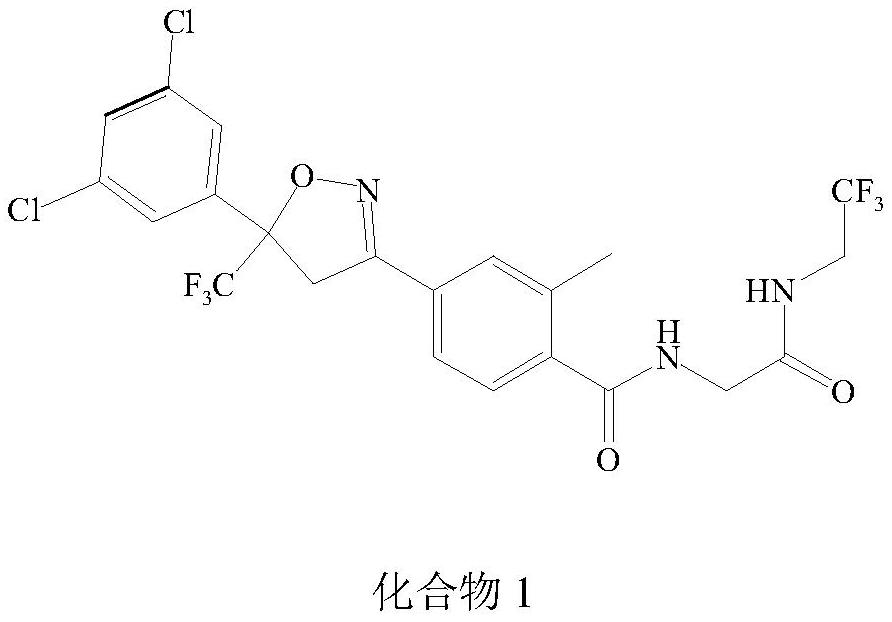
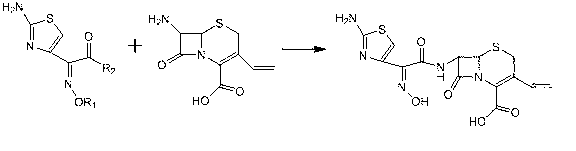
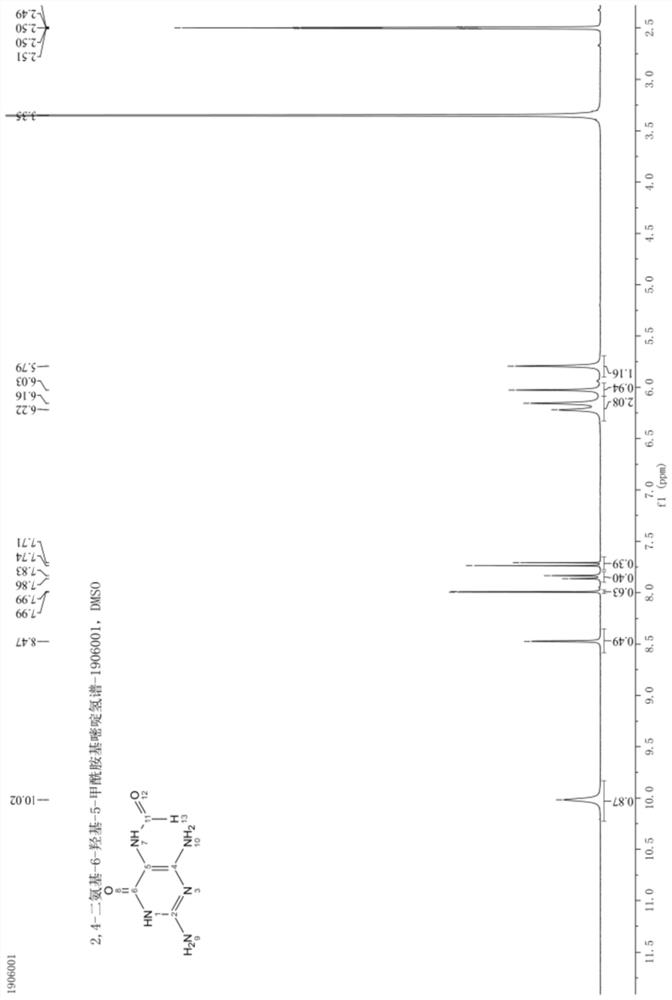
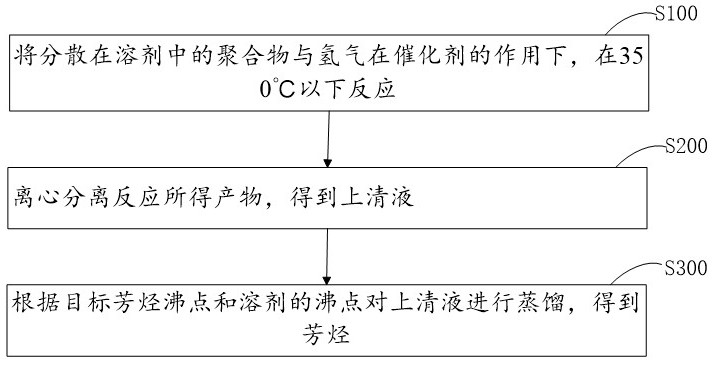
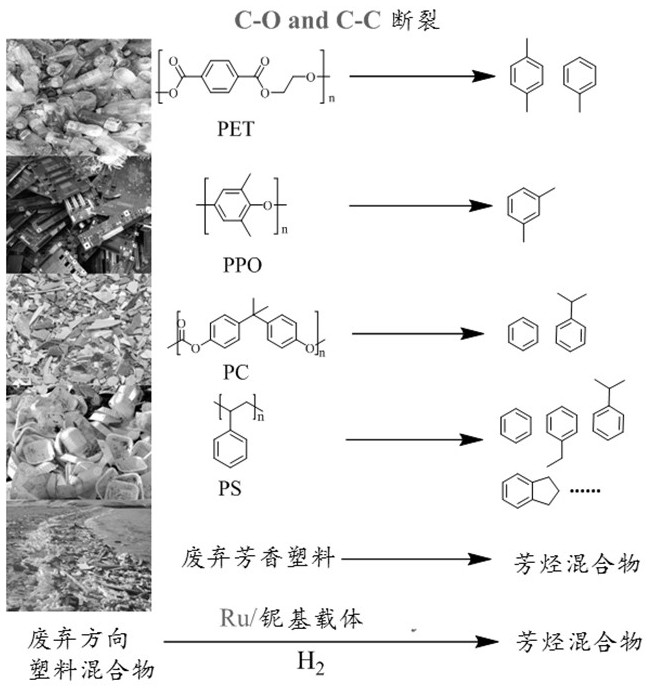
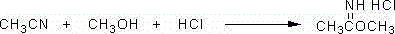Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
132results about How to "High molar yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Improved process for producing cyclohexanol and pimelinketone
InactiveCN101172931AGood dispersionIncrease interface areaOxygen compounds preparation by hydrocarbon oxidationCyclohexanoneHydrogen peroxide breakdown
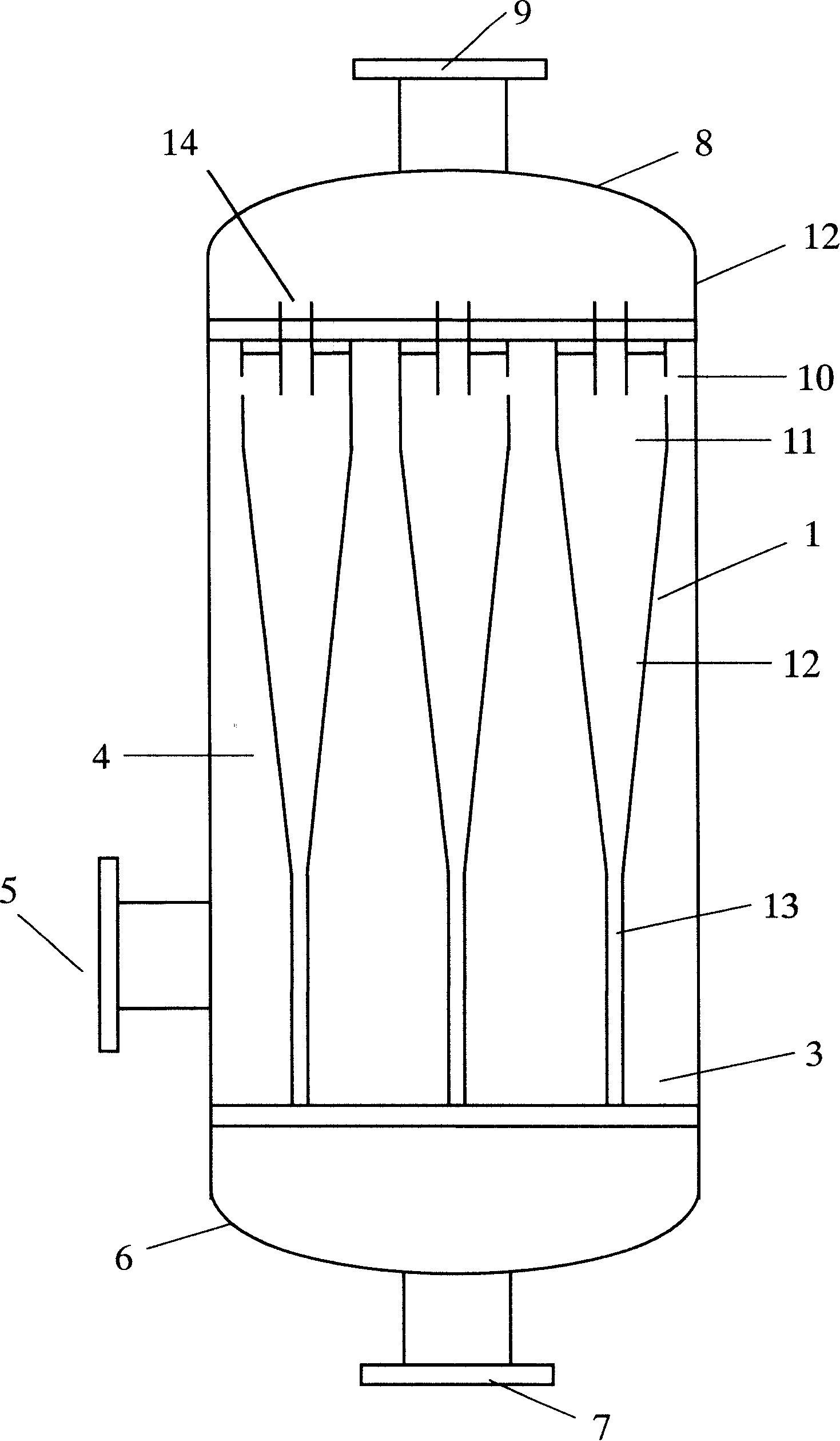
The invention discloses an improved method for preparing cyclohexanol and cyclohexanone. The invention is characterized in that the decomposition reaction of cyclohexyl peroxide is accomplished with two steps; during the first decomposition reaction, the flow of circulated alkaline is equal to or larger than that of cyclohexane oxydic liquid, thereby ensuring the alkaline water phase to become a continuous phase of the material in the first decomposition reaction; the cyclohexane phase is dispersed; the decomposition reaction is accomplished under an emulsification state, or is called even phase decomposition; the compound from the decomposition reaction is rough separated through a hydrocyclone separator; a large amount of alkaline liquid is separated from the lower outlet of the hydrocyclone separator to be recycled; the material of cyclohexane from the upper outlet of the hydrocyclone separator is changed to the continuous phase, while little alkaline liquid is changed into the dispersing phase; the waste alkaline liquid is separated by lifting a separation groove through gravity; the second decomposition reaction is then accomplished to ensure a full decomposition reaction. With the technical improvement, the total mol yield of the oxidation of cyclohexane prepared cyclohexanol and the cyclohexanone device is increased by 5 percent.
Owner:肖藻生
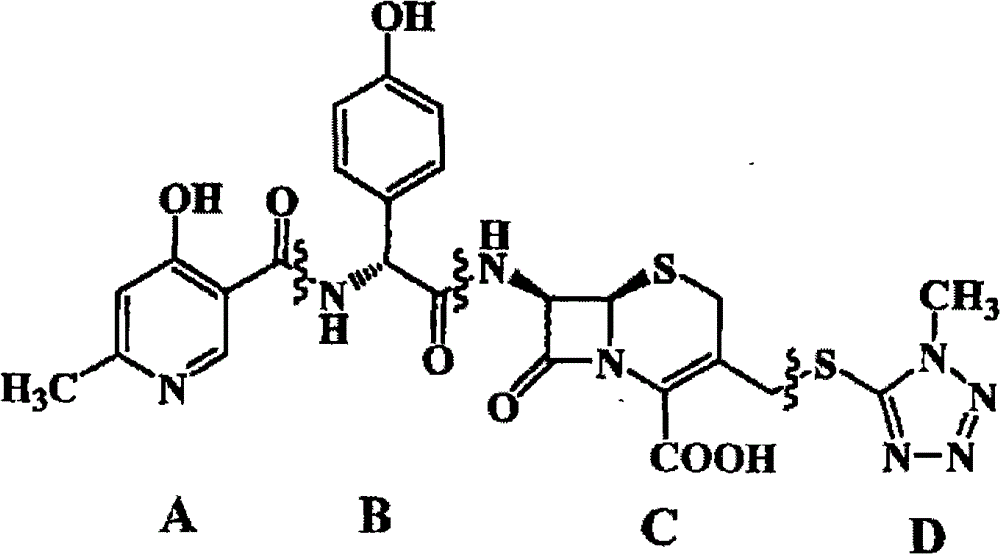
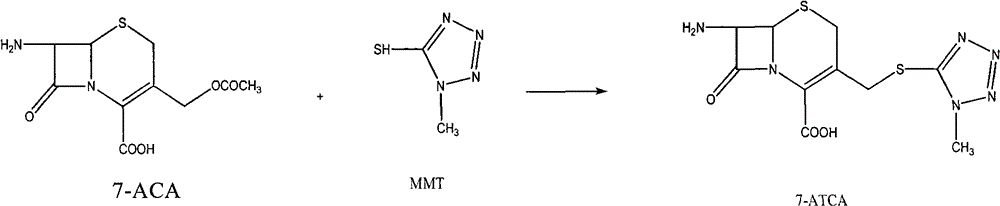
Process for enzymatic synthesis of cefprozil
InactiveCN104928340ASimple processEasy to operateOn/in organic carrierFermentationEnzymatic synthesisSynthesis methods
The invention relates to a medicine synthesis method, in particular to a process method for screening of immobilized cefprozil synthetase and enzymatic synthesis of cefprozil. In order to solve the problems that a conventional cefprozil enzymatic synthesis process has difficulties in screening and evaluating immobilized anzyme, tedious in production process step, poor in control point, long in reaction time, low in conversion ratio and the like, the invention provides an immobilized cefprozil synthetase and a novel process for cefprozil synthesis.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Preparation method of isoxazoline insectifuge
InactiveCN111675667AHigh molar yieldEasy to operateBiocideOrganic chemistryAnthelmintic drugFluralaner
The invention belongs to the technical field of chemical drug synthesis, and particularly relates to a preparation method of an isoxazoline insectifuge. The preparation method is characterized in thatthe isoxazoline insectifuge is fluralaner; an intermediate I is sequentially subjected to an amidation condensation reaction, a substitution reaction and a ring closing reaction in the same reactioncontainer; and after the reactions are finished, a reaction product is poured into water, stirring and filtering are conducted to obtain a solid, and the obtained solid is recrystallized to obtain theisoxazoline insectifuge. The method has the beneficial effects that the method uses a solvent and one-pot method to synthesize fluralaner, technological operation of a synthesis process is reduced, treatment time and reaction time are shortened, product yield is improved, and the method is suitable for large-scale production.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Preparation method of methyl glucoside
InactiveCN101659683AHigh molar yieldRich sourcesSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsCelluloseNitrogen
A preparation method of methyl glucoside relates to preparation of methyl glucoside, in particular to a new way for directly preparing the methyl glucoside by taking cellulose as raw material, then combing the cellulose in a methanol medium, and then conducting catalyzed reaction between the cellulose and the methanol under the existence of an acid catalyst, and a related acid catalyst. The invention provides the preparation method of methyl glucoside, which has rich resource of adopted reactants, does not compete with grain such as starch, and has simple technological process and the highestmolar yield of the methyl glucoside of 65 percent. The cellulose, the methanol and the acid catalyst are mixed and added in a high pressure autoclave, then nitrogen is led in for evacuation, and afterreaction, cooling is conducted to obtain the methyl glucoside. The molar yield of the methyl glucoside is higher, which is 40 percent generally, and can reach 65 percent maximally.
Owner:XIAMEN UNIV
P-methoxyphenol hydrogenation catalyst, preparation method of p-methoxyphenol hydrogenation catalyst and hydrogenation reaction
ActiveCN105413681AHigh activityHigh reactivityPreparation by hydroxy compound hydrogenationOrganic compound preparationRheniumHydrogenation reaction
The invention relates to the field of catalysts, in particular to a p-methoxyphenol hydrogenation catalyst, a preparation method of the p-methoxyphenol hydrogenation catalyst and a hydrogenation reaction. The p-methoxyphenol hydrogenation catalyst comprises carriers and active components which adhere to the carriers, wherein the active components comprise components A and components B , the components A are precious metals, and the components B are selected from one or more of ferrum (Fe), cobalt (Co), nickel (Ni), silver (Ag) and rhenium (Re). The p-methoxyphenol hydrogenation catalyst is high in activity, excellent in selectivity and long in service life.
Owner:SHENYANG RES INST OF CHEM IND
Preparation method of cefotaxime sodium crystal
ActiveCN103275101APrevent gelGranular concentrationOrganic chemistrySodium acetateSodium acetrizoate
The invention relates to a preparation method of a cefotaxime sodium crystal. The preparation method comprises the following steps of: dissolving sodium acetate in a mixed solvent of organic solvent and water below 10-40 DEG C, wherein the volume fraction of organic solvent in the mixed solvent is 30-70%; adding cefotaxime acid, and stirring until the cefotaxime acid reacts in the solution; adding a cefotaxime sodium crystal in the solution, then adding elution agents with a feeding time of 2-8 hours, cooling to 5 DEG C below zero to 5 DEG C; and filtering, washing and drying a crystal slurry to obtain the cefotaxime sodium crystal. By the preparation method, the phenomenon of gelatinization frequently seen in the crystallization process of the cefotaxime sodium crystal is avoided, the size distribution of the products is centralized and the major particle size is adjustable from 5mu m to 60my m, the liquidity of the product is good, the process yield is higher than 87%, and the product purity is higher than 95.5%.
Owner:TIANJIN UNIV
Method for preparing bisphenolmonoacryates compounds antioxidant
InactiveCN101148408AHigh molar yieldImprove product qualityOrganic compound preparationCarboxylic acid esters preparationReaction temperatureSolvent
The present invention is technological process of preparing bisphenol monoacrylate compound as oxidant with bisphenol compound, acrylic acid and phosphorus oxychloride as materials, triethylamine as acid absorbent and aliphatic hydrocarbon as solvent, and through acyl chlorination and esterification in a reactor. The technological conditions include the molar ratio between bisphenol compound and acrylic acid of 1 to 1.05, the molar ratio between acrylic acid and phosphorus oxychloride of 3 to 1.05, the molar ratio between phosphorus oxychloride and triethylamine of 1 to 3.5; reaction temperature of 63-66 deg.c and reaction time of 150-155 min. After reaction, the resultant is filtered to eliminate solid triethylamine hydrochloride, the filtrate is cooled to re-crystallize to produce white crystal product of smelting point 117.5-18.6 deg.c. The present invention is simple, and has high bisphenol monoacrylate compound yield and low material consumption.
Owner:CHANGZHOU UNIV
Tilmicosin phosphate preparation method
ActiveCN105837648ASimple production processShorten the production cycleSugar derivativesSugar derivatives preparationOrganic solventPhosphorylation
The invention relates to a tilmicosin phosphate preparation method. The preparation method comprises that through hydrolysis under acidic conditions, alkalization extraction, amination, phosphorylation with phosphoric pentoxide and a small amount of water, full stirring salification, centrifugation separation after solid precipitation and vacuum drying, tilmicosin phosphate is prepared from tylosin tartrate as a raw material. According to the preparation method, phosphoric pentoxide and a small amount of water are directly added into an amination liquid, the mixture undergoes a phosphorylation reaction and tilmicosin phosphate is precipitated from the organic solvent. The preparation method has a final mole yield of 95% or more and a simple process route, can be operated easily, is free of spray drying production equipment, has a low production cost and realizes recycle of an organic solvent.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
Preparation method of ethylene glycol bis(propionitrile) ether
ActiveCN106146344AReduce alkalinityReduce generationCarboxylic acid nitrile preparationOrganic compound preparationAlkalinityEthylene glycol bis
The invention discloses a preparation method of ethylene glycol bis(propionitrile) ether. The preparation method includes: using ethylene glycol and acrylonitrile as reaction raw materials, and allowing the two to have reaction under the reaction temperature of 30-70 DEG C and the catalyzing of a catalyst aqueous solution to generate the ethylene glycol bis(propionitrile) ether, wherein the catalyst aqueous solution is a composite alkali catalyst aqueous solution mixed by a triethyl benzyl ammonium hydroxide aqueous solution and a sodium hydroxide aqueous solution. Due to the fact that the composite alkali catalyst aqueous solution low in alkalinity is used, the alkalinity in the reaction solution is weakened greatly, the generation of the byproduct bis-propionitrile ether can be reduced greatly, the mole yield of the ethylene glycol bis(propionitrile) ether can be increased greatly to reach above 90%, purification can be performed easily to obtain the high-purity ethylene glycol bis(propionitrile) ether, and the purity of the ethylene glycol bis(propionitrile) ether can reach above 95%.
Owner:ZHANGJIAGANG HICOMER CHEM CO LTD
Preparation method of cefdinir
InactiveCN103319503AGood for pH adjustmentDissolution Control and ConditioningOrganic chemistrySulfurNitrogen
The invention discloses a preparation method of cefdinir, which comprises following steps: (1) letting 7-amino-3-vinyl-8-oxo-5-sulfur heterocyclic-1-aza-bicycle [4.2.0] symplectic-2-alkene-2-carboxylic acid (7-AVCA) react with (Benzothiazol-2-yl)-(Z)-2-trityloxyimino-2-(2-aminothiazole-4-yl)-thioacetate with the existence of Tributylamine in a dichloromethane system to obtain a cefdinir ester solution, then conducting steaming to remove the dichloromethane in order to obtain a sepia and thick cefdinir ester solid; (2) purifying the cefdinir ester solid obtained in (1) to obtain the cefdinir. In the invention, a cefdinir synthetic product with high recovery rate and high purity can be produced in the method without the application of a counter solvent. The preparation process of the product has the advantages that reaction conditions are gentle, operation processes are simple and pollutions are fewer etc. Therefore, the method is applicable in industrial production.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
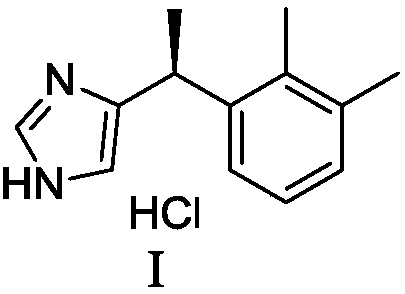
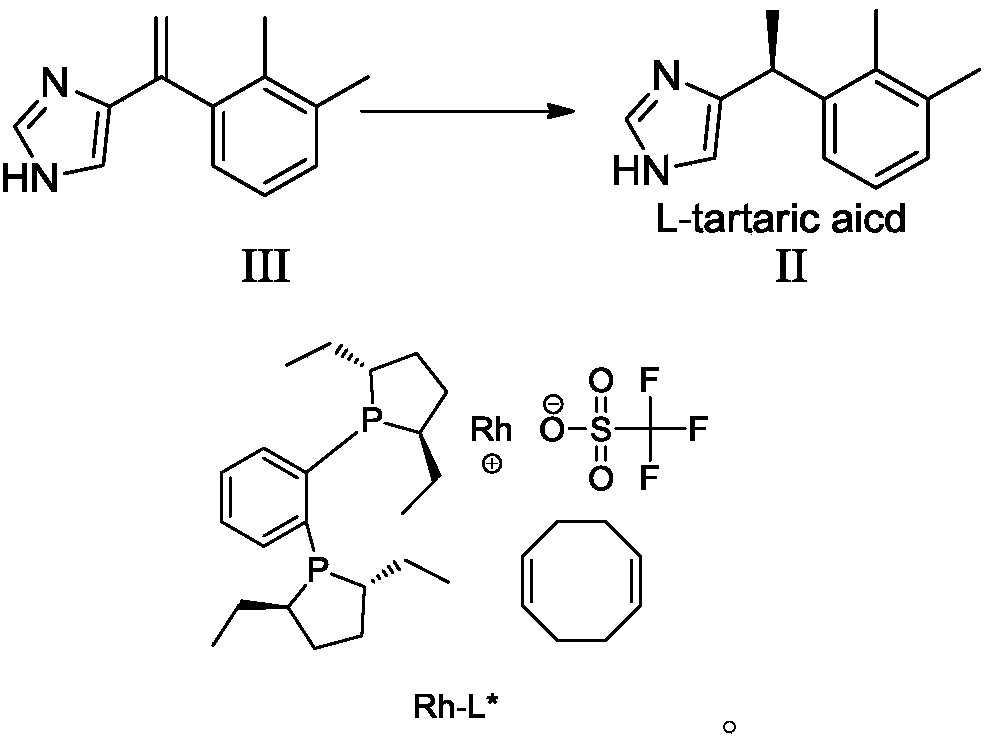
Preparation method of dexmedetomidine hydrochloride and its intermediate
ActiveCN108147999AShort route stepsHigh molar yieldSilicon organic compoundsPreparation by halogen halide additionBenzeneHydrogen
The invention discloses a preparation method of dexmedetomidine hydrochloride and its intermediate. A preparation method of dexmedetomidine L-tartrate comprises the steps of subjecting dexmedetomidineintermediate III and hydrogen to reduction reaction in an organic solvent in the presence of a chiral catalyst, and subjecting the reduced product and tartaric acid to neutralization reaction to obtain dexmedetomidine L-tartrate II, wherein the chiral catalyst is (+)-1,2-bis(2S-5S)-diethylphospholano-benzene(1,5-cyclooctadiene)rhodium trifluoromethanesulfonate. The preparation method herein has ashort step path, has no need for chiral splitting, and has high total molar yield; the product prepared herein has high purity, reaches the standard for bulk pharmaceutical chemicals and is suitablefor industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Silanization reaction catalyst
ActiveCN101623655AQuick responseReduce usageSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsTrimethylsilylErythromycin Oxime
The invention mainly discloses a silanization reaction catalyst. Etherate is obtained by erythromycin oxime through etherification, (2', 4'')-O-bis(trimethylsilyl)erythromycin A-9-O-(1-ethoxy-1-methylethyl) oxime of intermediate for synthesizing clarithromycin is prepared by the silanization reaction, the catalyst is added into the silanization reaction and is hydrobromide with a general formula being RHBr, wherein R is organic alkali, or the catalyst is strong acid and weak base salt with a general formula being XBr, wherein X is weak base cations. The consumption of the catalyst is 0.01-0.4 of the erythromycin oxime according to the mol, the temperature of the silanization catalysis reaction is 0-40 DEG C and the time of the silanization catalysis reaction is 1-10h. The invention enables the process for preparing the (2', 4'')-O-bis(trimethylsilyl)erythromycin A-9-O-(1-ethoxy-1-methylethyl) oxime to be stable, the yield to be high, the cost to be low and the three wastes to be less.
Owner:ZHEJIANG GUOBANG PHARMA
Method for preparing atorvastatin calcium intermediate
InactiveCN102766136AHigh molar yieldIncrease profitOrganic chemistryTert-ButylamineOrganic synthesis
The invention provides a method for preparing atorvastatin calcium intermediate and belongs to the technical field of drug organic synthesis. The method includes the following steps: 1) a compound A and a compound B are mixed according to weight ratio of 1:1.5-2.0; 2) tetrahydrofuran and n-butyl ether are added in the material obtained in the step 1) according to weight ratio of the compound A, the tetrahydrofuran and the n-butyl ether as 1:6-10:6-10 and stirred evenly; 3) trimethylacetic acid occupying 15-25% of the weight of the compound A is added in the material obtained in the step 2), and backflow reaction is performed at the temperature of 94-96 DEG C to obtain a compound C; 4) concentration of the compound C is detected in a reaction process, triethylamine or tert-butylamine is added equivalently in two batches, total addition of the triethylamine is 7-13% of the weight of the compound A, and total addition of the tert-butylamine is 12.8-24% of the weight of the compound A. The method for preparing the atorvastatin calcium intermediate can improve conversion rate of products and reduce pollutant generated in reaction remarkably.
Owner:ZHEJIANG HONGYUAN PHARMA
Method for preparing methyl heptenone by using 3-methylcrotonaldehyde
ActiveCN105218339AHigh molar yieldHigh selectivityOrganic compound preparationCarbonyl compound preparationPtru catalystHydrogenation reaction
The invention provides a method for preparing methyl heptenone by using 3-methylcrotonaldehyde, which comprises the steps of condensation and selective reduction, wherein the step of condensation comprises the sub-steps of adding acetone and a catalyst, and dropwise adding 3-methylcrotonaldehyde; and the step of selective reduction comprises the sub-steps of adding materials and carrying out hydrogenation reaction; and the sub-step of adding materials, namely adding 1 part of methyl diheptenone, 0-10 parts of a solvent, and 0.001-0.05 part of transition metal Pd / Rh catalyst precursor compounds and diphosphine ligand compounds into a reaction kettle. According to the invention, through two-step reaction, the mole yield of the methyl diheptenone to 3-methylcrotonaldehyde is high, and the methyl heptenone product content reaches 98.0-98.7%; in the step of selective reduction, the conversion rate of methyl diheptenone is greater than or equal to 99.0%, and the selectivity reaches 90.8-94%; and in the step of condensation, the conversion rate of 3-methylcrotonaldehyde is 99.0%, and the mole yield of the methyl diheptenone to 3-methylcrotonaldehyde reaches 89.8-92.7%.
Owner:SHANDONG NHU PHARMA +1
Method for the preparation of halogenated benzonitriles
InactiveUS20050176984A1Improve responseHigh molar yieldOrganic compound preparationOrganic chemistry methodsPtru catalystPhysical chemistry
The present invention relates to a method for preparing halogenated benzonitriles by vapor phase ammoxidation at a reaction temperature in the range of 300 to 500° C. in a fixed bed reactor using a three-component catalyst. More particularly, the method of the invention relates to a method for preparing 2,6-dichlorobenzonitrile (2,6-DCBN) from 2,6-dichlorotoluene (2,6-DCT) by vapor phase ammoxidation. The invention also relates to a three-component catalyst provided on a carrier and its use in a vapor phase ammoxidation reaction according to the invention. In addition, the invention provides a method for preparing the three-component catalyst.
Owner:TESSENDERLO CHEM
Cefuroxime sodium freeze dry power and preparation method thereof
InactiveCN101491502ASimple processReduce labor intensityAntibacterial agentsPowder deliveryActivated carbonPenicillin
The invention discloses a Cefuroxime sodium lyophilized powder and a method for preparing the same. The method is characterized in that a Cefuroxime sodium solution is added with active carbon, decolored, subjected to decarbonization and aseptic filtration and washed by purified water; a mixture of a filtrate and an eluant is pressed into a feed tray or a penicillin bottle of a freezedryer; a product with the moisture of Cefuroxime sodium of less than 2 percent is prepared by a lyophilization method; and the method simplifies a process, reduces labor intensity, aseptic risk, production cost and pollution and has rapid dissolution speed, good stability and high yield.
Owner:邢建荣
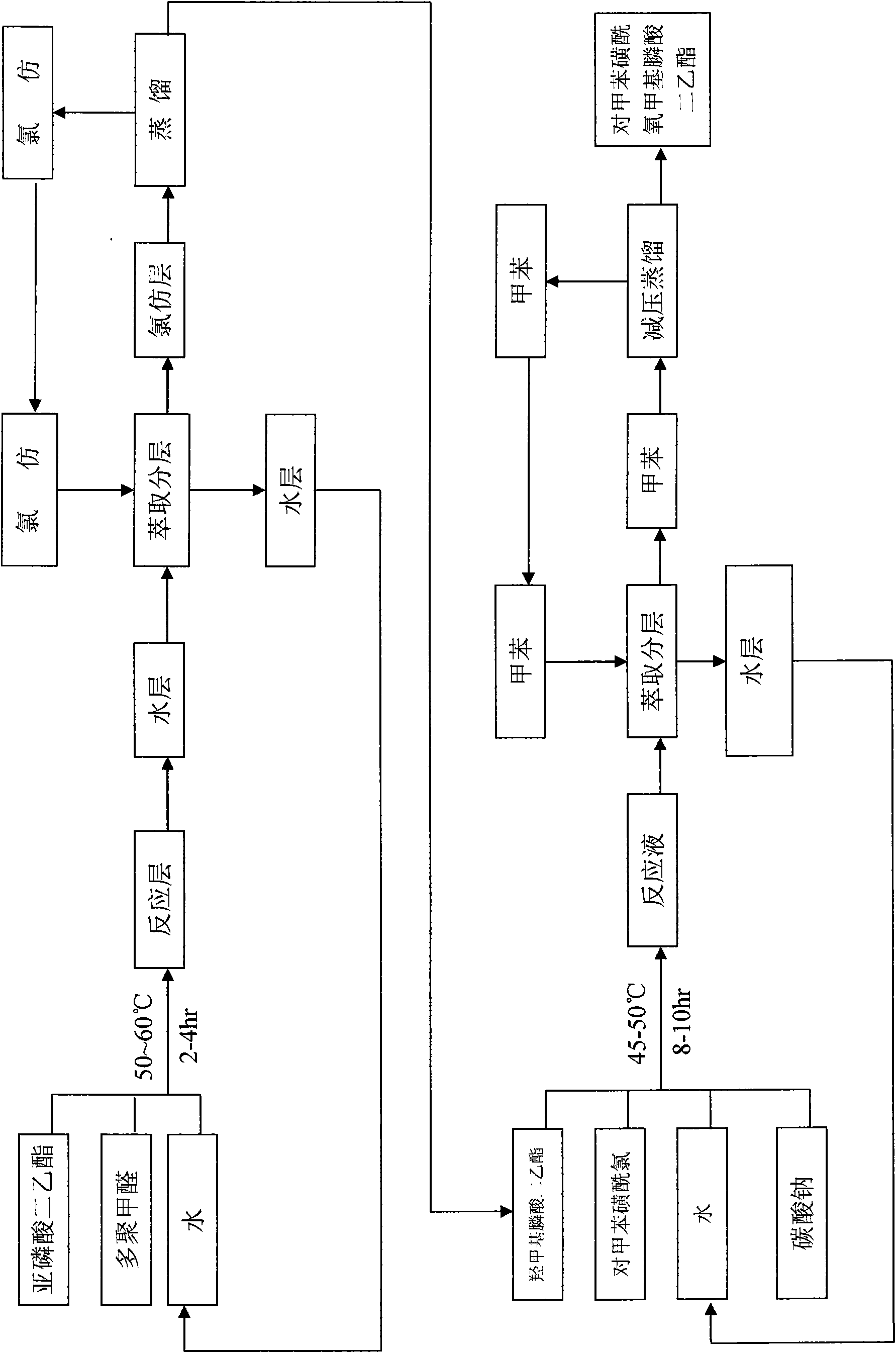
New production technology of diethyl (tosyloxy)methylphosphonate
InactiveCN101565433ALow costWeaken corrosionGroup 5/15 element organic compoundsChemistryParaformaldehyde
The invention discloses a production technology of nucleoside AIDS virus resistant drug key intermediate-diethyl (tosyloxy)methylphosphonate. The diethyl (tosyloxy)methylphosphonate is mainly prepared by taking diethyl phosphite and paraformaldehyde as raw materials to react for 2-4 hours under the temperature ranging from 50 DEG C to 60 DEG C to prepare the diethyl(hydroxymethyl)phosphonate, then taking the diethyl(hydroxymethyl)phosphonate as a raw material, adding paratoluensulfonyl chloride into the diethyl(hydroxymethyl)phosphonate, taking an acid-binding agent as a catalyst to react for 8-10 hours under the temperature ranging from 45 DEG C to 55 DEG C, and then adding an organic solution to the reacted materials to extract, separate and distill. The production technology has short reaction time, high production yield coefficient and little environment pollution, and the obtained product has high purity.
Owner:吴小峰
Synthesis method of novel anticoagulation drug
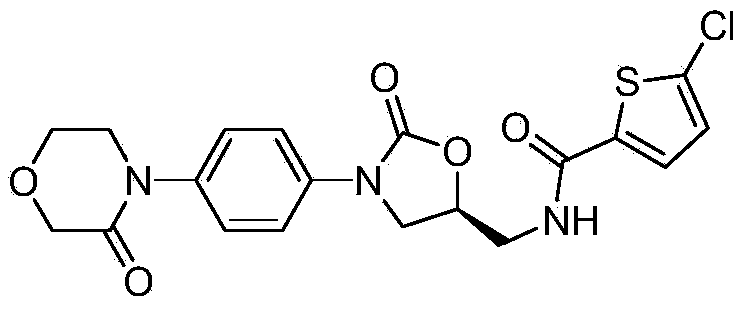
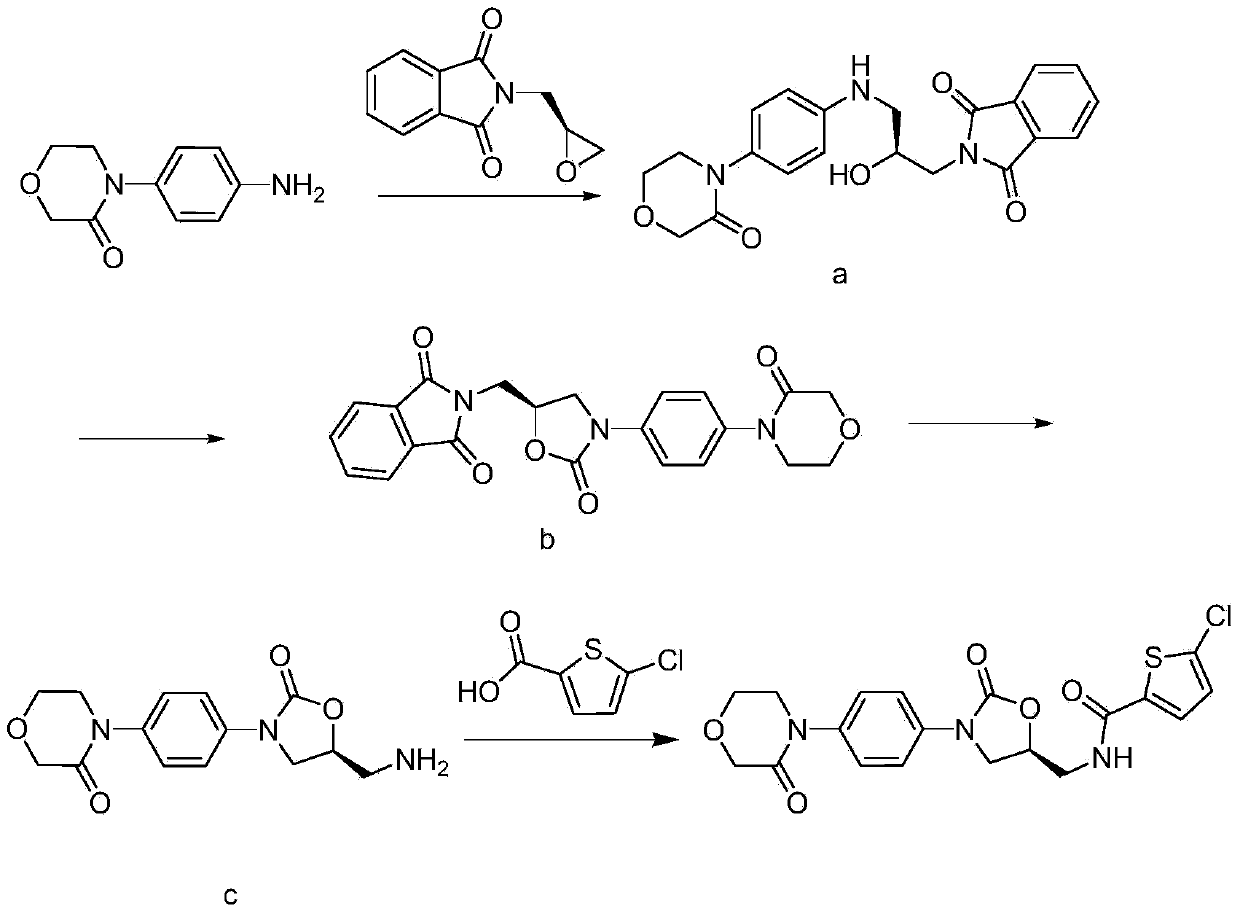
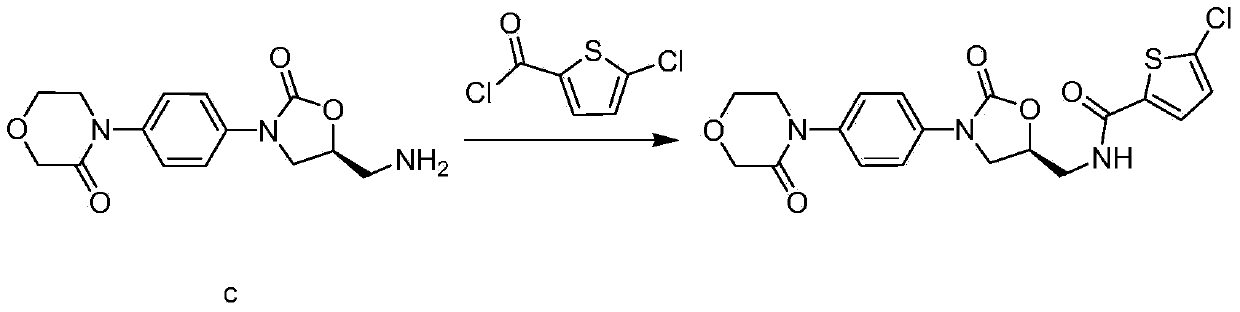
The invention relates to a synthesis method of a novel anticoagulation drug, which comprises the step that 4-[4-[(5S)-5-(aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-2-molindone or salt thereof reacts with 5-penphene-2-carboxylic acid in the presence of a condensing agent N', N-carbonyldiiazole (CDI) or N, N'-dicyclohexyl carbodiimide (DCC).
Owner:YOUCARE PHARMA GROUP
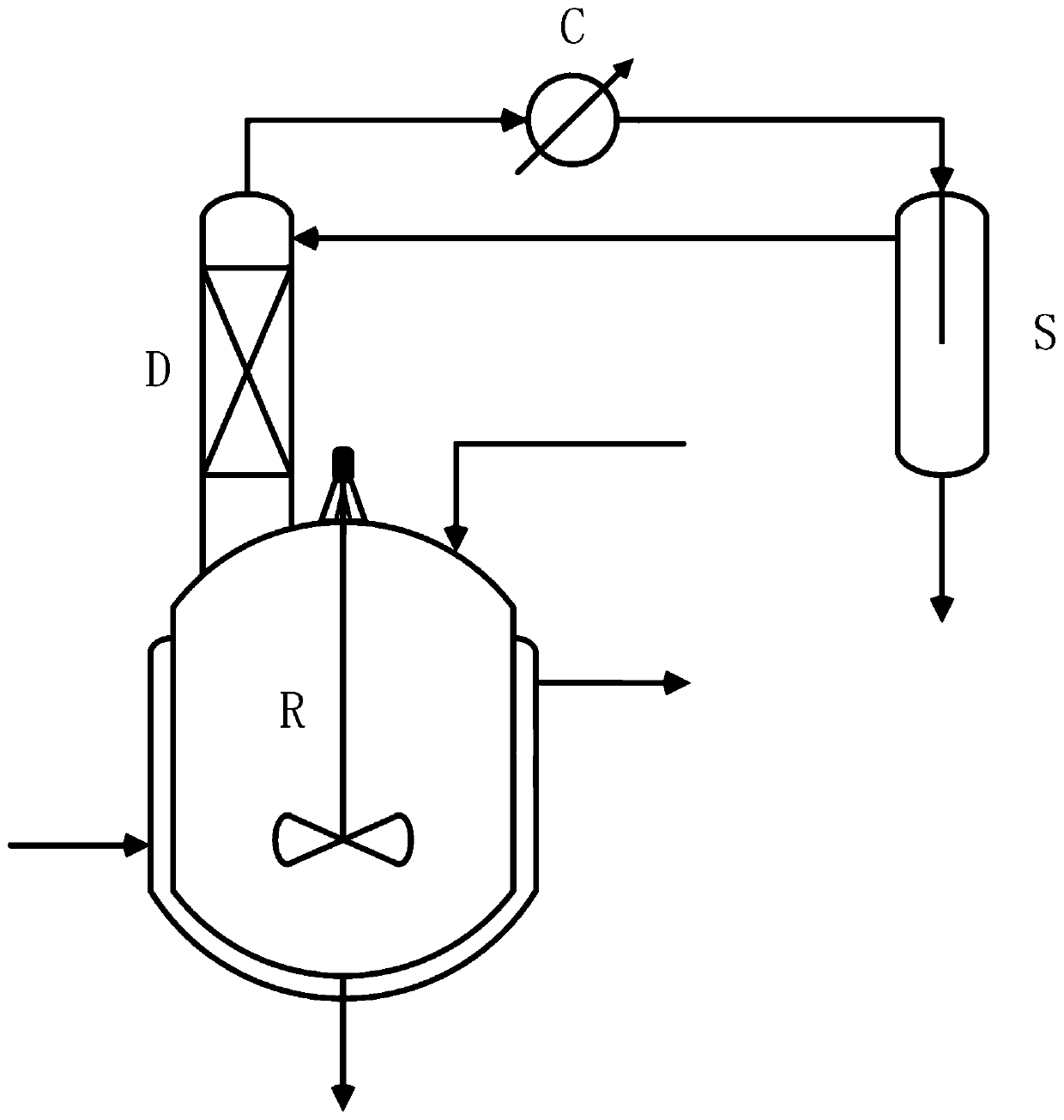
Device and method for preparing 2, 5-furandicarboxylic acid from hexose diacid (hexose diacid salt) by coupling dehydration cyclization reaction and azeotropic distillation dehydration
The invention discloses a device and a method for preparing 2, 5-furandicarboxylic acid from hexose diacid (hexose diacid salt) by coupling dehydration cyclization reaction and azeotropic distillationdehydration. The device integrates dehydration cyclization reaction and azeotropic distillation dehydration. The method comprises the following steps of 1) opening a reaction kettle for stirring anda reaction kettle jacket for heating steam, sequentially adding a reaction solvent, hexose diacid (salt), a catalyst and an entrainer into a dehydration cyclization reaction kettle, enabling water generated by reaction and the entrainer to form azeotrope, enabling the azeotrope to slip out of the tower top and reacting for 15-48h at the reaction temperature of 100-130 DEG C, and 2) after the reaction is finished, recovering the entrainer from a phase splitter, then cooling, neutralizing with alkali, recovering the reaction solvent by reduced pressure distillation, and then crystallizing and recrystallizing to obtain the 2, 5-furandicarboxylic acid product. By coupling azeotropic distillation, water generated in the reaction is removed in time, so that side reactions are reduced, and the yield of the product 2, 5-furandicarboxylic acid is greatly increased.
Owner:ZHEJIANG HENGYI PETROCHEMICAL RES INST CO LTD +1
Method for preparing cefpiramide acid
InactiveCN103059048AHigh purityImprove responseOrganic chemistryTrimethylsilyl chlorideCarboxylic acid
The invention provides a method for preparing cefpiramide acid. The method provided by the invention adopts (6R,R7)-3-[(1-methyl-1H-tetrazolyl-5-yl)thiomethyl]-7-amino-8-oxo-5-thia-1-azabicyclo-[4.2.0]octyl-2-alkenyl-2-carboxylic acid as a trimethylchlorosilicyl protective agent. The method provided by the invention is simple to operate, obviously enhances the product purity and yield, and can easily implement industrial production.
Owner:珠海保税区丽珠合成制药有限公司 +1
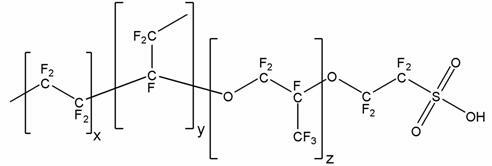
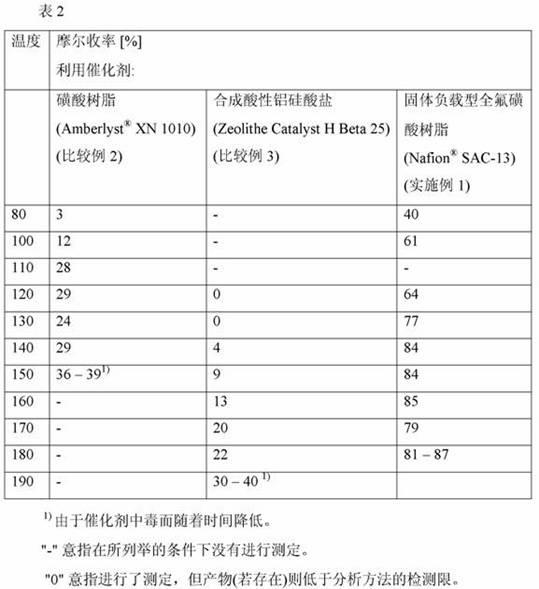
Process of producing 1,1 diaryl alkanes and derivatives thereof
InactiveCN102126916AHigh molar yieldReduce foulingCatalystsHydrocarbon preparation catalystsAlkaneAryl
The invention relates to a process of producing 1,1 diaryl alkanes and derivatives thereof. A process of producing a 1,1-diaryl alkane comprises a condensation reaction of an aromatic compound having at least one aromatic hydrogen with an acetal, in the presence of a perfluorinated sulfonic acid in polymeric form as catalyst.
Owner:EVONIK DEGUSSA GMBH
Method for preparing 17 beta-HSD1 inhibitor
The invention relates to a method for preparing a 17 beta-HSD1 inhibitor, belonging to the technical field of pharmaceutical synthesis. In order to solve the technical problem of low yield in the prior art, the invention provides a method for preparing a 17 beta-HSD1 inhibitor. The method comprises the following steps: A. under the action of an inorganic alkali, reacting bromopyridine disclosed as Formula II with 5-chloro-2-thienylboronic acid pinacol cyclic ester in the presence of a palladium-containing catalyst and a phosphorous compound to obtain an intermediate compound; and under the action of an inorganic weak alkali, in the presence of the palladium-containing catalyst and phosphorous compound, carrying out Suzuki reaction on the intermediate compound and meta-hydroxyphenylboric acid in a nitrile-alcohol mixed solvent to obtain the 17 beta-HSD1 inhibitor. The method provided by the invention has the advantages of high yield, high purity of the final product, and short process route, and can easily implement industrial production.
Owner:LINHAI LIANSHENG CHEM
Process for producing 3,4-enedioxy thiophene
The invention provides a method for preparing 3, 4-ethylenedioxythiophene (EDOT for short). Firstly, thiodiglycolic acid and methanol generate dimethyl 2, 2'-thiobisacetate through an esterification reaction under the catalysis of an acid, and then the dimethyl 2, 2'-thiobisacetate is condensed with diethy-aceto oxalate under the catalysis of sodium methoxide to obtain 2, 5-dimethyl carboxylate-3, 4-dihydroxy-thiophene; then the 2, 5-dimethyl carboxylate-3, 4-dihydroxy-thiophene and a cyclic esterification reagent are cyclic-esterified to generate 2, 5-dimethyl carboxylate-3, 4-ethylenedioxythiophene under the cocatalysis of copper powder or cuprous oxide and potassium carbonate, and 2, 5-dicarboxylic acid-3, 4-ethylenedioxythiophene is obtained through alkaline saponification and acid regulation; and finally a copper catalyst remained in the cyclic esterification reaction is used for catalytic decarboxylation in specific pyridine solvents, and the high-purity EDOT is obtained throughreduced pressure distillation. The method has higher yield in the preparation of the EDOT, has low impurity content, and meets the requirement of electronic chemicals.
Owner:APELOA PHARM CO LTD +1
Preparation method and application of 2, 4-diamino-6-hydroxy-5-formamidopyrimidine
The invention discloses a preparation method of 2, 4-diamino-6-hydroxy-5-carboxamido pyrimidine. The preparation method comprises the following step: carrying out an acylation reaction on 2, 4-diamino-5-nitroso-6-hydroxypyrimidine in formamide and water under the catalytic action of a catalyst A to obtain the 2, 4-diamino-6-hydroxy-5-carboxamido pyrimidine. The invention also discloses a preparation method of guanine formate or guanine. The preparation method of guanine formate or guanine comprises the following step: reacting the 2, 4-diamino-6-hydroxy-5-formamidopyrimidine in formic acid toobtain guanine. According to the synthesis methods of the 2, 4-diamino-6-hydroxy-5-formamidopyrimidine and guanine, the production process is greatly shortened, the generation amount of three wastes is greatly reduced, the product quality of the guanine product meets related quality requirements, and the molar yield is higher than that of the guanine product prepared by the prior art. Therefore, the preparation methods disclosed by the invention are efficient, economic, green and environment-friendly preparation methods.
Owner:潍坊奥通药业有限公司
Method for preparing gamma-butyrobetaine ester
InactiveCN101538215AReduce manufacturing costHigh molar yieldOrganic compound preparationAmino-carboxyl compound preparationPropanolAlcohol
The invention discloses a method for preparing gamma-butyrobetaine ester, which uses gamma-chloro butyric ester and trimethylamine as raw materials and alcohol as a solvent. The raw materials and the solvent are heated to react in a high-pressure autoclave, and products of the gamma-butyrobetaine ester are obtained by concentrating processing and fine purification. The gamma-butyrobetaine ester is methyl ester or ethyl ester. The feeding mol ratio of the gamma- chloro butyric ester to the trimethylamine is 1:1.2-3.0, wherein the degree of purity of the gamma-chloro butyric ester is not less than 97 percent, and the degree of purity of the trimethylamine is more than 98 percent. The alcohol as the solvent is one of methanol, absolute ethyl alcohol, propanol, or isopropanol, and the use level of the alcohol is 1-5 times higher than that of the gamma-chloro butyric ester. The reaction is carried out at the temperature of 50-120 DEG C and the high pressure for 5-24h in an airtight mode. Because the technical scheme is adopted, the preparing cost is decreased, the domestic blank for preparing the gamma-butyrobetaine ester is replenished, and the mol yield of the products is enhanced and can reach more than 90 percent.
Owner:潍坊祥维斯化学品有限公司
Method for preparing aromatic hydrocarbon by hydrocracking aromatic ring-containing polymer
PendingCN111892477AHigh molar yieldHigh selectivityMolecular sieve catalystsMolecular sieve catalystMolecular sievePolymer science
The invention provides a method for preparing aromatic hydrocarbon by hydrocracking an aromatic ring-containing polymer. The method comprises the following steps: reacting crushed polymer with hydrogen at the temperature of 350 DEG C or below under the action of a catalyst; separating reaction products to obtain aromatic hydrocarbons. The catalyst comprises a carrier and an active component loadedon the carrier, wherein the active component is selected from at least one of Ru, Rh, Pt, Pd, Fe, Ni, Cu and Co; wherein the carrier is selected from at least one of a metal oxide, a phosphate, a molecular sieve, SiO2 and sulfonated carbon, and the metal oxide is selected from at least one of Al2O3, Nb2O5, Nb2O5-Al2O3, Nb2O5-SiO2, TiO2, ZrO2, CeO2 and MoO3; the phosphate is selected from at leastone of NbOPO4 and ZrOPO4; wherein the molecular sieve is selected from at least one of Nb-SBA-15, Nafion, H-ZSM-5, H-Beta and H-Y in a molecular screening manner.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation process for cyanomethyl ester
The invention relates to the chemical field and in particular to a preparation process for cyanomethyl ester. The cyanomethyl ester is prepared with a high yield by taking acetonitrile, methanol, anhydrous hydrogen chloride and a 28% cyanamide aqueous solution as main reaction raw materials and taking toluene as a solvent. The preparation process has the advantage that according to the preparation method for the cyanomethyl ester, the molar yield of the cyanomethyl ester is increased to 85%; content of product cyanomethyl ester is greater than or equal to 99.5%; and the reaction process is gentle and safe.
Owner:NANTONG TENDENCI CHEM
Amycolatopsis sp. and application thereof
ActiveCN113637607AHigh molar yieldHigh vanillinBacteriaMicroorganism based processesBiotechnologyVanillic acid
The invention relates to amycolatopsis sp. and application thereof. The amycolatopsis sp. HM-141 (the preservation number is CGMCC (China General Microbiological Culture Collection Center) No.22871) disclosed by the invention is applied to production of vanillin by taking ferulic acid as a substrate. Experiments confirm that the amycolatopsis sp. HM-141 uses ferulic acid as a substrate to produce vanillin, the molar conversion rate can reach up to 87%, impurities (or byproducts) in the product are obviously lower than that in the prior art, the detection amount of vanillin alcohol is 0, the detection amount of vanillic acid is as low as 0.25 g / L, and the amycolatopsis sp. HM-141 has obvious advantages.
Owner:SHAANXI HEALTHFUL BIOLOGICAL ENG
Method for preparing propionate by means of microreactor
InactiveCN109020811AImprove uniformityGreatly increase the specific surface areaOrganic compound preparationCarboxylic acid esters preparationPropanoic acidReaction temperature
The invention discloses a method for preparing propionate by means of a microreactor. Production equipment comprises a first metering pump, a second metering pump and a third metering pump, wherein the first metering pump and the second metering pump are communicated with a first blender, the third metering pump is communicated with a second blender, the blenders are communicated with the microreactor, a material output pipe with a sampling stop valve is arranged at a discharging end of the microreactor, the material output pipe is connected with a filter, a drainage pipe and a discharging pipe are connected with an output end of the filter in parallel, the discharging pipe is connected with a rectifying tower, and an output pipe of the rectifying tower is connected with a dehydration tank; a preparation process comprises the steps that propionic acid and monohydric alcohol are pumped by the first metering pump and the second metering pump respectively, an acidic ion liquid catalyst ispumped by the third metering pump, a reaction temperature is controlled to be 50 to 100 DEG C, and reaction time is controlled to be 5 to 60 min. The method for preparing the propionate by means of the microreactor has the advantages that the material diffusion speed can be further improved, the evenness of material mixing is further improved, the reaction time and a production cycle are greatlyshortened, energy consumption is low, and the conversion rate is high.
Owner:ZHANGJIAGANG HICOMER CHEM CO LTD
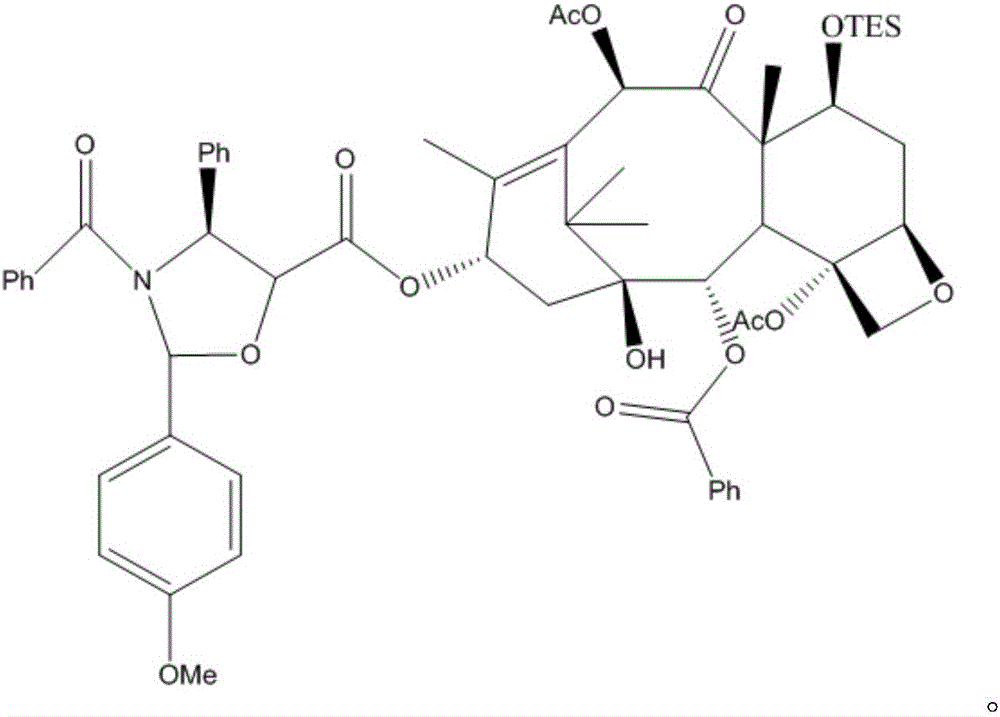
Methods for preparing semi-synthetic paclitaxel and intermediate thereof
The invention relates to methods for preparing semi-synthetic paclitaxel and an intermediate thereof. The method for preparing the semi-synthetic paclitaxel comprises the following steps: dissolving 10-DABIII (10-deacetylbaccatinIII) into pyridine, protecting hydroxyl on position-7 carbon on the 10-DABIII with triethyl silicyl to obtain an intermediate I, acetylating hydroxyl on position-10 carbon of the intermediate I to obtain an intermediate II, reacting the intermediate II, a side-chain radical compound and 4-dimethylaminopyridine in an organic solvent to prepare an intermediate III, and reacting the intermediate III with trifluoroacetic acid under an acidic condition to obtain a crude paclitaxel product. The preparation methods are simple and easy for industrialization; the active hydroxyl of the raw material 10-DABIII is effectively protected, so that fewer byproducts are finally generated, and a prepared paclitaxel product has high molar yield of 70 to 81 percent and high paclitaxel purity of 99.5 to 99.9 percent; the residual rate of the raw material in the steps of synthesizing the intermediate II and synthesizing the paclitaxel is low, side reactions are reduced, and the raw material is high in reaction selectivity and utilization rate; the product can be directly used as a raw material for the medical field of treatment of ovarian cancer, breast cancer and the like.
Owner:CHONGQING BEISHENG PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com