Preparation method of cefdinir
A technology for cefdinir and cephalosporin, which is applied in the field of preparation of cefdinir, can solve the problems of affecting the purity of the final compound, difficult to realize large-scale production, complicated operation steps and the like, and achieves the effects of high recovery, low pollution and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
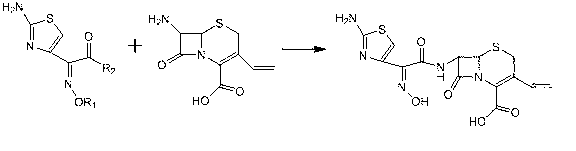
[0027]A preparation method of cefdinir, comprising the following steps: (1) 7-amino-3-vinyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene -2-carboxylic acid (7-AVCA) in dichloromethane system, in the presence of tri-n-butylamine, with (Z)-2-(2-aminothiazol-4-yl)-2-trityloxyimino Thioacetic acid (S-2-benzothiazole) ester reacts to obtain cefdinirate liquid, dichloromethane is evaporated to obtain tan viscous cefdinirate solid; (2) cefdinirate obtained in (1) The ester solid was purified to obtain cefdinir. The specific operation is to add 7-AVCA, (Z)-2-(2-aminothiazol-4-yl)-2-trityloxyiminothioacetic acid (S-2-benzothiazole) into the reaction flask Esters, dichloromethane and tri-n-butylamine, start stirring reaction. In the preparation method of this cefdinir, the mol ratio of tri-n-butylamine and 7-AVCA is in the range of 2:1~3:1; the volume ratio range of dichloromethane and 7-AVCA is in the range of 15:1~20:1 In (1), the range of the reaction temperature in (1) is 20-30°C as...
Embodiment 2
[0029] As a further optimization, on the basis of Example 1, in this example, the purification in (2) is specifically divided into two steps: (21) Use the cefdiniroid solid prepared in (1) to Dissolve the acetonitrile, add phosphoric acid, after the solid is precipitated, filter and vacuum dry to obtain cefdinir phosphate; (22) dissolve the cefdinir phosphate obtained in (21) in a weak base, add activated carbon for decolorization and filtration, Cefdinir was obtained by acid gradient crystallization. The volume ratio of acetonitrile in (21) to 7-AVCA in (1) described in this implementation is 13:1~18:1; the molar ratio of phosphoric acid to 7-AVCA in (1) is 8:1~ 12:1, (21) The temperature of the vacuum oven in (21) is 20-40°C and the pressure is below -0.08Mpa to carry out the purification process. The selectivity of the purification process is strong, various impurities can be removed more effectively, and the molar yield of the synthetic compound of cefdinir is guaranteed ...
Embodiment 3
[0031] On the basis of Example 2, the reaction parameters were further optimized. In this example, 8.5g 7-AVCA, 22.92g (Z)-2-(2-aminothiazol-4-yl)- 2-trityloxyiminothioacetic acid (S-2-benzothiazole) ester, this ester is referred to as active ester, 183g methylene chloride, 18.16g tri-n-butylamine, start stirring. The temperature was 26°C, the temperature was kept and stirred for 5 hours, and the sample was taken to detect that 7-AVCA was less than 1%, and the reaction was stopped. At a pressure of -0.085Mp and a temperature of 24°C, dichloromethane was distilled off to obtain a tan viscous cefdiniroid solid.
[0032] Dissolve the cefdiniril solid with 127.8ml of acetonitrile, add 39.2g of phosphoric acid, stir until a large amount of light yellow solid precipitates, filter with suction, rinse the filter cake twice with a small amount of acetonitrile, the filter cake is at a pressure of -0.08Mpa and a temperature of 28°C After drying, 23g of yellow-white solid was obtained, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com