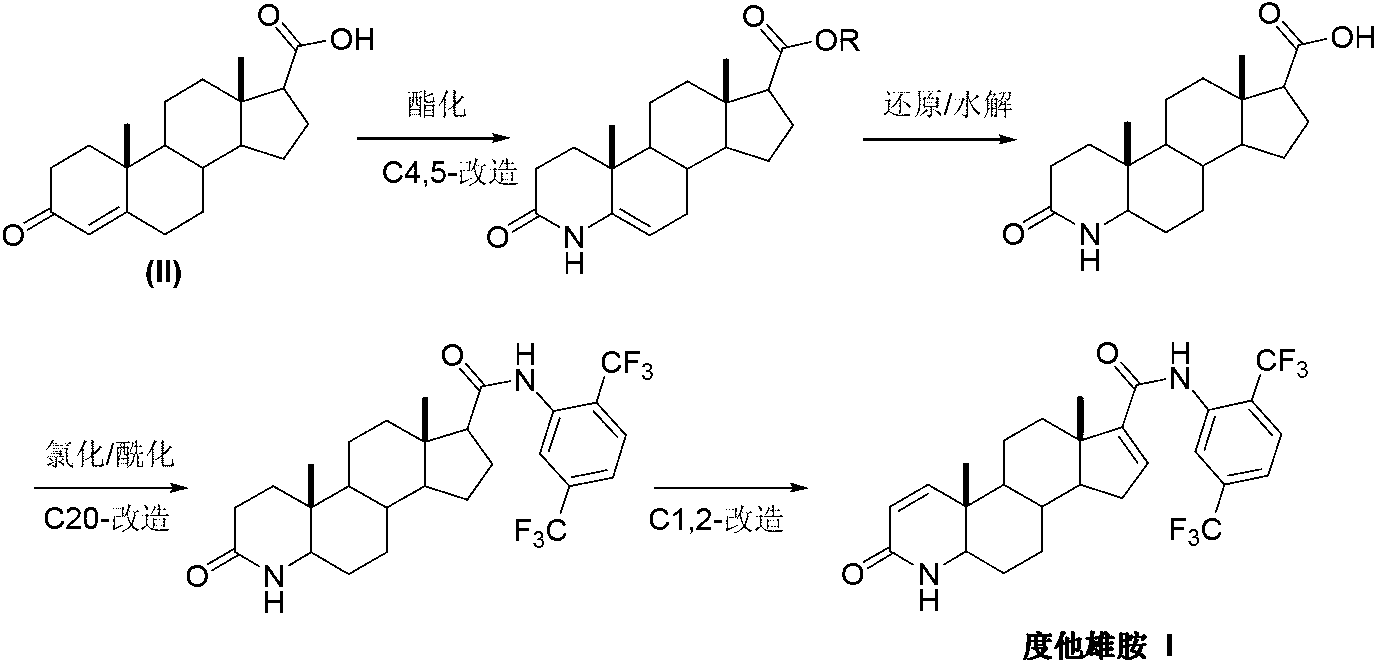
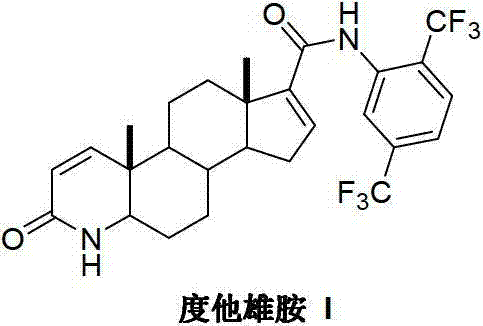
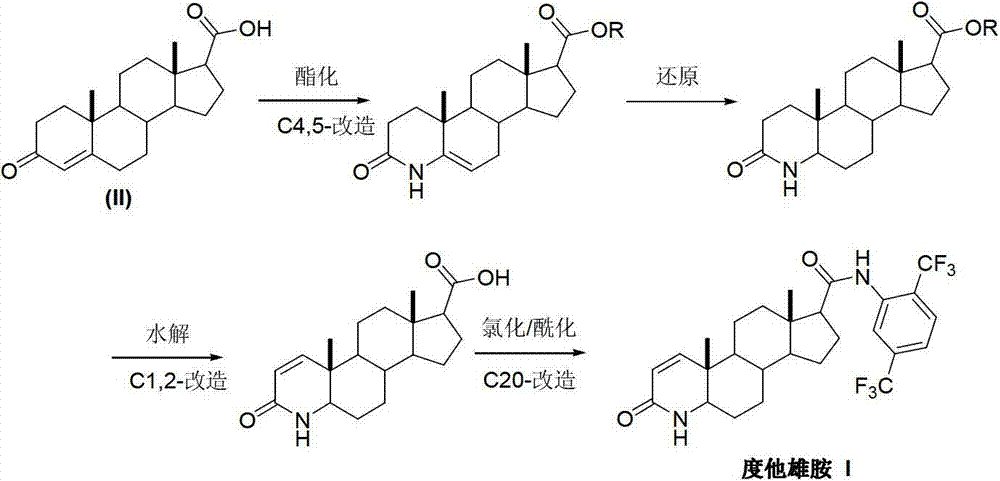
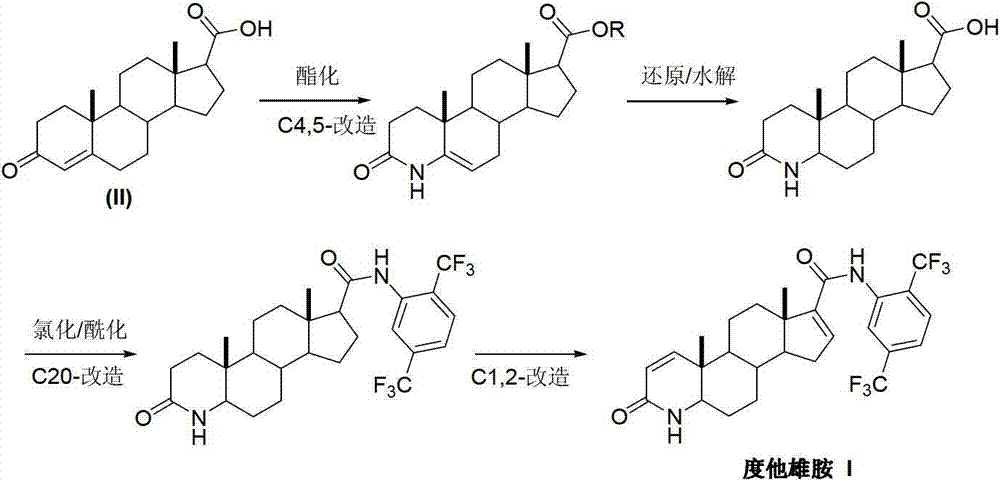
InactiveCN103254271AThe production process is easy to controlImprove product qualitySteroidsKetonic acidsDehydrogenation
The invention discloses a preparation method of dutasteride (N-(2,5-di(trifluoromethyl)phenyl)-4-aza-5alpha-androstane-1-ene-3-keto-17beta-formamide, I). The preparation method comprises the following steps that: pregnene ketonic acid (II) is taken as a raw material to convert carboxylic acid to amide through amidation to prepare androstane-4-ene-3-keto-17beta-formamide (III); the compound (III) is subjected to oxidative ring opening and ammonolysis cyclization to prepare 4-aza-androstane-5-ene-3-keto-17beta-formamide (IV); the compound (IV) is subjected to reductive hydrogenation reaction to generate 4-aza-5alpha-androstane-3-keto-17beta-formamide (V); the compound (V) is subjected to oxidative dehydrogenation to generate 4-aza-5alpha-androstane-1-ene-3-keto-17beta-formamide (VI); and the compound (VI) and 2,5-di(trifluoromethyl phenylamine) (VII) carry out amine exchange reaction in the presence of a catalyst to prepare dutasteride (I). The preparation method has the advantages of concise process, easiness in obtaining raw materials and controllable quality, and is suitable for industrial production.
Owner:ANHUI OURUIDA ELECTRICAL APPLIANCE TECH
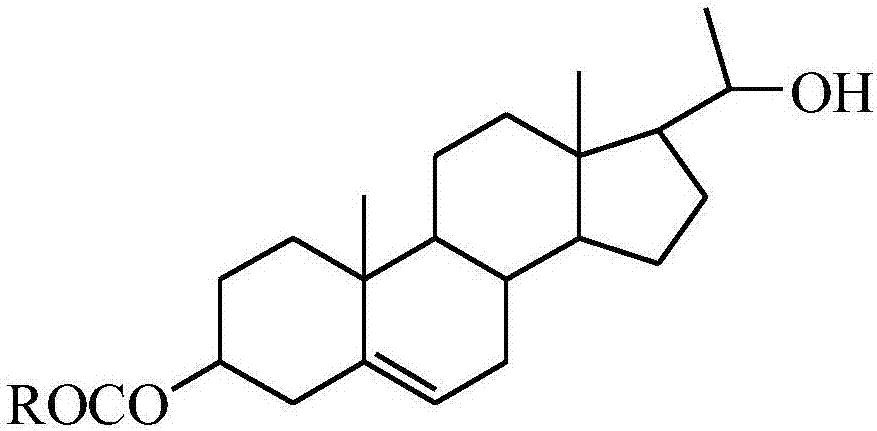
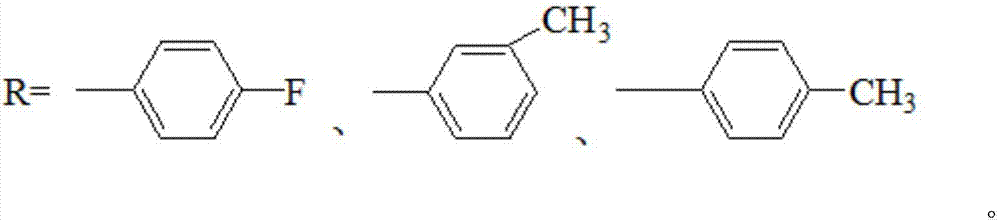
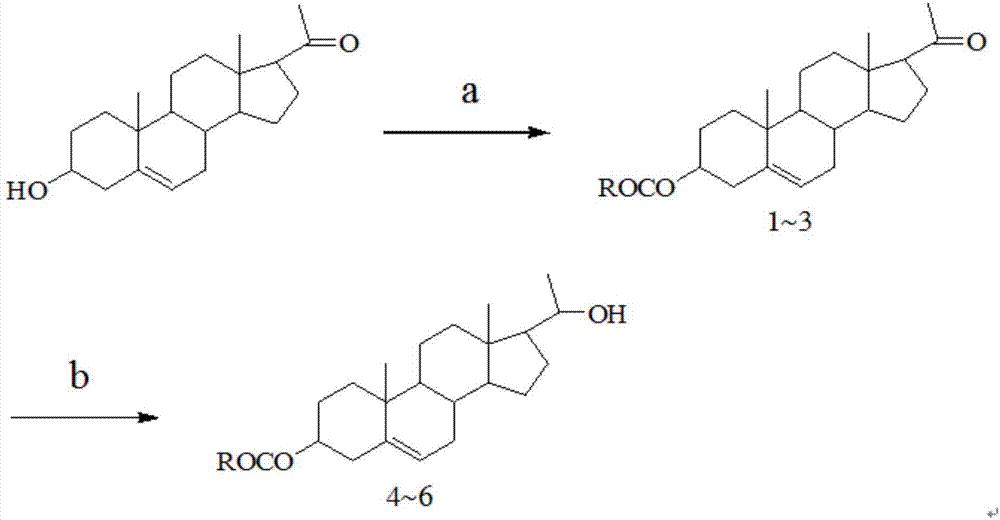
20-hydroxy-pregnen-3-aryl ester base pregnene compound, synthetic method thereof and application thereof in preparation of anti-tumor drugs
The invention discloses a 20-hydroxy-pregnen-3-aryl ester base pregnene compound. A steroid nucleus structural formula of the compound is as follows: formula (shown in the description), wherein an R- group can be the formula as follows. The invention further discloses a synthetic method of the 20-hydroxy-pregnen-3-aryl ester base pregnene compound. The synthetic method comprises the following steps: with a steroidal compound pregnenolone as a raw material, firstly esterifying 3-site hydroxide radical of pregnenolone by virtue of aroyl chloride, reacting so as to obtain an intermediate, and reducing 17-site carbonyl of the intermediate, so as to obtain the 20-hydroxy-pregnen-3-aryl ester base pregnene compound. The 20-hydroxy-pregnen-3-aryl ester base pregnene compound has an inhibiting effect to the growth and proliferation of certain cancer cells and can be used as a drug intermediate or a drug to be applied to the preparation of different anti-tumor drugs.
Owner:GUANGXI TEACHERS EDUCATION UNIV
Method for preparing 17 alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7 alpha, 9 alpha)-dicarboxylic acid lactone
The invention discloses a method for preparing 17alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7alpha,9alpha)-dicarboxylic acid lactone. Firstly, a compound I dissolved in a solvent and methanesulfonyl chloride are subjected to 11-hydroxyl sulfonylation reaction under the action of an acid-binding agent, and a compound II is obtained; then the compound II and inorganic alkali are added into the solvent and react to obtain the target compound 17alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7alpha,9alpha)-dicarboxylic acid lactone; finally, the target product high in purity is obtained through re-crystallization and column chromatography. The 17alpha-hydroxyl-3-oxo-gamma-lactone-pregnene-4-alkene-(7alpha,9alpha)-dicarboxylic acid lactone can be prepared to serve as a reference substance, and accordingly the quality of eplerenone can be better controlled.
Owner:ZHEJIANG XIANJU PHARMA
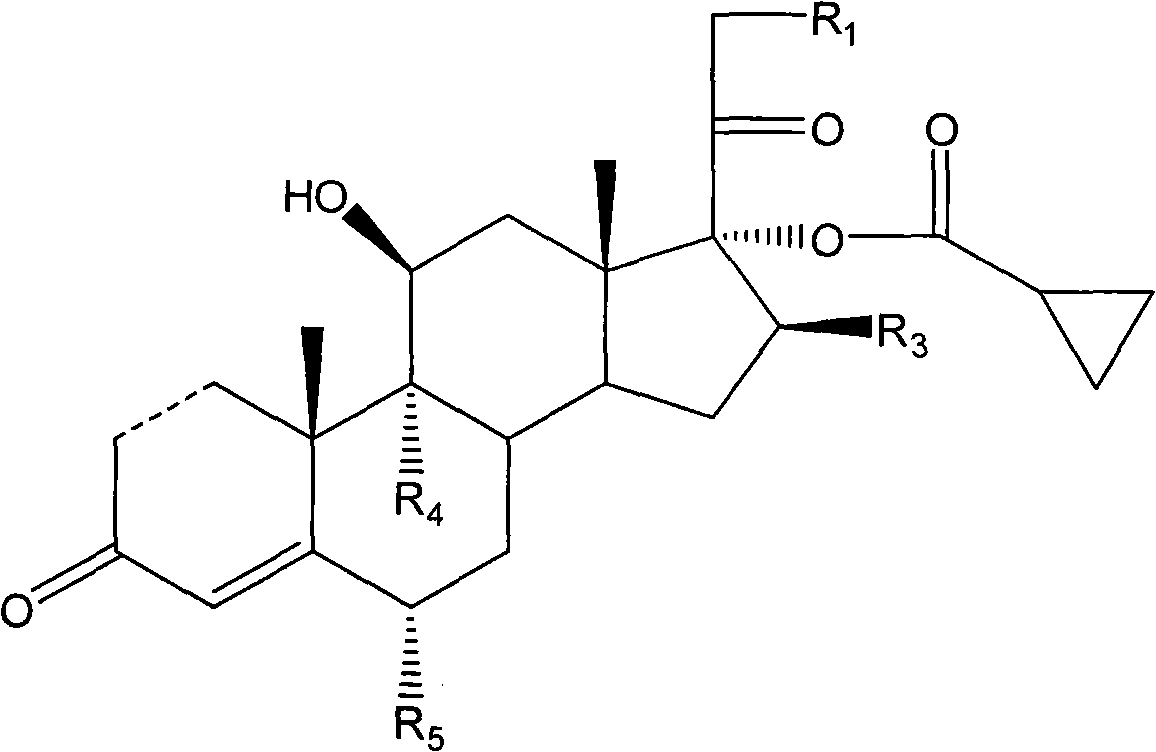
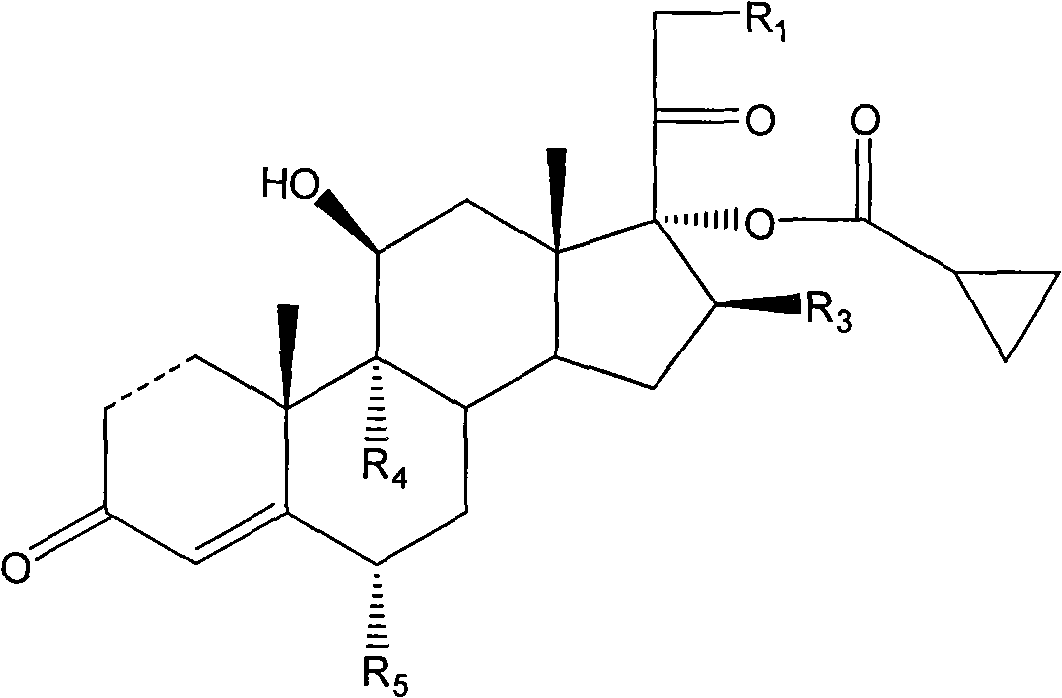
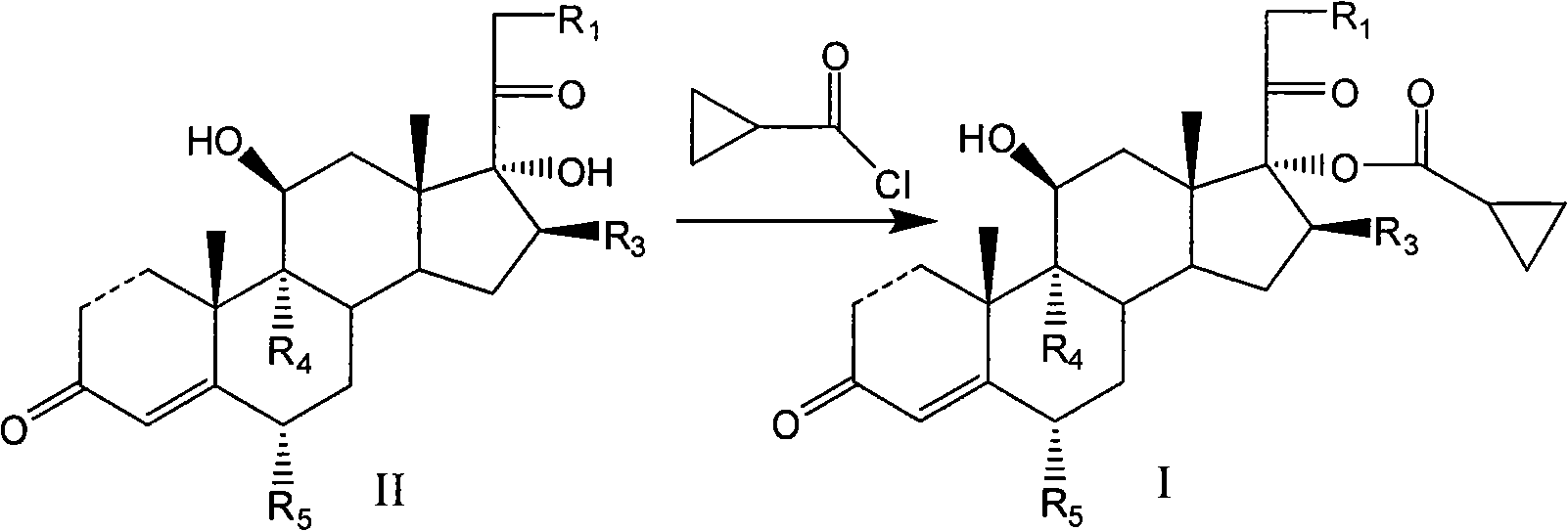
Cyclopropyl pregnene compound and application thereof
The invention discloses a cyclopropyl pregnene compound and application thereof, and discloses a novel adrenocortical hormone compound shown in a formula (I), a medicine composition containing the compound shown in the formula (I) as an active component and one or more pharmaceutic adjuvants, and application of the compound shown in the formula (I) or physiologically acceptable salt or solvate thereof as a medicine for treating of the diseases of mammal especially human, especially local inflammation.
Owner:TIANJIN JINYAO GRP
A method for synthesizing 6-methyl-17α-hydroxyl-19-norpregna-4,6-diene-3,20-dione
ActiveCN105017365BReduces the possibility of metal residueThe reaction is easy to operateSteroidsDiketoneAlcohol
The invention relates to the field of pharmaceutical synthesis, in particular to a method for synthesizing 6-methyl-17alpha- hydroxyl-19-nor-pregnene-4,6-diene-3,20-diketone. The method comprises the steps of reacting norethisterone with ethylorthoformate to obtain 3-ethoxy-17alpha-acetenyl-19-demethyl-pregnene-3,5-diene-17beta-alcohol (2); causing the compound (2) to undergo Vilsmeier reaction to obtain 3-ethoxy-6-formoxyl-17alpha-acetenyl-19- demethyl-pregnene-3,5-diene-17beta-alcohol (3); reacting the compound (3) with NaBH4 and obtaining 6-methine-17alpha-acetenyl-17beta-hydroxyl-19-demethyl-pregnene-4-en-3-ketone (4) through acidification and dehydration; causing the compound (4) to undergo double bond shift under the effect of Pd-C / cyclohexene to obtain a product 6-methyl-17alpha-acetenyl-17beta-hydroxyl-19-demethyl-pregnene-4,6-diene-3-ketone (5); and reacting the compound (5) with benzene sulfenyl chloride to obtain 6-methyl-19-demethyl-21-phenylsulfinyl-pregnene-4,6,17(20)20-tetraene-3-ketone.
Owner:丽江华映激素药物科技开发有限公司
Fragrance Compositions and Other Compositions Which Contain Naturally Occurring Substances Found in Corals
InactiveUS20140045805A1Improve friendlinessImprove the level ofCosmetic preparationsOrganic active ingredientsFlavorPregnene
This invention is generally related to the fields of fragrance compositions, personal care products, and home consumer products. This invention also relates to 20-pregnenes, in particular those found naturally occurring in corals and which affect mood in humans, to the incorporation of these 20-pregnene compounds into various compositions, and to methods of affecting the mood of individuals using such compounds.
Owner:HUMAN PHEROMONE SCI
Method for synthesizing progesterone midbody 3beta-hydroxy-5-pregnene-20-ketone
The invention relates to a method for synthesizing progesterone midbody 3beta-hydroxy-5-pregnene-20-ketone. By the method, a refining procedure and a by-product oxidation recovery processing procedure of a traditional synthesizing process are saved. The obtained midbody does not need to be refined, the once quality yield is more than 86%, and the product purity is more than 99.0%.
Owner:HUAZHONG PHARMA
The preparation method of steroid medicine intermediate
ActiveCN108559766BHigh purityReduce the ratioMicroorganism based processesSteroidsMicrobial transformationDehydrogenation
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
A kind of synthetic method of pregna-1,4,9(11),16(17)-tetraene-3,20-dione and its intermediate
ActiveCN104628808BReduce usageRaw materials are cheap and easy to getSteroids preparationBulk chemical productionSynthesis methodsSide chain
The invention relates to a synthesis method and main intermediates of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. The synthesis method sequentially comprises the following steps of reacting a second intermediate and tosylmethyl isocyanide in an organic solvent at the temperature of lower than 35 DEG C below zero to generate a third intermediate; reacting the third intermediate and a methylated reagent in an organic solvent at the temperature of 70-90 DEG C, and then, removing methyl ether protecting groups and tosylmethyl isocyanide under the action of an acid to obtain a fourth intermediate; and reacting the fourth intermreidate under the action of 3-ketosteroid-1-dehydrogenase to generate pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone. Raw materials of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone are cheap and available; the yield of pregnene-1,4,9 (11),16 (17)-tetraenol-3, 20-diketone is relatively high; a C17-position side chain is introduced by using tosylmethyl isocyanide, so that acetone cyanohydrin serving as a highly-toxic reagent is prevented from being used; and the synthesis method is safe, environment-friendly and suitable for industrial production.
Owner:山东国九堂制药集团股份有限公司
Synthetic method for hormone drug intermediate 9(11)-pregnene-16beta-methyl-17alpha,21-diol-3,20-dione-21-acetate
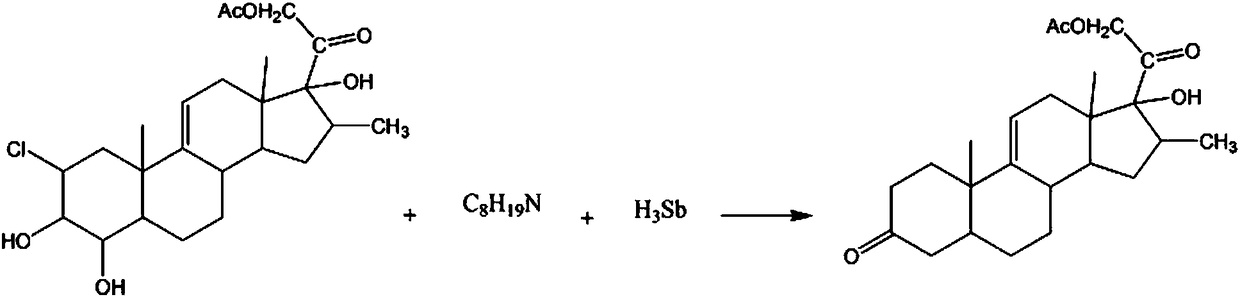
InactiveCN108239130AAvoid pollutionAvoid the risk of increased hazards in the reaction processSteroidsPotassiumKetone
The invention discloses a synthetic method for the hormone drug intermediate 9(11)-pregnene-16beta-methyl-17alpha,21-diol-3,20-dione-21-acetate. The synthetic method comprises the following steps: adding 2-chloro-4-hydroxy-9(11)-pregnene-16beta-methyl-3beta,17alpha,21-triol-20-one-21-acetate, a potassium chloride solution and a 2-methylpyridine solution into a reaction vessel, lowering solution temperature, controlling a stirring speed and continuing a reaction; and adding a diisobutylamine solution, raising a solution temperature, carrying out a reaction, adding antimony hydride powder, raising the temperature, carrying out a reaction, then carrying out standing and filtering successively so as to obtain a crystal, washing the crystal with a sodium nitrate solution, then washing the crystal with a benzonitrile solution, washing the crystal with a p-bromochlorobenzene solution, carrying out recrystallization in a diethylene glycol dibutyl ester solution, and then carrying out dehydration with a dehydrating agent so as to obtain the finished 9(11)-pregnene-16beta-methyl-17alpha,21-diol-3,20-dione-21-acetate.
Owner:CHENGDU QIANYE LONGHUA PETROLEUM ENG TECH CONSULTING
A kind of preparation method of dutasteride
InactiveCN103254271BThe production process is easy to controlImprove product qualitySteroidsKetonic acidsDehydrogenation
Owner:ANHUI OURUIDA ELECTRICAL APPLIANCE TECH
Synthesis process of a pregnane compound
The invention discloses a synthesizing method of Delt9,11-pregnene compound, which is characterized by the following: adopting 21-non-hydroxylating pregnene compound as starting material; reacting with phosphorus pentachloride, phsophorus trichloride or phosphorus oxytrichloride in the organic solvent of tetrahydrofuran and acetonitrile; eliminating C11-hydroxy; obtaining the product with purity over 95% and the content of impurity isomer between 2 and 4%.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
A method for synthesizing 6-methylene-17α-hydroxyl-19-norpregna-4-ene-3,20-dione
The invention provides a method for synthesizing 6-methylene-17 alpha-hydroxy-19-desmethyl pregnene-4-alkene-3,20-diketone. The method comprises the steps of (1) using 17 alpha-hydroxy-19-desmethyl pregnene-4-alkene-3,20-diketone for preparing 3-ethyoxyl-17 alpha-hydroxy-19-desmethyl pregnene-4-alkene-20-ketone; (2) using the product in the step (1) for preparing 3-ethyoxyl-6-(N-methyl-N-phenyl) aminomethyl-17 alpha-hydroxy-19-desmethyl pregnene-4,6-diene-20-ketone; (3) using the product in the step (2) for preparing the 6-methylene-17 alpha-hydroxy-19-desmethyl pregnene-4-alkene-3,20-diketone. According to the method provided by the invention, the 6-methylene-17 alpha-hydroxy-19-desmethyl pregnene-4-alkene-3,20-diketone is synthesized through three-step reaction, a synthetic process is simple and short, and low in cost, and the yield achieves 55 percent or above.
Owner:丽江映华生物药业有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






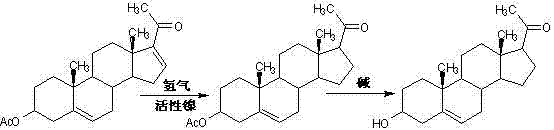




![Method for preparing pregna-5-ene-3[beta], 21-diol by resting cell method Method for preparing pregna-5-ene-3[beta], 21-diol by resting cell method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e22a9e3-1d66-4038-8c36-20ff24d26b0a/BDA0002598201690000061.png)
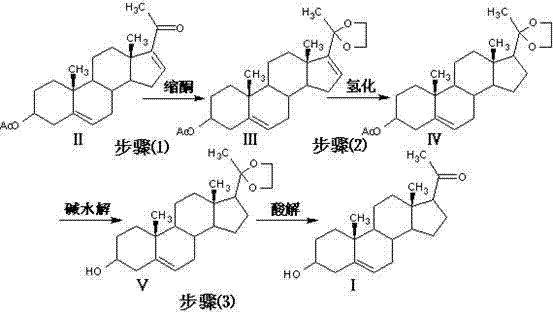
![Method for preparing pregna-5-ene-3[beta], 21-diol by resting cell method Method for preparing pregna-5-ene-3[beta], 21-diol by resting cell method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e22a9e3-1d66-4038-8c36-20ff24d26b0a/BDA0002598201690000062.png)
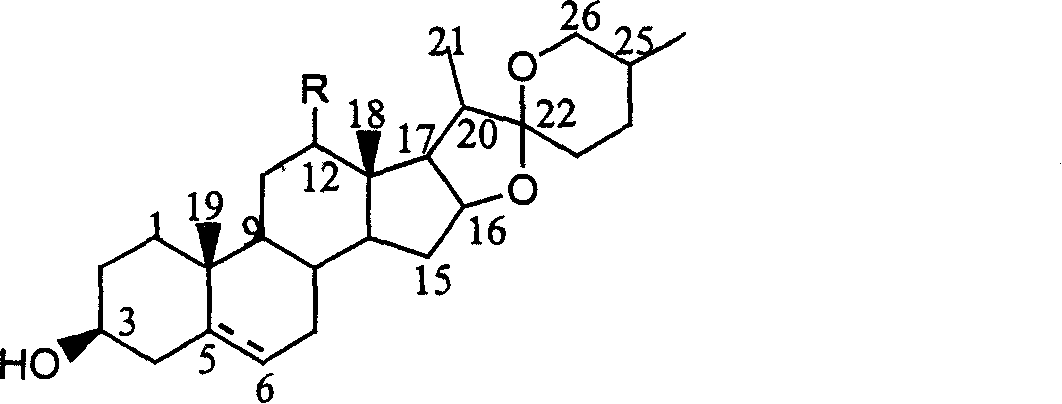
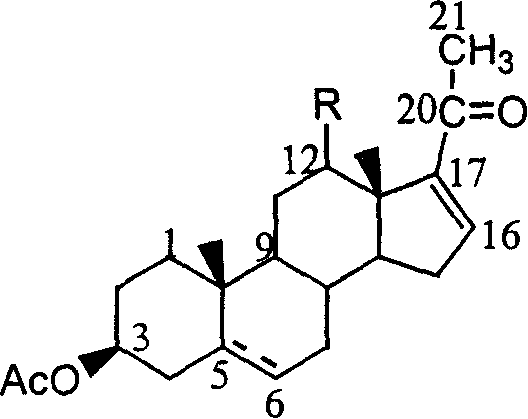
![Method for preparing pregna-5-ene-3[beta], 21-diol by resting cell method Method for preparing pregna-5-ene-3[beta], 21-diol by resting cell method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e22a9e3-1d66-4038-8c36-20ff24d26b0a/BDA0002598201690000071.png)