Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35results about How to "Short route steps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
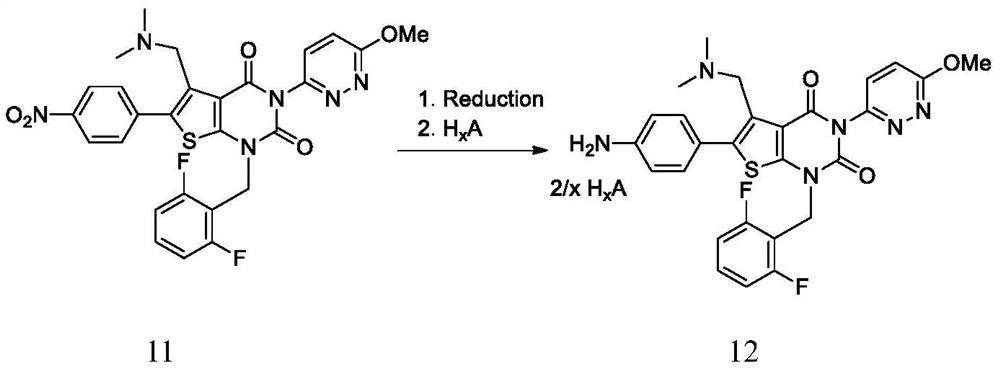
Elagolix synthesis method
The invention provides an elagolix synthesis method. The elagolix synthesis method comprises enabling a compound 5 and a compound 10 to participate in a condensation reaction to finish N-alkylation reaction to obtain a compound 11, and then implementing alkaline hydrolysis to obtain elagolix 12. The invention further discloses two synthesis methods of the compound 5: the method I comprises enabling a 5-bromine-6-methylpyrimidine-2,4(1H,3H)-diketone compound 1 and a 2-(brooethyl)-1-fluorin-3-(trifluoromethyl) benzene compound 2 to have a condensation reaction to obtain an intermediate 3, and then having a coupling reaction; the method II comprises enabling 1-halide-3-fluorin-2-anisole and acetoacetate 7 to have a coupling reaction to obtain a compound 8, and then having a condensation cyclization reaction with a compound 9; the improvements greatly shorten the route steps, the route efficiency is improved, the use of a noble metal catalyst is avoided, and the process cost is greatly lowered. The operation of the route is simple, the total yield is high, the purity of an obtained product is also relatively high, and the method is suitable for the enlarged production.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
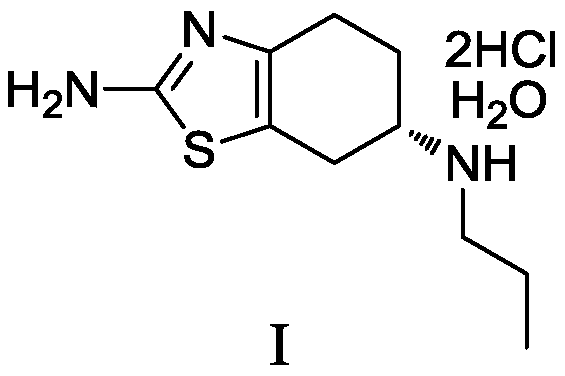
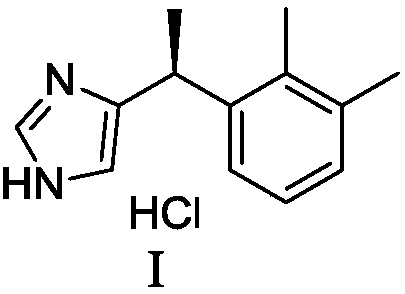
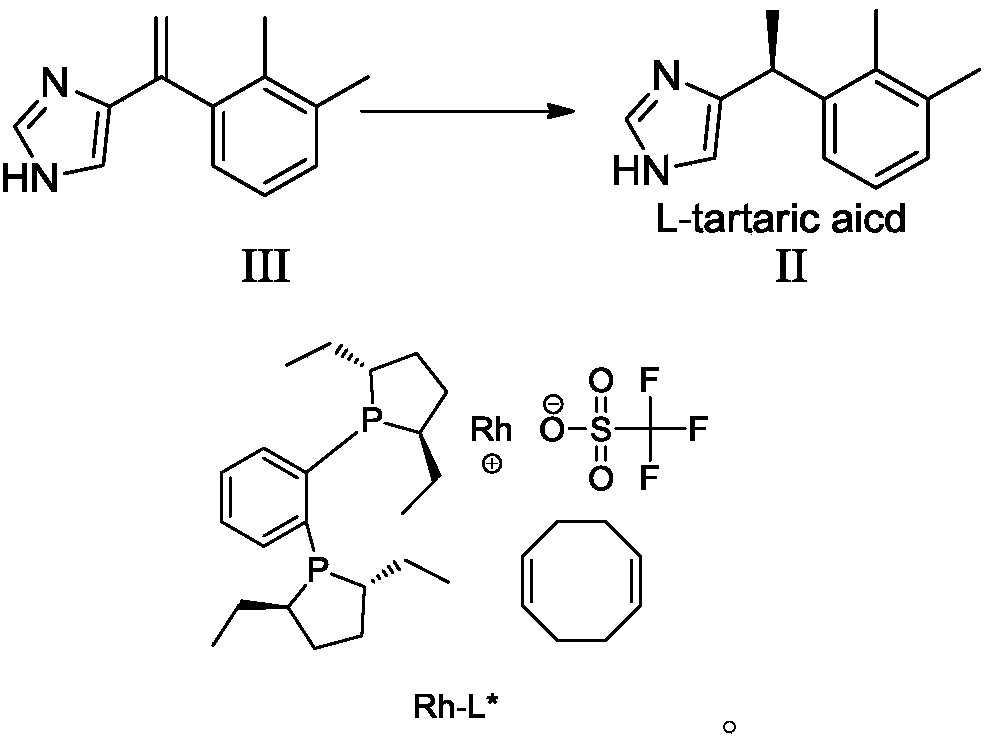
Preparation method of dexmedetomidine hydrochloride and its intermediate
ActiveCN108147999AShort route stepsHigh molar yieldSilicon organic compoundsPreparation by halogen halide additionBenzeneHydrogen
The invention discloses a preparation method of dexmedetomidine hydrochloride and its intermediate. A preparation method of dexmedetomidine L-tartrate comprises the steps of subjecting dexmedetomidineintermediate III and hydrogen to reduction reaction in an organic solvent in the presence of a chiral catalyst, and subjecting the reduced product and tartaric acid to neutralization reaction to obtain dexmedetomidine L-tartrate II, wherein the chiral catalyst is (+)-1,2-bis(2S-5S)-diethylphospholano-benzene(1,5-cyclooctadiene)rhodium trifluoromethanesulfonate. The preparation method herein has ashort step path, has no need for chiral splitting, and has high total molar yield; the product prepared herein has high purity, reaches the standard for bulk pharmaceutical chemicals and is suitablefor industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
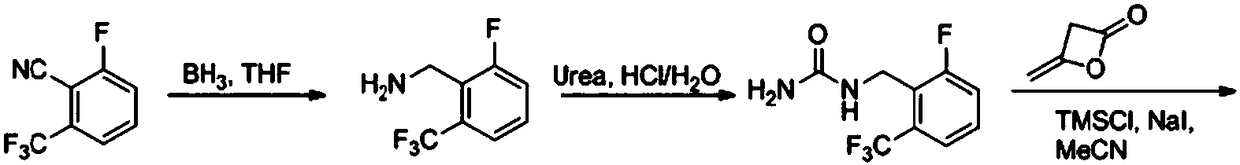
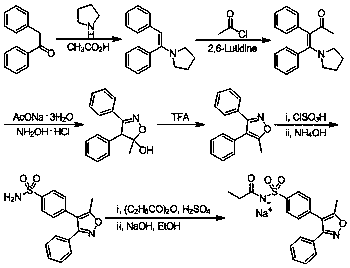
Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof
ActiveCN101928277ASimple processLow costOrganic chemistryOrganic compound preparationBenzoic acidMethyl palmoxirate
The invention provides a preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid. The preparation method comprises the following steps of: carrying out a guanidine-forming reaction on 3-amino-4-methyl toluic acid and cyanamide under the acidic condition of hydrochloric acid to generate 3-[(aminoiminomethyl)amino]-4-methyl-benzoic acid-hydrochloride, and then carrying out a cyclization reaction on the 3-amino-4-methyl toluic acid and cyanamide and 3-(dimethylamino)-1-(3-pyridyl)-2-propylene-1-one to generate the 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, wherein a structural formula of the 3-[(aminoiminomethyl)amino]-4-methyl-benzoic acid-hydrochloride is shown as the description. The method has short route, simple operation, safe and environmentally-friendly process, repeatability, low cost, high yield, high stability and safety of a guanidine hydrochloride intermediate, suitability for large-scale industrial production and higher economic benefit and social benefit.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Preparation method of 2,6-pyridinedimethanol
The invention relates to a preparation method of a compound 2,6-pyridinedimethanol. The preparation method comprises the steps: a raw material 2,6-dimethylpyridine is oxidized by using potassium permanganate, 2,6-pyridinedicarboxylic acid is generated and is reduced by a sodium borohydride / iodine system, and the 2,6-pyridinedimethanol with higher yield is obtained. Compared with the prior art, the method has the advantages that the steps are simplified, the reaction condition is mild, the operation is safe, simple and convenient, the products are easy to separate, the yield is higher and the like.
Owner:TIANJIN POLYTECHNIC UNIV
Synthetic method of bempedoic acid and intermediate thereof
InactiveCN112110828AHigh puritySimple structurePreparation from carboxylic acid saltsCarboxylic acid nitrile preparationBempedoic acidMorpholine
The invention discloses new intermediate compounds 6 and 7 of bempedoic acid, and also discloses a synthetic method of bempedoic acid. The method shortens the route steps, improves the yield, lowers the process cost, improves the structure of the bempedoic acid intermediate, enhances the crystallinity of the intermediate compounds, and is beneficial to enhancing the purity of the final product, and suitable for enlarged production. The structural formulas of the compound 6 and the compound 7 are shown in the specification, wherein -N(R2)2 represents N,N-dimethylamino, cyclohexylamino, morpholinyl or amino; and M is an inorganic base or organic alkali, and comprises sodium, potassium, calcium, cyclohexanediamine or morpholine.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Synthesis method of relugolix or salt thereof
The invention provides a synthesis method of relugolix or a salt thereof, wherein thesynthesis method comprises the steps: taking 1-(dimethylamino)-3-(4-nitrophenyl)-2-acetone and cyanoacetate as initial raw materials, and carrying out condensation cyclization, alkylation reaction, Ullmann reaction and coupling reaction to obtain a relugolix 4 free alkali form or salt form. The synthetic route optimized the process, the route steps are shortened, the route efficiency is improved, the use of noble metal catalysts can be reduced, and the process cost is greatly reduced. The route is simple to operate, the total yield is high, the purity of the obtained product is high, and the method is suitable for large-scale production. The forms of the three salts of relugolix are also found, the crystallinity is good, the purification is easy, and the product purity is favorably improved.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
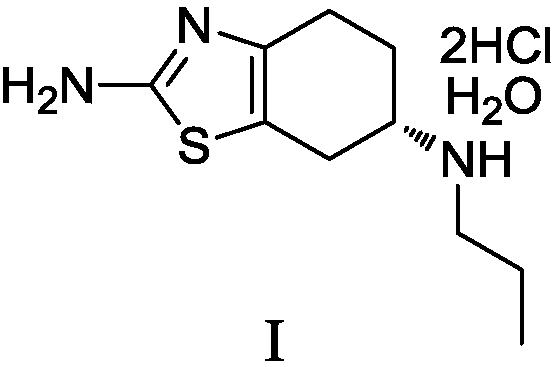
Preparation method of pramipexole dihydrochloride and intermediate thereof
The invention discloses a preparation method of pramipexole dihydrochloride and an intermediate thereof. The invention provides a preparation of pramipexole II. The preparation method of the pramipexole II comprises the following steps: performing condensation reaction and reduction reaction on a pramipexole intermediate III, propylamine and hydrogen in an organic solvent and under the existence of a chiral catalyst, and performing one-pot method to obtain the pramipexole II. According to the preparation method provided by the invention, the route step is short, chiral resolution is not neededand the total molar yield is high; furthermore, the prepared product has high purity, can reach to the standard of raw material medicines and is suitable for industrialized production. (The formula is shown in the description).
Owner:SHANGHAI BOCIMED PHARMA CO LTD
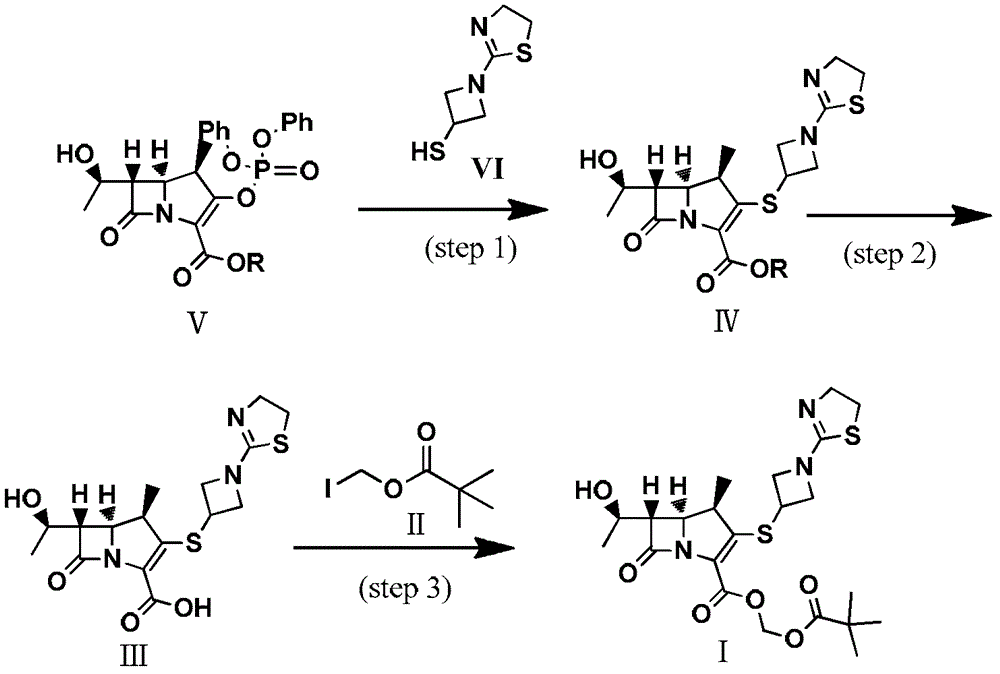
Preparation method of tebipenem ester
InactiveCN103059026ASimple and fast operationMild reaction conditionsOrganic chemistryBulk chemical productionHydrogenPalladium catalyst
The invention discloses a preparation method of tebipenem ester. The method comprises the following steps of: 1), carrying out reaction on the compound of the formula (IV) under acidic or alkaline condition, removing protective groups, so as to obtain tebipenem acid, wherein the pH (potential of hydrogen) value of the acidic condition is 2-6 and the pH value of the alkaline condition is 9-13; and 2), reacting the tebipenem acid with iodomethyl pivalate, so as to obtain the tebipenem ester. The method disclosed by the invention is easy to operate and mild in reaction condition, the noble metal palladium catalyst is not required, and thus the method is more beneficial for industrial production and the cost is greatly reduced. Formula (IV) is shown in the specification.
Owner:BEIJING KANGJIANYUAN TECH
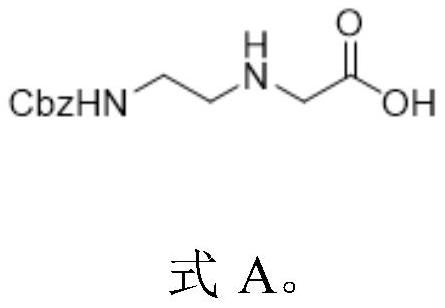
Preparation method of N-(2-aminoethyl) glycine derivative
ActiveCN113501771AReduce usageOxidation reaction conditions are mildCarbamic acid derivatives preparationOrganic compound preparationGlycineBiochemical engineering
The invention discloses a preparation method of an N-(2-aminoethyl) glycine derivative, which comprises the steps of by taking hydroxyethyl ethylenediamine as a raw material, carrying out Cbz protection reaction of primary amine, and then carrying out oxidation reaction to obtain an N-(2-aminoethyl) glycine derivative product compound. According to the preparation method of the N-(2-aminoethyl) glycine derivative, reaction conditions for obtaining carboxylic acid groups through primary alcohol oxidation are optimized, primary amine protected hydroxyethyl ethylenediamine is subjected to one-step oxidation reaction to directly obtain the N-(2-aminoethyl) glycine derivative product compound, the route is short, the reaction conditions are mild, the safety is good, the purification is easy, and the yield is greatly improved; and the method is mild in condition, good in operation safety, green and environment-friendly in post-treatment, capable of achieving environment-friendly industrial production and wide in application prospect.
Owner:成都泰和伟业生物科技有限公司
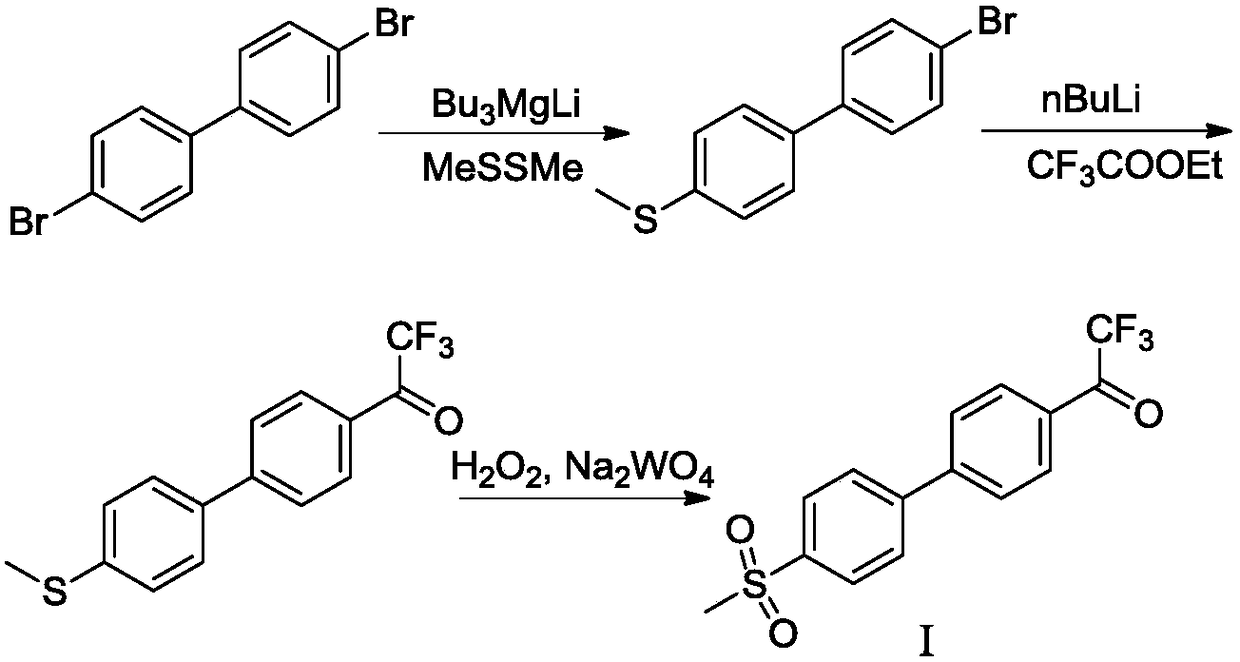
Preparation method of odanacatib, and preparation method of odanacatib intermediate
ActiveCN108912020AHigh purityStandards compliantOrganic compound preparationGroup 3/13 element organic compoundsMetal catalystMethyl sulfones
The invention discloses a preparation method of odanacatib, and a preparation method of an odanacatib intermediate. The preparation method of the trifluoroethylketone biphenyl methylsulfone intermediate I comprises the following step: 2,2,2-trifluoro-1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethanone and 4-bromophenyl methyl sulfone undergo a coupling reaction in a solvent in the presence of a metal catalyst and an inorganic alkali to obtain the trifluoroethylketone biphenyl methylsulfone intermediate I. The preparation methods have the advantages of simplicity and safety in operation, short route steps, environmental friendliness, high reaction yield, and high purity of the obtained products, and the odanacatib II obtained by using the trifluoroethylketone biphenyl methylsulfone intermediate I as the intermediate has the advantages of high purity, meeting of bulk drug standards, and suitableness for industrial production.
Owner:上海新礼泰药业有限公司
A kind of synthetic method of parecoxib sodium
ActiveCN106008385BShort route stepsMild conditionsOrganic chemistryParecoxib sodiumSynthesis methods
The invention belongs to the technical field of medical production, and particularly relates to a preparation method of parecoxib sodium. The invention aims to provide a synthesis method of parecoxib sodium, which has the advantages of short synthesis route, mild and controllable conditions and low cost and is simple to operate. By using 5-methyl-3,4-diphenylisooxazole as the initial raw material, chlorosulfonation, acylation and salification are carried out to synthesize the target product parecoxib sodium. The synthesis method provided by the invention has the characteristics of short route steps, mild and controllable conditions, small environmental pollution, higher yield and the like, and is simple to operate and suitable for industrial production.
Owner:HONGGUAN BIO PHARMA CO LTD
A kind of synthetic method of Relugoli or its salt
The synthetic method of relugolix provided by the invention or its salt comprises taking 1-(dimethylamino)-3-(4-nitrophenyl)-2-acetone and cyanoacetate as starting materials, After condensation cyclization, alkylation reaction, Ullmann reaction, coupling reaction, Relugolix 4 free base form or salt form is obtained: this synthetic route optimizes the process, shortens the route steps, improves the route efficiency, and The use of precious metal catalysts can be reduced, which greatly reduces the process cost. The route is simple to operate, not only the total yield is high, but also the obtained product has high purity, which is suitable for scale-up production. We also found three salt forms of relugolix, which have better crystallinity and are easy to purify, which is beneficial to improve product purity.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
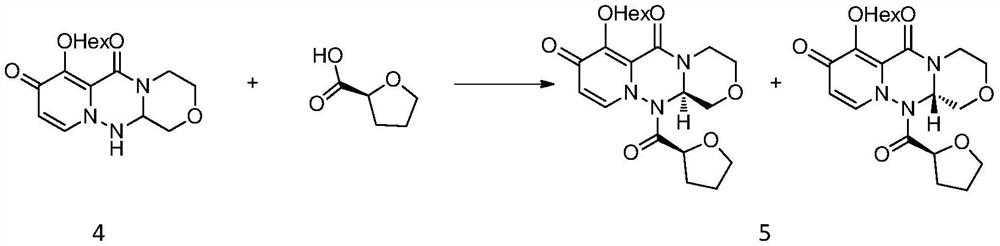
A kind of synthetic method of key mother core intermediate of baloxavir dipivoxil
ActiveCN109912624BImprove protectionAvoid substitutionOrganic chemistryAnnulationCombinatorial chemistry
The invention provides a method for synthesizing the key mother nucleus intermediate formula 6 of baloxavir dipivoxil, which uses compound formula 1 as the starting material, undergoes a condensation reaction with compound formula 2 to obtain the intermediate compound formula 3, and then directly reacts with hydrazine hydrate in one step Ring forming to obtain the racemic compound formula 4, followed by condensation with (S)-tetrahydrofuran-2-carboxylic acid, crystallization and resolution to obtain the intermediate compound formula 5, and finally removal of the chiral prosthetic group to obtain the target product compound formula 6. This route avoids unnecessary protection and replacement of substituents, combined with a one-step ring-forming reaction, greatly reduces the steps of the reaction route, improves the route efficiency and yield, and greatly reduces the cost. The route is as follows:
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
A kind of preparation method of ambrisentan
ActiveCN109705042BHigh purityEfficient removalOrganic chemistryBulk chemical productionMethanol waterDrugs synthesis
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Synthetic method of Jjar mycin
PendingCN112851719AShort route stepsSimple reaction conditionsSugar derivativesSugar derivatives preparationSodium methoxideFuran
The invention relates to a synthetic method of jjar mycin. The technical problems of long steps and high cost of the existing synthesis method are mainly solved. The synthesis method comprises the following synthesis steps: generating a compound 1 from 4-chloro-5-iodine-7H-pyrrolo [2, 3-d] pyrimidine and 1-acetyl-2, 3, 5-tribenzoyloxy-1-beta-D-ribofuranose under the action of N, O-bis (trimethylsilyl) acetamide and trimethylsilyl trifluoromethanesulfonate, and pulping and purifying the product; reacting the compound 1 with sodium methoxide in methanol to obtain a compound 2 without purification; heating and reacting the compound 2 in a sodium hydroxide aqueous solution to generate a compound 3, and pulping and purifying the product; heating and reacting the compound 3 and cuprous cyanide in pyridine, pulping an obtained crude product, and recrystallizing to obtain a target product.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
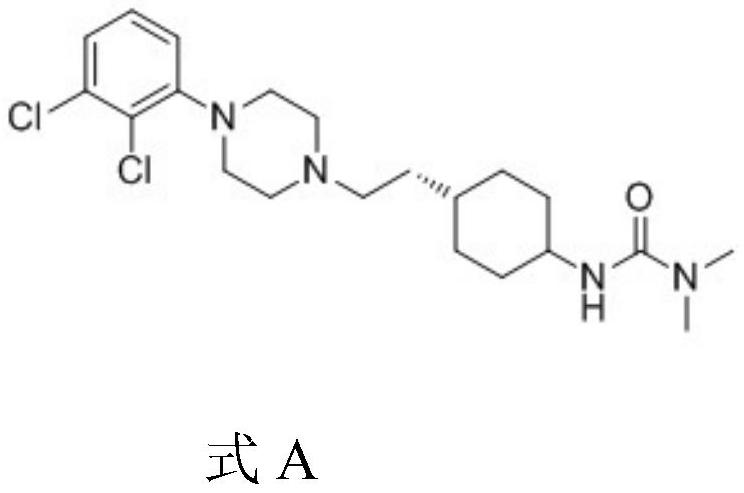
A kind of preparation method of cariprazine
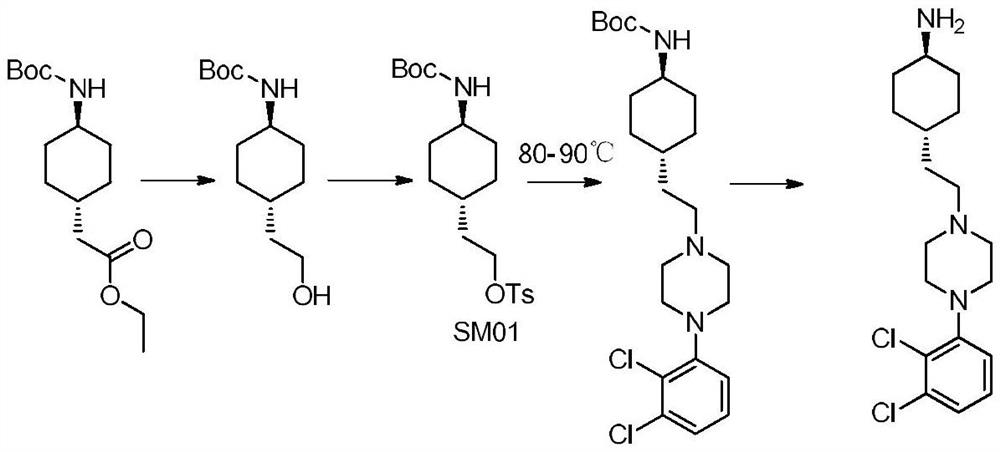
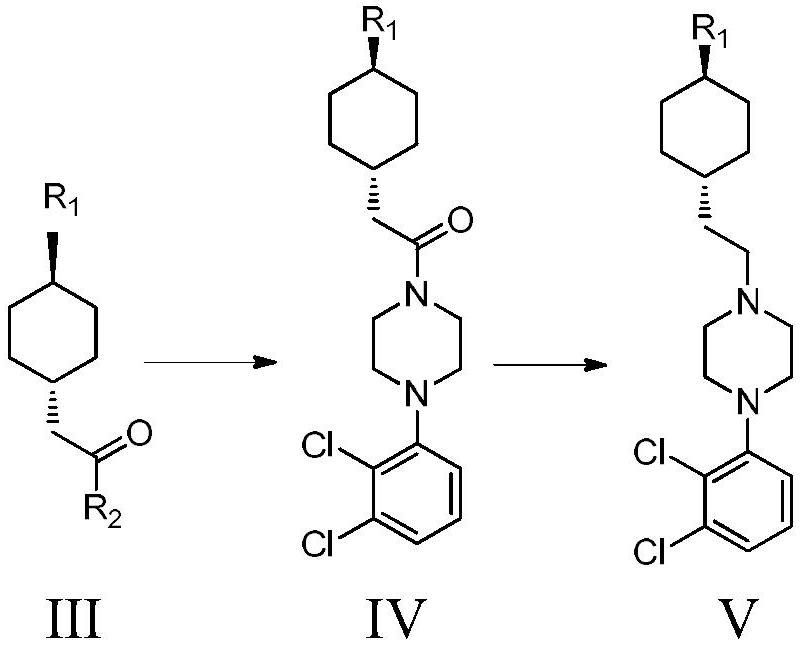
The invention provides a kind of preparation method of cariprazine, it comprises the steps: trans-2-(trans-4-(3,3-dimethylureido) cyclohexyl) derivative reacts in acid-binding agent Reaction with 1‑(2,3‑dichlorophenyl)piperazine or its salts under conditions, followed by reaction with reducing agent to generate cariprazine. The synthesis route of cariprazine of the present invention has few steps, simple process and meets production requirements.
Owner:SHANGYU JINGXIN PHARMA +2
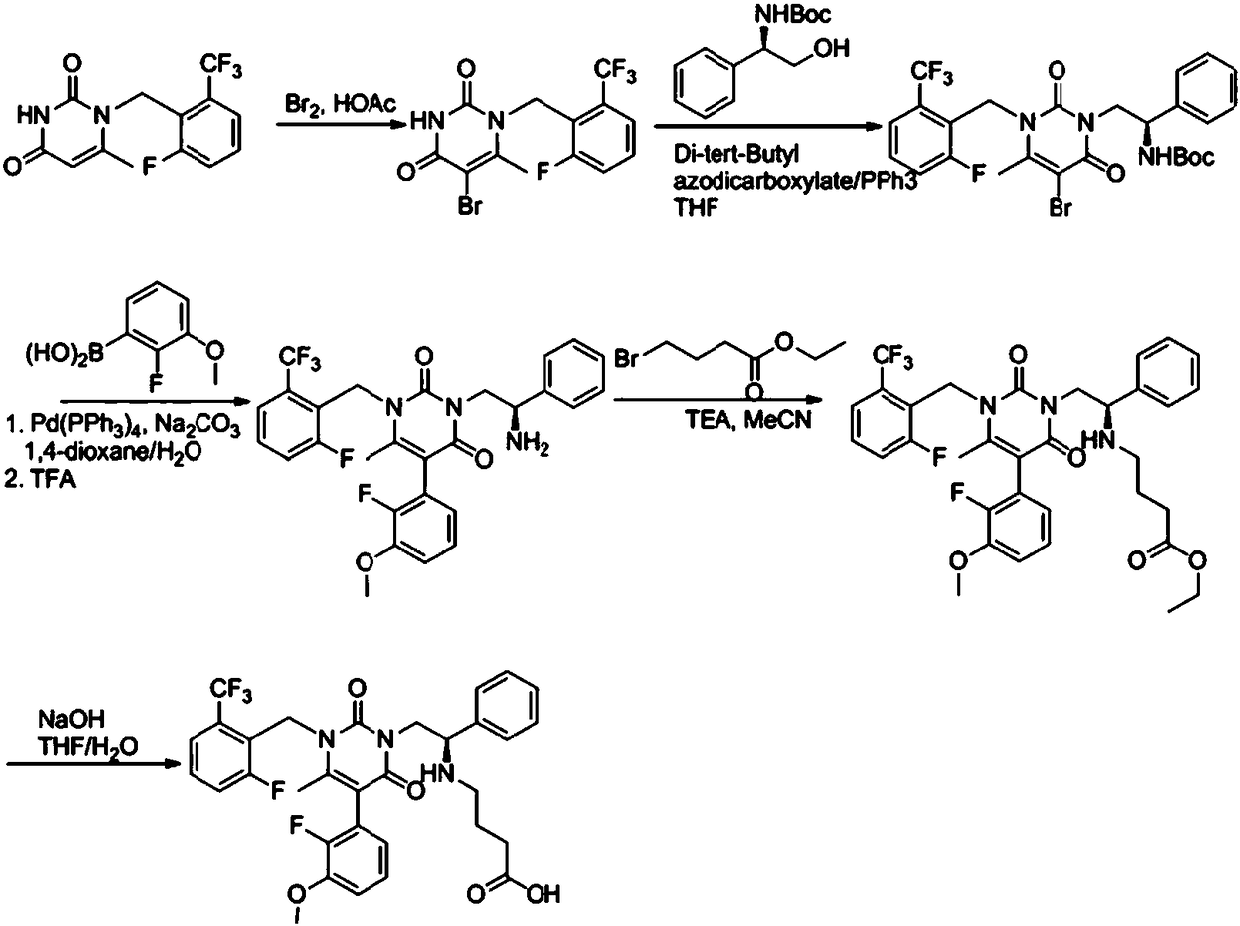
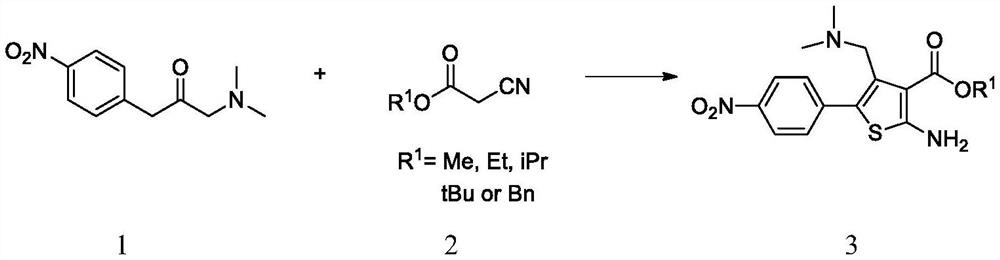
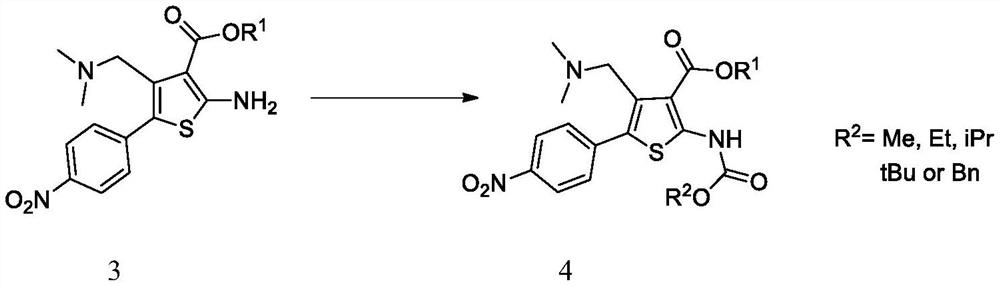
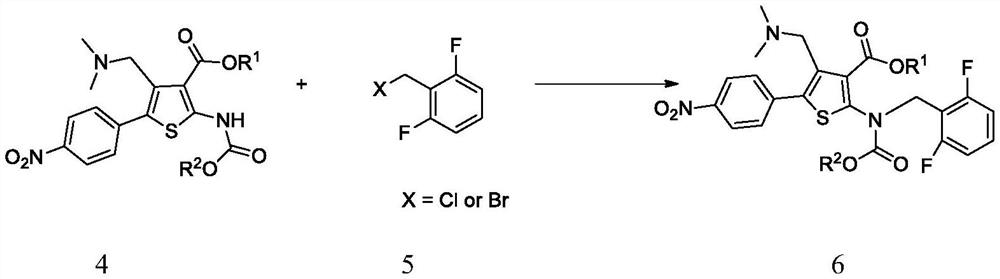
Intermediate of (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and preparation method of intermediate
The invention discloses an intermediate of a (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and a preparation method of the intermediate. The preparation method comprises the following steps of: taking a compound II as a raw material, firstly performing an amidation reaction to protect amido groups of the compound II and obtain a compound III, and conducting a reduction reaction between the compound III and hydrogen in the presence of a catalyst to obtain a compound IV; carrying out an amidation reaction to protect nitrogen atoms in a piperidine ring to obtain a compound V, then conducting areaction of the compound V with a reducing agent to obtain a compound VI, and then conducting a reaction between the compound VI with a halogenating reagent to obtain a compound VII, wherein the compound VII can also be subjected to a ring-closure reaction in the presence of a base so as to obtain a compound I.
Owner:PHARMABLOCK SCIENCES (NANJING) INC
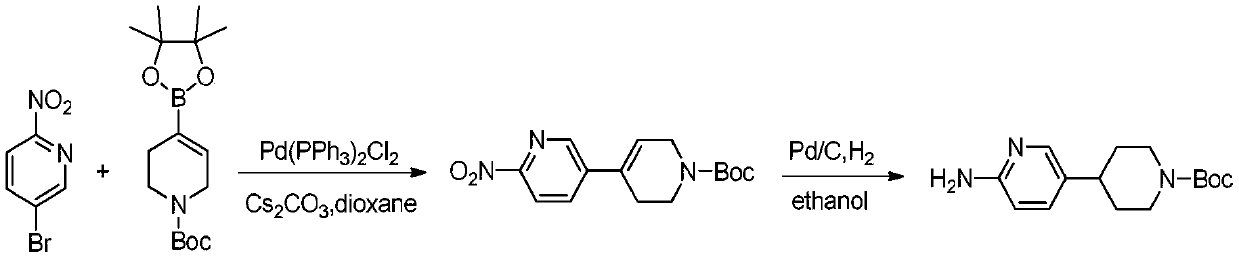
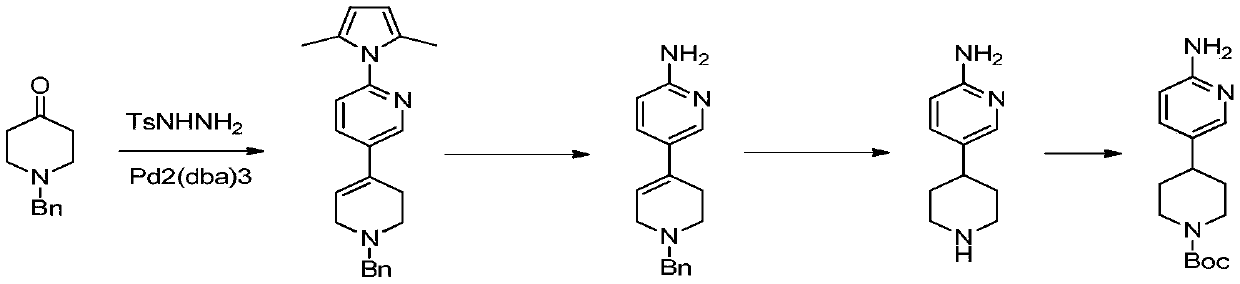
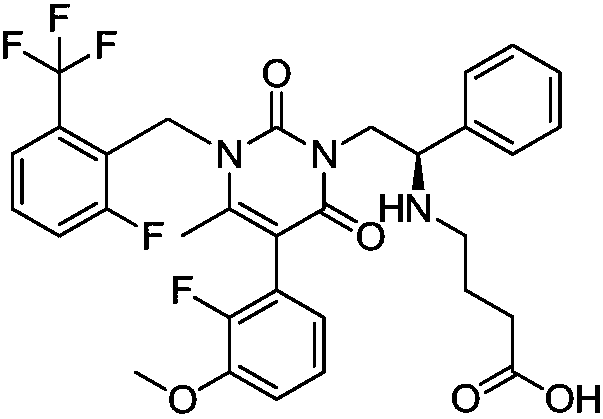
Process suitable for scale-up preparation of 4-(6-aminopyridin-3-yl) substituted piperidines
ActiveCN110540535BReduce pollutionSimple preparation processOrganic chemistryBulk chemical productionSulfohydrazidePtru catalyst
The invention discloses a process method suitable for amplification preparation of 4-(6-aminopyridin-3-yl) substituted piperidine, and belongs to the synthesis field of a medicine intermediate. N-substituted piperidone, aryl sulfohydrazide and 2-amino-5-bromopyridine are subjected to a coupling reaction under a palladium catalyst, and then are subjected to a hydrogenation to obtain 4-(6-aminopyridin-3-yl) substituted piperidine. According to the process, the raw materials in pyridine do not need to be protected; the condensation and the coupling are carried out in the same reaction kettle, sothat the operation cost is reduced, the steps of performing protection at first and then performing protection removal as in documents can be avoided, and production cost of the existing biological, medicine and chemical intermediates is greatly reduced; and the process is subjected to amplification verification in kilogram-scale, and the verification proves that the yield and the product purity are basically equal to those of gram-scale, so that the method can be used as a process for industrial scale production.
Owner:SHANGHAI ZAIQI BIO TECH
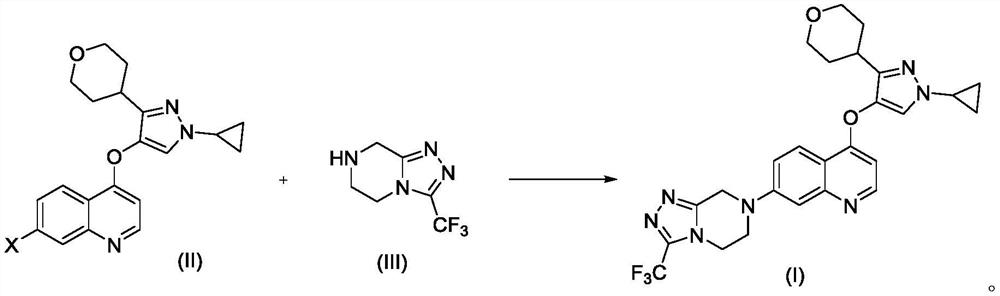
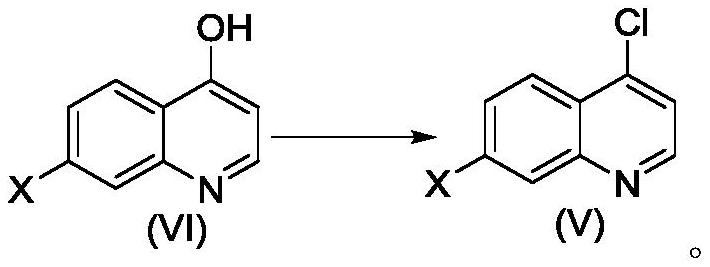
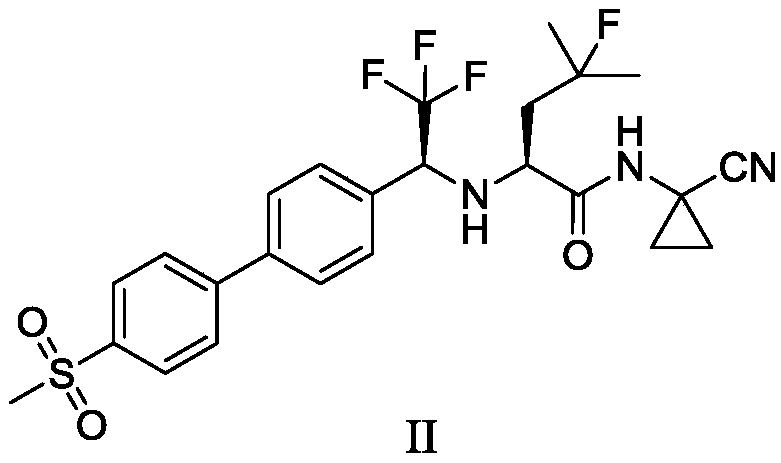
Preparation method of quinoline TGF-beta1 inhibitor
The invention belongs to the field of medical chemistry, and relates to a preparation method of a quinoline TGF-beta1 inhibitor, in particular to a preparation method of 4-((1-cyclopropyl-3-(tetrahydro-2H-pyran-4-yl)-1H-pyrazole-4-yl) oxy)-7-(3-(trifluoromethyl)-5, 6-dihydro-[1, 2, 4] triazolo [4, 3-a] pyrazine-7 (8H)-yl) quinoline as shown in a formula (I) or a salt, a hydrate, a solvate or a crystal thereof.
Owner:NANJING SANHOME PHARM RES & DEV CO LTD
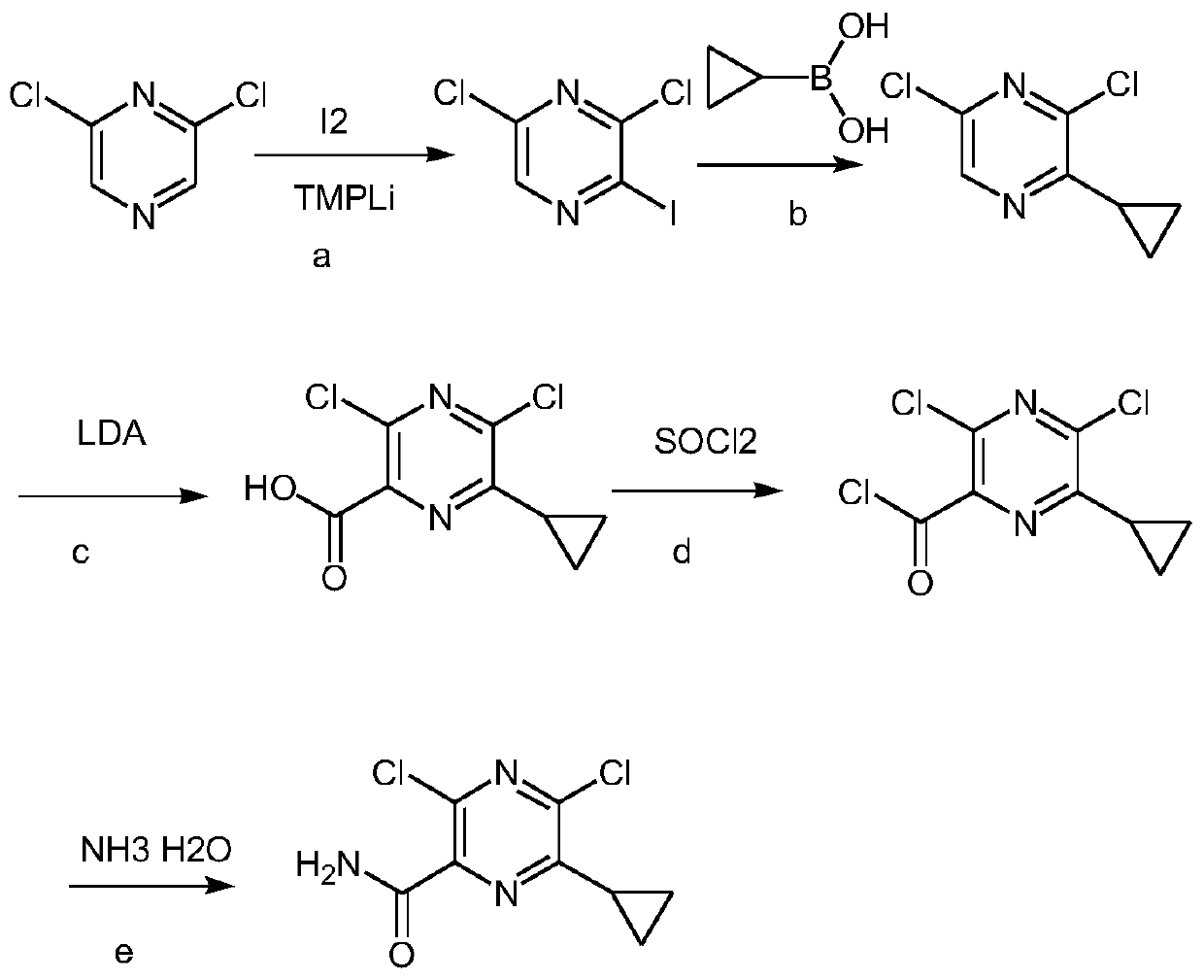
Preparation method of Gilteritinib key intermediate
The invention relates to the field of drug synthesis, and particularly discloses a preparation method of a Gilteritinib key intermediate, namely a method for synthesizing a 3,5-dichloro-6-ethylpyrazinecarboxamide intermediate (compound I). The method is novel in route, simple and convenient to operate, high in yield, good in safety and suitable for industrial production, and comprises the following steps: by taking ethyl propionyl acetate as an initial raw material, carrying out hydrolytic acylation to obtain a compound III; carrying out ring closing on the compound III and aminomalononitrilep-toluenesulfonate to obtain a compound V; then carrying out amino diazotization chlorination to obtain a compound VI; carrying out phosphorus oxychloride transposition on the compound VI to obtain 3,5-dichloro-6-ethylpyrazine-2-carbonitrile (a compound VII); and hydrolyzing the compound VII to obtain the 3,5-dichloro-6-ethylpyrazinecarboxamide intermediate (compound I).
Owner:苏州康纯医药科技有限公司
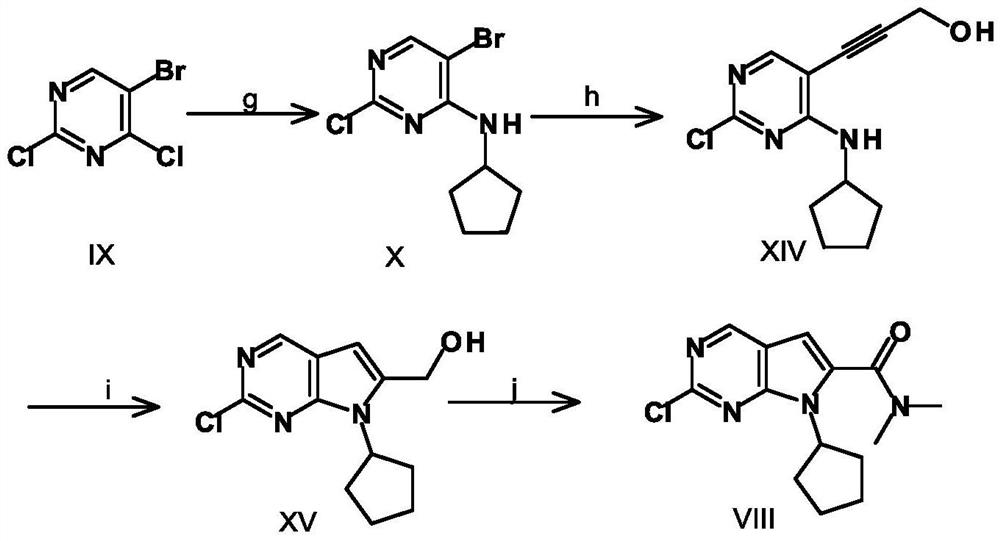
A key intermediate for synthesizing cdk4/6 dual inhibitor and its preparation method and application
ActiveCN110016024BHigh purityShort route stepsOrganic chemistryBulk chemical productionDual inhibitorCombinatorial chemistry
The invention discloses a synthetic 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide intermediate (compound I) and its The preparation method and application include the following steps: using 2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine (compound II) as a starting material, first preparing compound III by applying a protecting group; compound III Compound IV is obtained through carbonyl insertion reaction; compound V is obtained through deprotection group; compound VI is obtained by selective dechlorination; compound I is obtained by cyclopentyl on compound VI; compound VI is obtained by hydrolysis of compound I; -7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide (Compound VIII).
Owner:PHARMABLOCK SCIENCES (NANJING) INC
Preparation method of odancati and its intermediate
ActiveCN108912020BHigh purityStandards compliantOrganic compound preparationGroup 3/13 element organic compoundsPtru catalystBiochemical engineering
The invention discloses a preparation method of odancati and its intermediate. The invention provides a preparation method of trifluoroacetophenone biphenylmethyl sulfone intermediate I, comprising the following steps: in a solvent, in the presence of a metal catalyst and an inorganic base, 2,2,2-trifluoroacetophenone- 4-boronic acid pinacol ester and 4-bromophenyl methyl sulfone carry out coupling reaction, obtain trifluoroacetone biphenyl methyl sulfone intermediate I. The preparation method of the present invention has the advantages of simple and safe operation, short route steps, environmental friendliness, high reaction yield, and high purity of the prepared product, and is prepared by using the trifluoroacetone biphenylmethyl sulfone intermediate I of the present invention as an intermediate Odangcati II has high purity, meets the standards of raw materials, and is suitable for industrial production.
Owner:上海新礼泰药业有限公司
Preparation method of ambrisentan
ActiveCN109705042AHigh purityEfficient removalOrganic chemistryBulk chemical productionEther solventCrystallization
The invention belongs to the field of medicine synthesis and particularly relates to a preparation method of ambrisentan, wherein the ambrisentan is prepared through a benzyl protection reaction, a condensation reaction, and a benzyl protection group removal reaction in the method. A benzyl-protected intermediate is delivered to the next step of the condensation reaction without post-treatment. The debenzylation reaction is carried out through catalytic hydrogenation, which is green and environment-friendly and is simple in reaction operations and post-treatment. A reaction liquid is treated and pulped in an ether solvent, so that residual raw materials and intermediate in the solid are removed effectively. When an ambrisentan crude product is prepared, a recrystallization step with a methanol / water mixed solvent is carried out to obtain high-purity ambrisentan. The method is fewer in steps and high in yield, has short production cycle, simple operation, good reproducibility and mild conditions, and is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
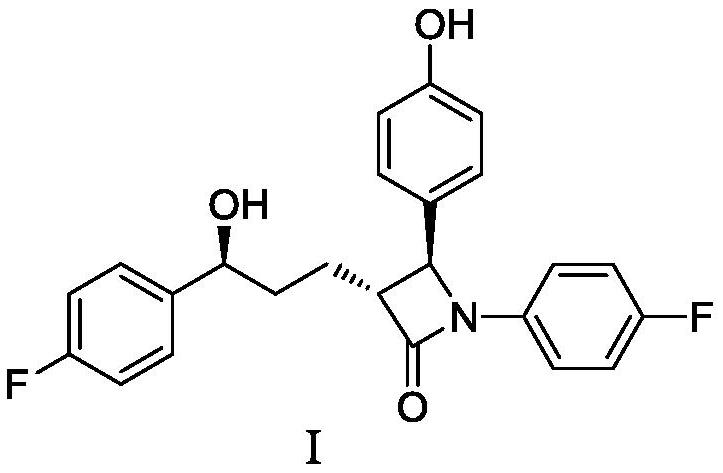
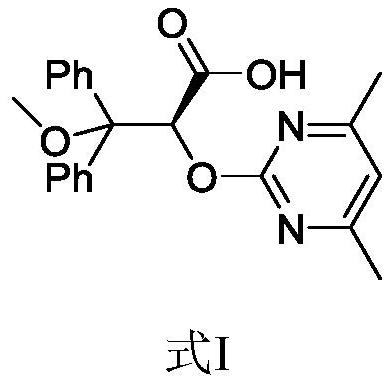
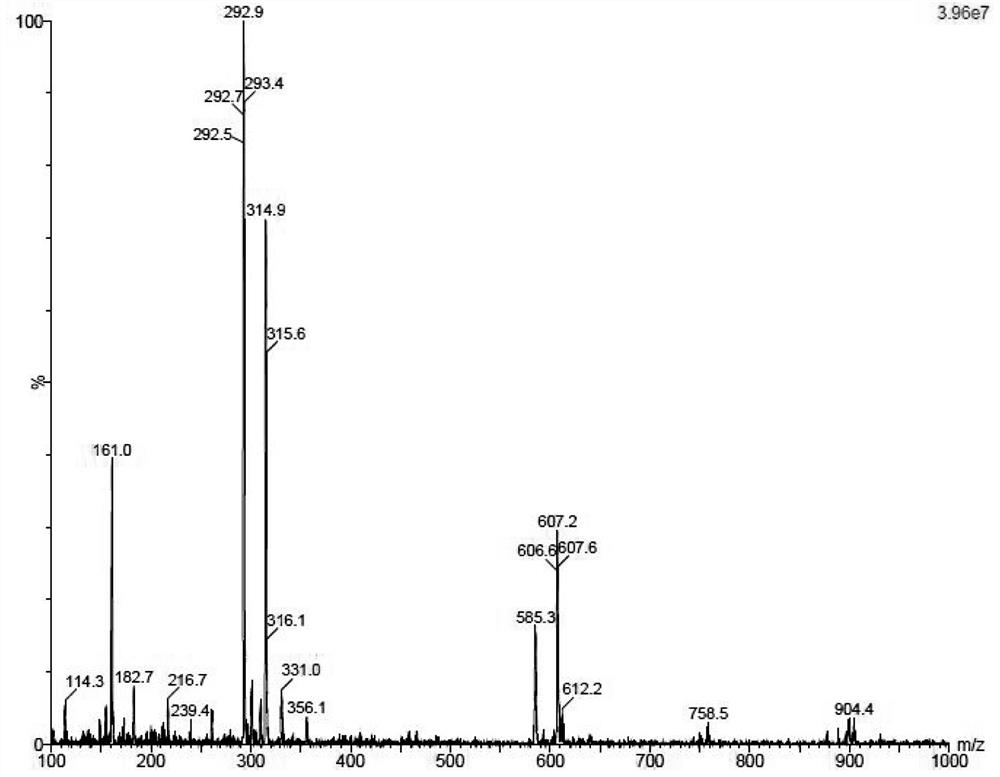
Preparation method of ezetimibe and its intermediate
ActiveCN110818606BShort route stepsMild reaction conditionsGroup 4/14 element organic compoundsPtru catalystOrganic base
The invention discloses a preparation method of ezetimibe and an intermediate thereof. The invention provides a preparation method of ezetimibe intermediate IV, comprising the following steps: in an organic solvent, in the presence of a trialkylchlorosilane, an organic base, a chiral catalyst and lithium diisopropylamide, the ezetimibe The ezetimibe intermediate II and the ezetimibe intermediate III undergo a ring closure reaction to obtain the ezetimibe intermediate IV; R is methyl, ethyl or propyl. The preparation method of the present invention has short route steps, mild reaction conditions, simple post-treatment steps, avoids substrates connected with chiral groups, and the prepared product has high purity, meets the standard of raw materials, high yield, low production cost, and atom utilization High efficiency, suitable for industrial production.
Owner:上海新礼泰药业有限公司
A kind of synthetic method of cis-1,4-cyclohexanediol
ActiveCN107759446BEasy to getEasy to purifyOxygen-containing compound preparationOrganic compound preparationChemical synthesisCyclohexanone
The invention discloses a method for synthesizing cis-1,4-cyclohexanediol, which belongs to the field of organic chemical synthesis. The method uses 4-(hydrocarbyl acyloxy)cyclohexanone as a raw material, and only undergoes reduction and hydrolysis. cis-1,4-cyclohexanediol can be easily synthesized. The method can ensure high selectivity of the reaction, and the obtained product yield is high, and is suitable for large-scale production.
Owner:PHARMABLOCK SCIENCES (NANJING) INC
Preparation method of 4-methyl-1-propyl-2-amino-1H-pyrrole-3-nitrile
PendingCN114085178AShort stepsThe reaction is easy to operateOrganic chemistryOrganic synthesisBiochemical engineering
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method of an intermediate 4-methyl-1-propyl-2-amino-1H-pyrrole-3-nitrile of uconafil. The method comprises the following steps: carrying out condensation on malononitrile and acetone under the action of alkali to obtain a compound shown in a formula (I), and brominating the compound shown in the formula (I) to obtain a compound shown in a formula (II); then performing condensation and ring closing on the compound in the formula (II) and propylamine to obtain a target product compound in a formula (III). The invention provides a simple and cost-reducing industrial production route for the uconafil intermediate, and has the advantages of short reaction steps, simple and convenient reaction, avoidance of use of pipe products, and realization of stable industrial production and preparation.
Owner:苏州楚凯药业有限公司
Preparation method of cinacalcet hydrochloride and intermediate thereof
ActiveCN111196759AShort route stepsShort stepsOrganic compound preparationOrganic chemistry methodsAlpha-naphtholCinacalcet
The invention discloses a preparation method of cinacalcet hydrochloride and an intermediate thereof. The invention provides a preparation method of an intermediate L-cinacalcet tartrate III of cinacalcet hydrochloride. The method comprises the following steps: step (1): in an organic solvent, in the presence of a chiral catalyst and a chiral ligand, an asymmetric hydrogenation reduction reactionis conducted on a cinacalcet intermediate II to obtain cinacalcet IV, wherein the chiral catalyst is bis (1, 5-cyclooctadiene)-rhodium trifluoromethanesulfonate, and the chiral ligand is (S)-3, 3'-bis(2, 4, 6-triisopropylphenyl)-1, 1'-di-2-naphthol cyclic phosphate; and (2) in an organic solvent, a neutralization reaction is conducted between cinacalcet IV and L-tartaric acid to obtain L-cinacalcet tartrate III. The preparation method disclosed by the invention has advantages of short route step, simple and safe operation and high total yield; and the prepared product has high purity, meets the requirements of bulk drugs, is low in production cost and is suitable for industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD +1
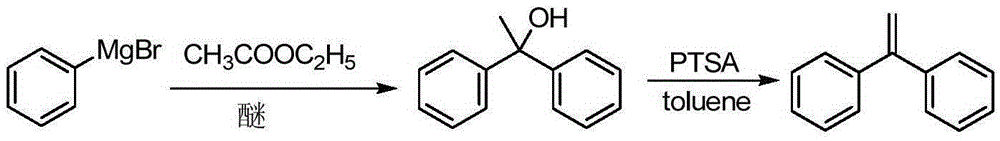
A kind of preparation method of 1,1-diphenylethylene
ActiveCN103755516BModerate boiling pointStrong Lewis alkalineHydrocarbonsBulk chemical productionPhenylmagnesium bromideGrignard reagent
The invention discloses a preparation method of 1,1-diphenylethylene shown in a formula (V). The method comprises the steps of firstly carrying out reaction on bromobenzene and magnesium chips in anhydrous 2-methyltetrahydrofuran to obtain a phenylmagnesium bromide Grignard reagent, and then dripping acetophenone into the phenylmagnesium bromide Grignard reagent to react, so as to generate 1,1-diphenylethanol; finally, dewatering 1,1-diphenylethanol in the presence of a sulfoacid functional ionic liquid catalyst, so as to obtain 1,1-diphenylethylene shown in the formula (V). The preparation method disclosed by the invention is short in reaction step, mild in condition, simple and convenient to operate, high in product yield, low in production cost, friendly to environment, and applicable to industrial production.
Owner:ZHEJIANG UNIV OF TECH +1
Method for synthesizing aromatic primary amine through OTf amination
PendingCN113387842AReduce usageImprove securityCarbamic acid derivatives preparationOrganic compound preparationBiochemical engineeringCombinatorial chemistry
The invention discloses a method for synthesizing aromatic primary amine through OTF amination. Amination reaction is carried out on OTF of an aromatic group and benzophenone imine, and then benzhydryl is removed to obtain an aromatic primary amine compound. According to the method for synthesizing the primary aromatic amine through OTF amination, the raw materials are cheap and easy to obtain, the preparation cost is greatly reduced, meanwhile, the operation condition is mild, the operation difficulty is reduced, the energy consumption is low, no pollution is caused to the environment, and the method is suitable for green and environment-friendly industrial production and beneficial to enlarged production and industrial popularization.
Owner:成都泰和伟业生物科技有限公司 +1
Preparation method of pramipexole hydrochloride and its intermediate
The invention discloses a preparation method of pramipexole dihydrochloride and an intermediate thereof. The invention provides a preparation of pramipexole II. The preparation method of the pramipexole II comprises the following steps: performing condensation reaction and reduction reaction on a pramipexole intermediate III, propylamine and hydrogen in an organic solvent and under the existence of a chiral catalyst, and performing one-pot method to obtain the pramipexole II. According to the preparation method provided by the invention, the route step is short, chiral resolution is not neededand the total molar yield is high; furthermore, the prepared product has high purity, can reach to the standard of raw material medicines and is suitable for industrialized production. (The formula is shown in the description).
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000001.png)
![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000002.png)
![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000003.png)




















![Intermediate of (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and preparation method of intermediate Intermediate of (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and preparation method of intermediate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3056cc3c-1b9f-401a-8d4e-51fec7777b7b/FDA0001397788310000011.png)
![Intermediate of (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and preparation method of intermediate Intermediate of (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and preparation method of intermediate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3056cc3c-1b9f-401a-8d4e-51fec7777b7b/FDA0001397788310000012.png)
![Intermediate of (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and preparation method of intermediate Intermediate of (1S,4S)-2,5-diazabicyclo[2,2,2]octane derivative and preparation method of intermediate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3056cc3c-1b9f-401a-8d4e-51fec7777b7b/FDA0001397788310000013.png)