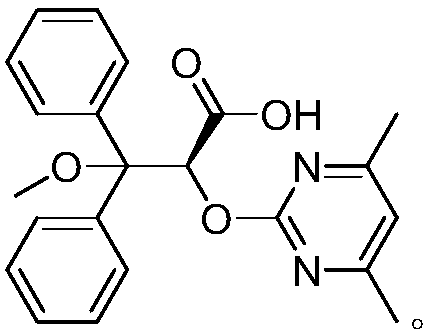
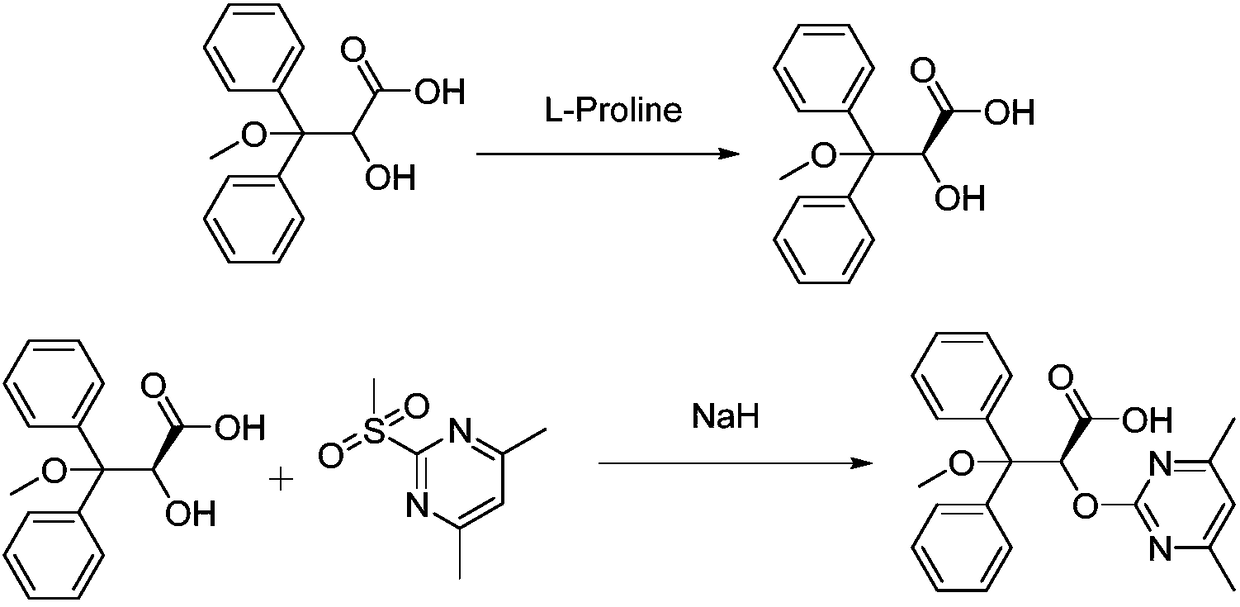
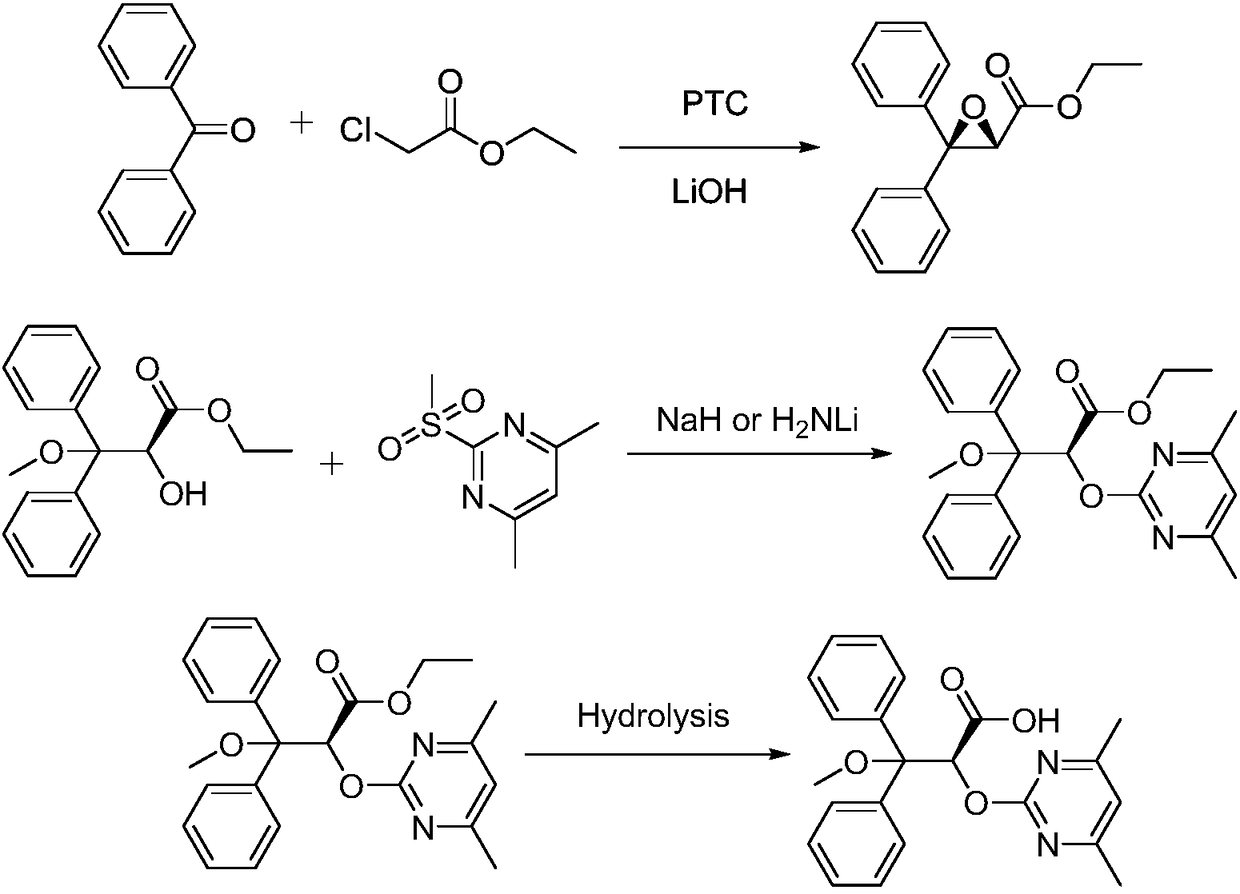
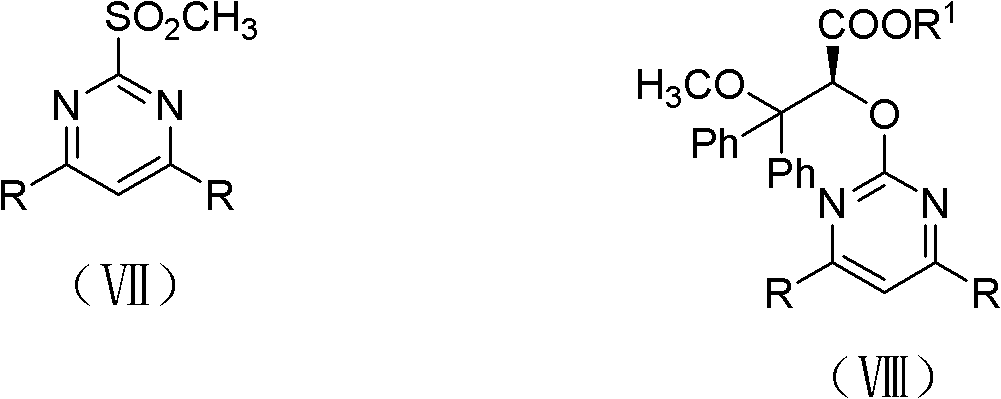Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Ambrisentan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
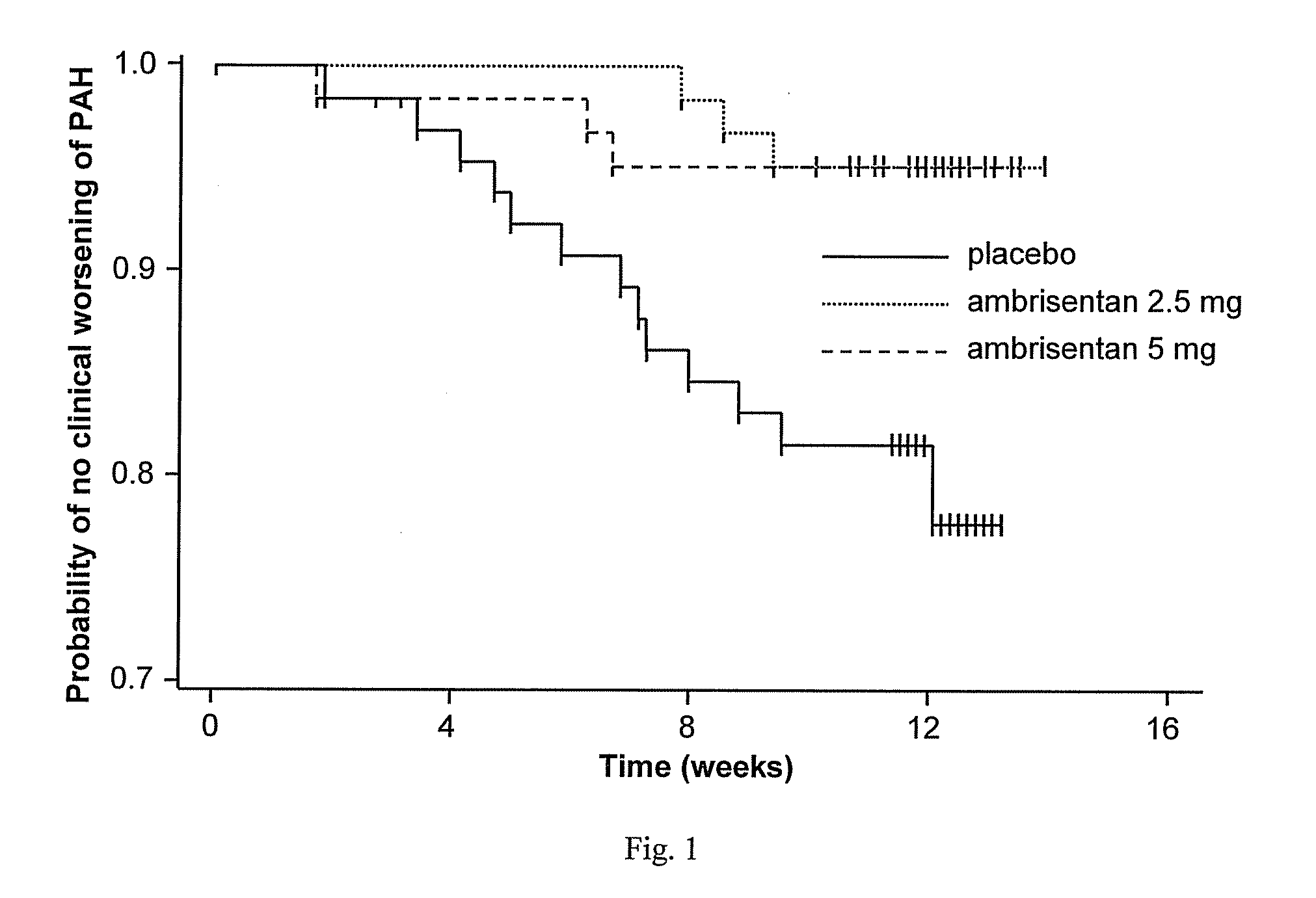
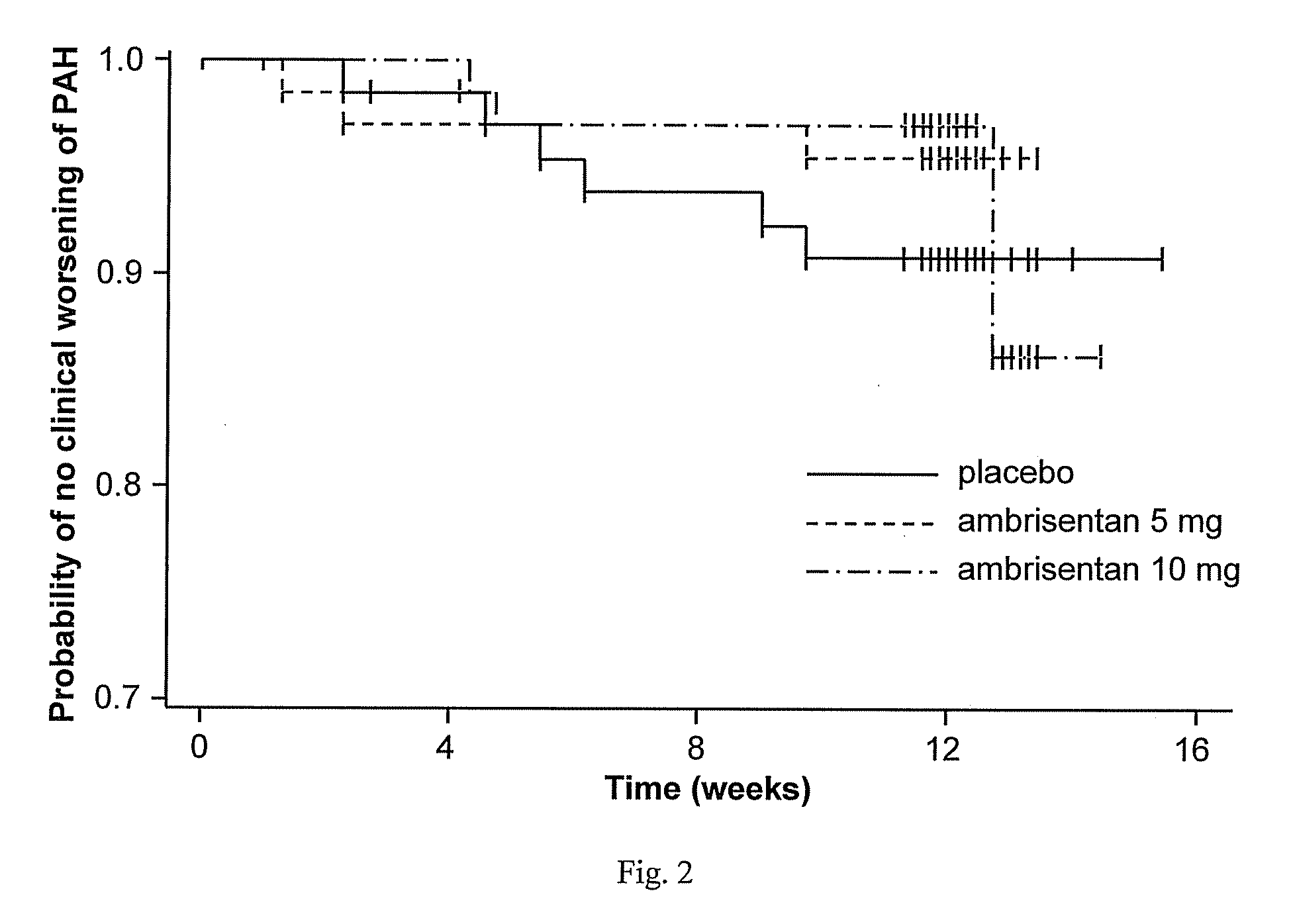
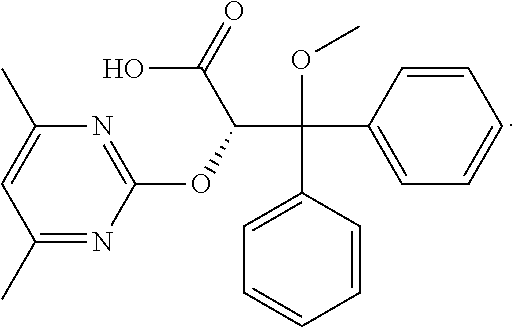
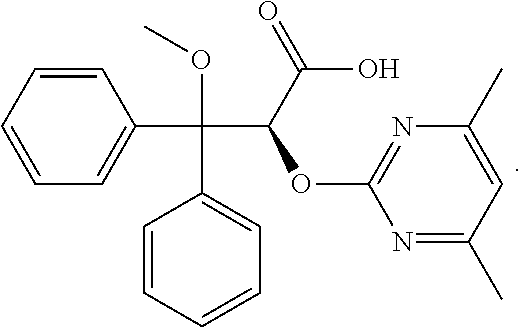
Ambrisentan is used to treat high blood pressure in the lungs (pulmonary arterial hypertension). This condition is thought to be caused by increased levels of a certain natural substance (endothelin-1).
Method for treating pulmonary arterial hypertension in a patient not having idiopathic pulmonary fibrosis
InactiveUS20130225595A1Therapeutic utilityGreat riskBiocideAnimal repellantsIdiopathic pulmonary fibrosisAmbrisentan
There is provided a method of treating pulmonary hypertension in a patient in need thereof, said method comprising: administering a therapeutically effective amount of ambrisentan to the patient with pulmonary arterial hypertension, wherein the patient has been determined not to have idiopathic pulmonary fibrosis.
Owner:GILEAD SCI INC
Method for treating a pulmonary hypertension condition
InactiveUS20080139593A1Prolong lifeImprove life expectancyBiocideRespiratory disorderAmbrisentanPulmonary hypertension
A method for treating a pulmonary hypertension condition such as pulmonary arterial hypertension (PAH) in a subject comprises administering to the subject a therapeutically effective amount of ambrisentan, wherein, at baseline, time from first diagnosis of the condition in the subject is not greater than about 2 years.
Owner:GILEAD SCI INC
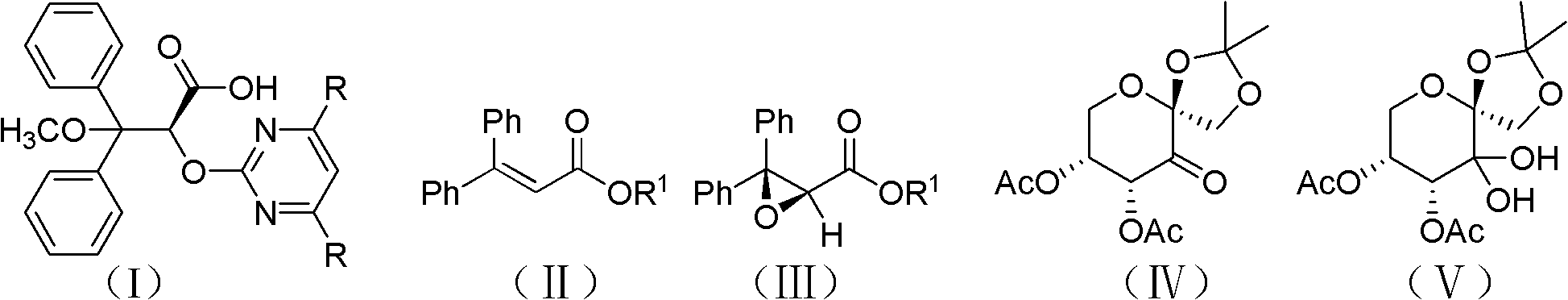
A preparation method of optically pure (+)-ambisentan and optically pure (+)-dalusentan
ActiveCN102276536AEnvironmentally friendlyHigh yieldOrganic chemistryFructoseEnantioselective synthesis
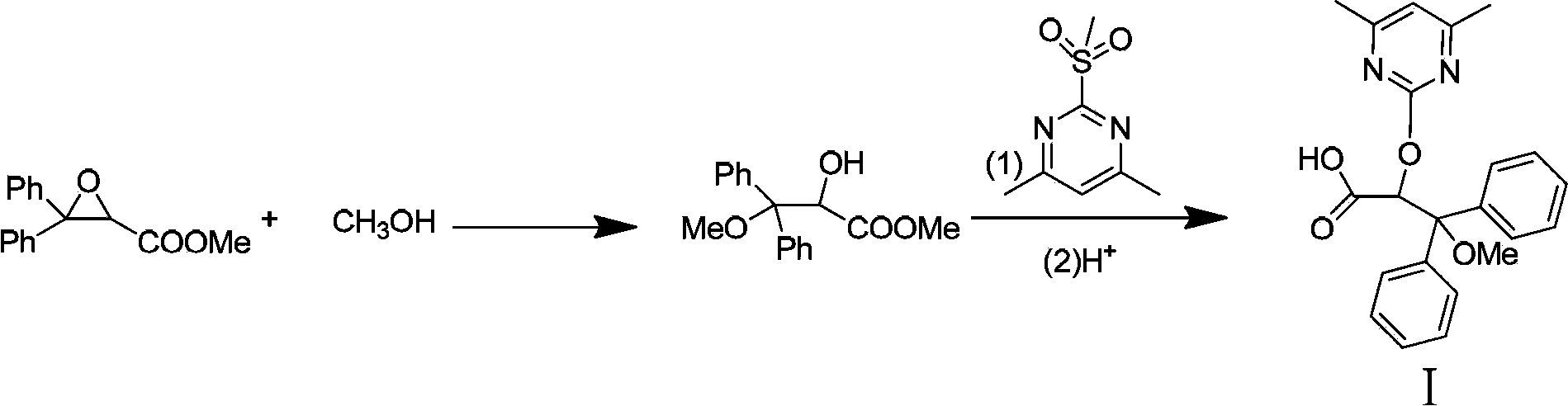
Disclosed is a method for preparing optically pure (+)-ambrisentan and (+)-darusentan, comprising: firstly catalyzing the asymmetric epoxidation of a β-unsaturated alkene using a chiral ketone derived from fructose or a hydrate thereof as a catalyst, and then subjecting the product to an epoxy compound ring-opening reaction and substitution reaction successively to obtain optically pure (+)-ambrisentan and (+)-darusentan.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Compositions and methods of treating pulmonary hypertension
InactiveUS20120269898A1Preventing pulmonary hypertensionGood treatment effectBiocideElcosanoid active ingredientsTadalafilPharmacology
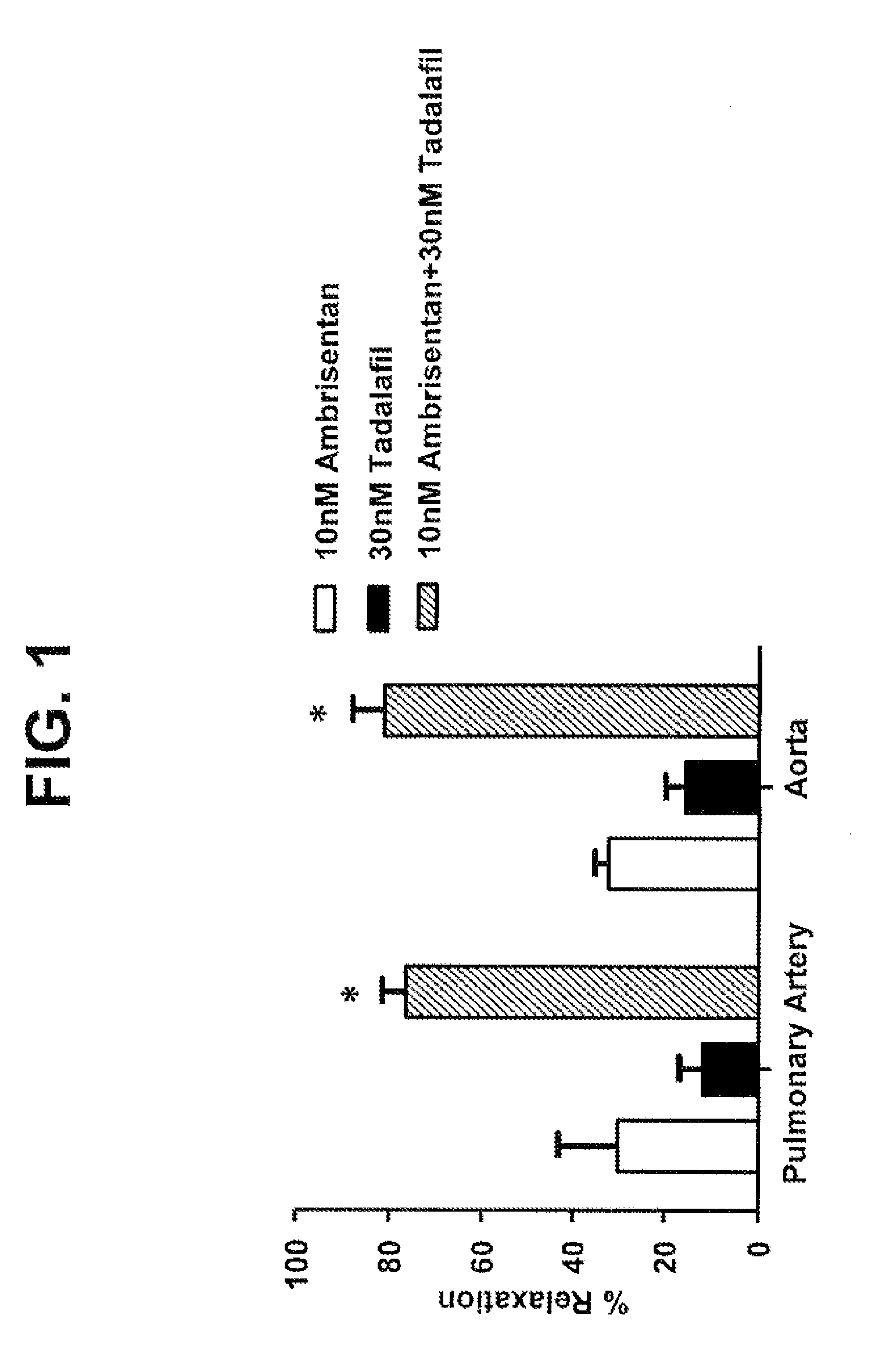
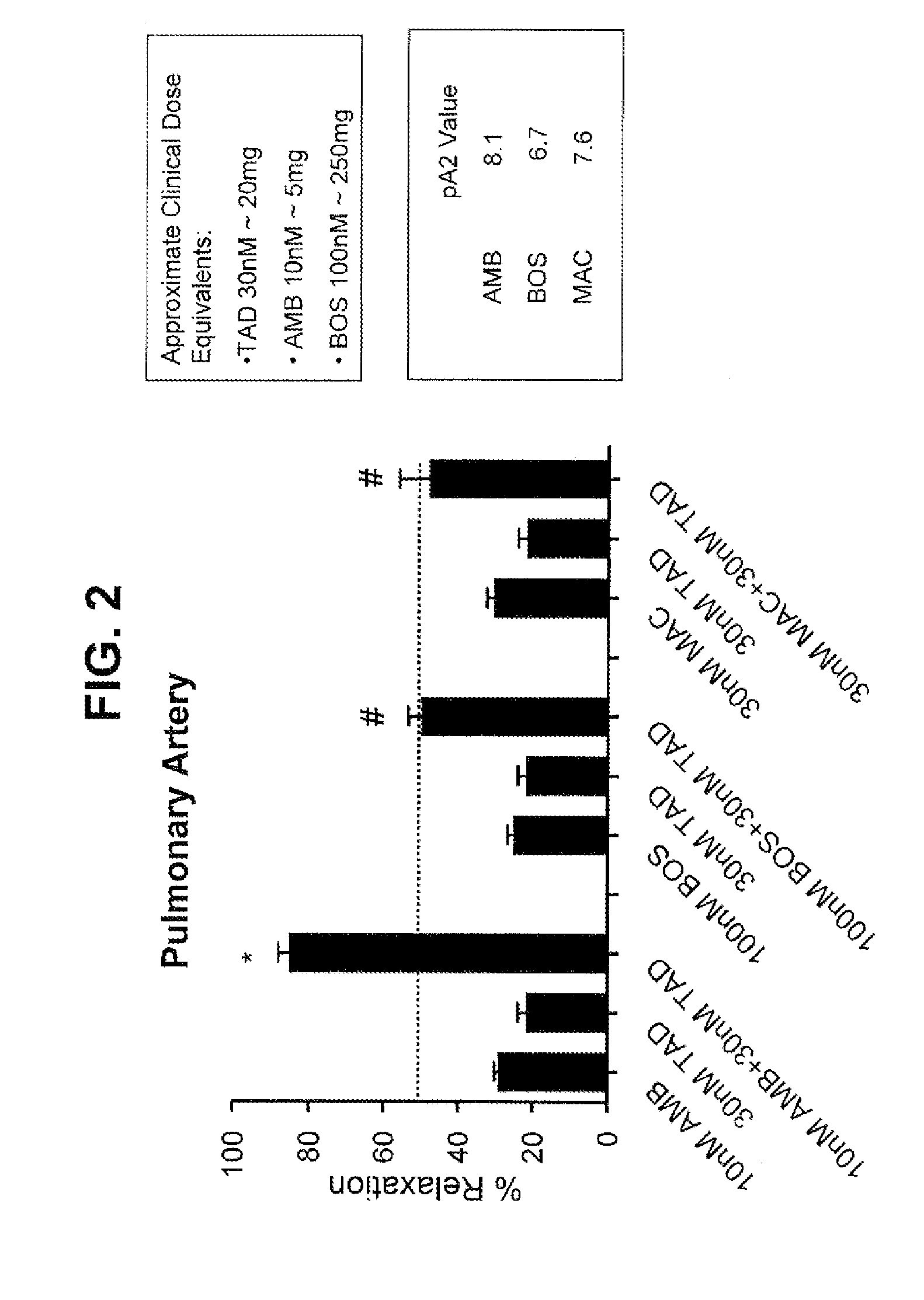
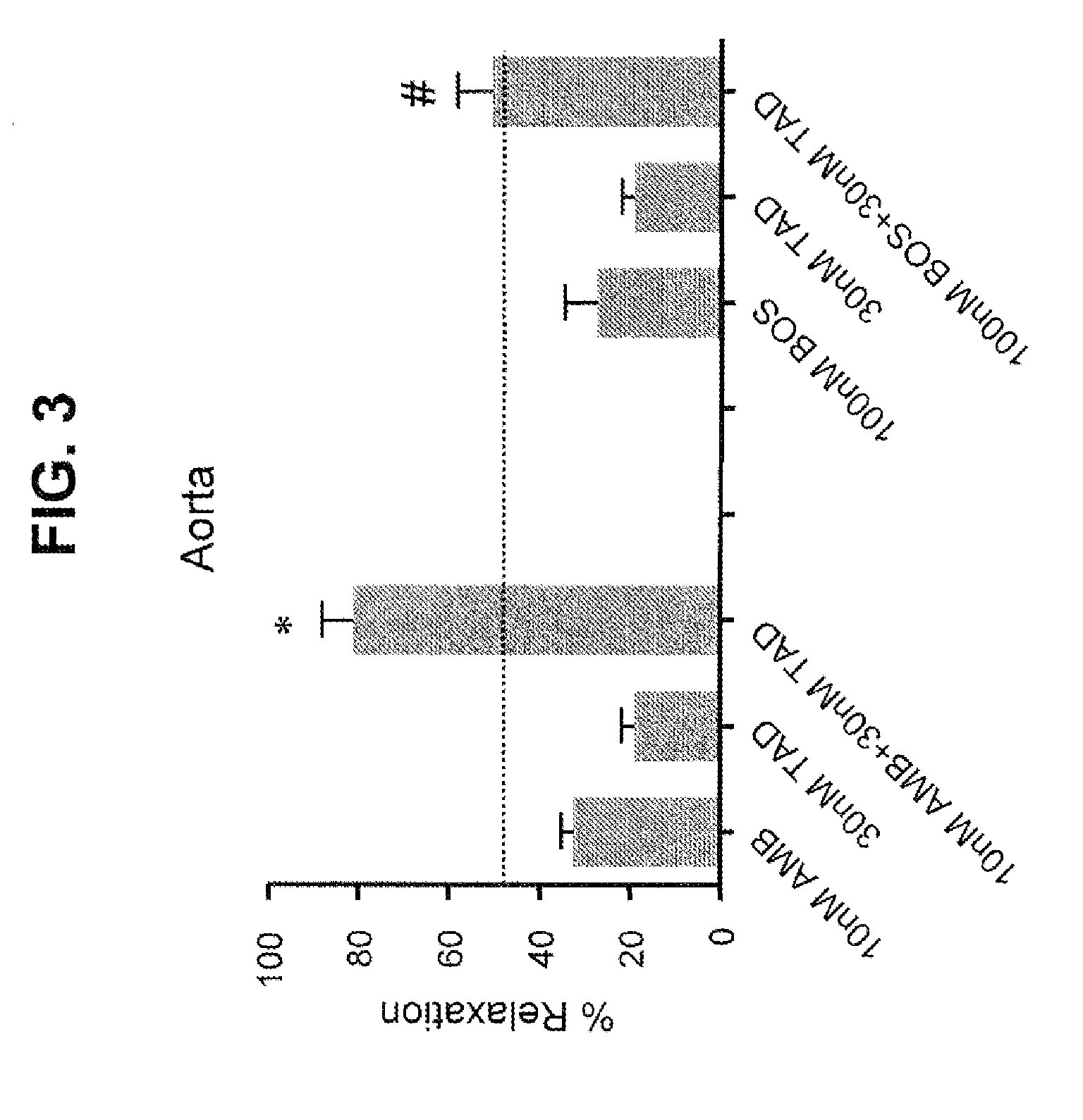
Provided are formulations comprising therapeutically effective amounts of ambrisentan or a pharmaceutically acceptable salt thereof and tadalafil or a pharmaceutically acceptable salt thereof and methods of treating and / or preventing pulmonary hypertension by administration of the formulations.
Owner:GILEAD SCI INC
Method for treating a pulmonary hypertension condition
InactiveUS20100152217A1Prolong lifeImprove life expectancyBiocideRespiratory disorderAmbrisentanPulmonary hypertension
A method for treating a pulmonary hypertension condition such as pulmonary arterial hypertension (PAH) in a subject comprises administering to the subject a therapeutically effective amount of ambrisentan, wherein, at baseline, time from first diagnosis of the condition in the subject is not greater than about 2 years.
Owner:GILEAD SCI INC
Compositions and methods of treating pulmonary hypertension
ActiveUS20140275098A1Preventing pulmonary hypertensionGood treatment effectBiocideUrinary disorderTadalafilPharmacology
Provided are formulations comprising therapeutically effective amounts of ambrisentan or a pharmaceutically acceptable salt thereof and tadalafil or a pharmaceutically acceptable salt thereof and methods of treating and / or preventing pulmonary hypertension by administration of the formulations.
Owner:GILEAD SCI INC
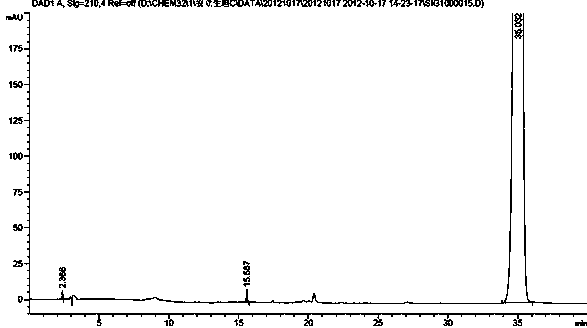
Detection method of substances relative to raw material and preparation of ambrisentan
ActiveCN104515816AAccurate and effective determinationPrecisely control product qualityComponent separationStationary phaseHplc method
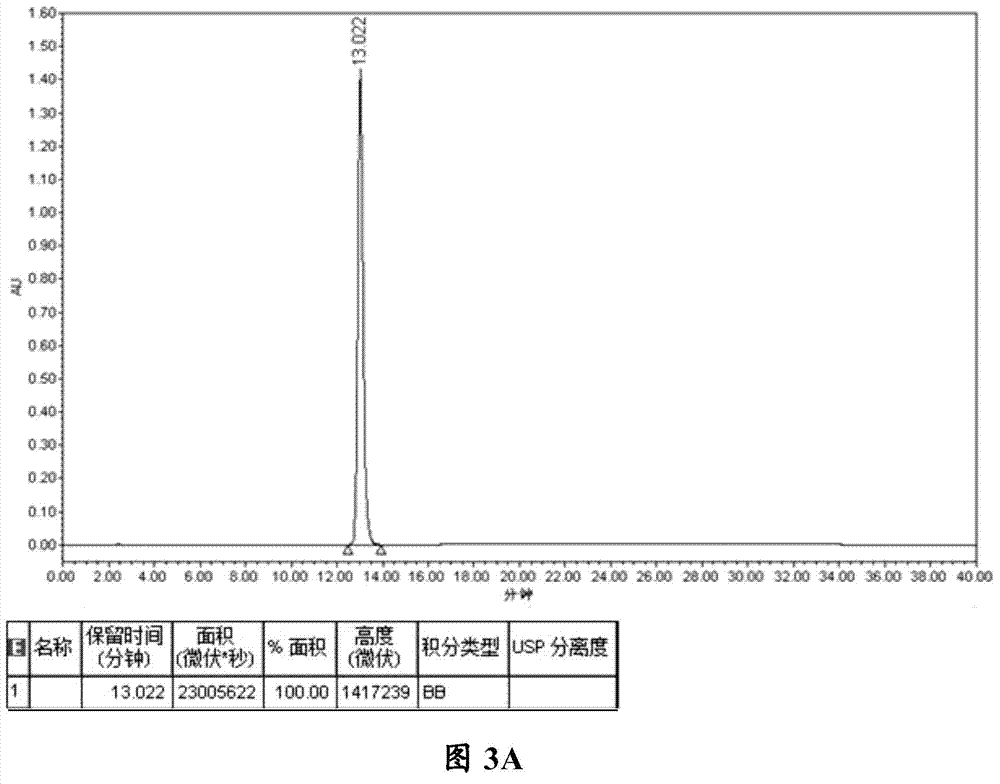
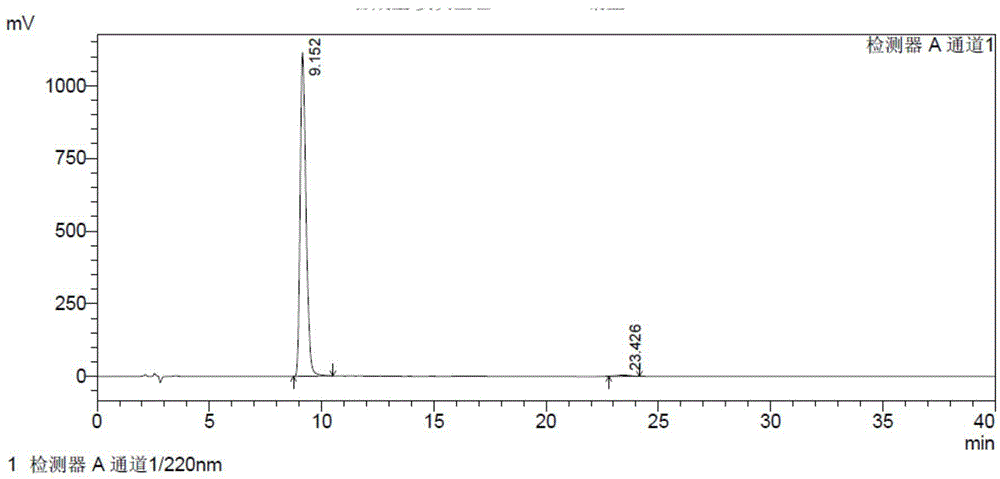
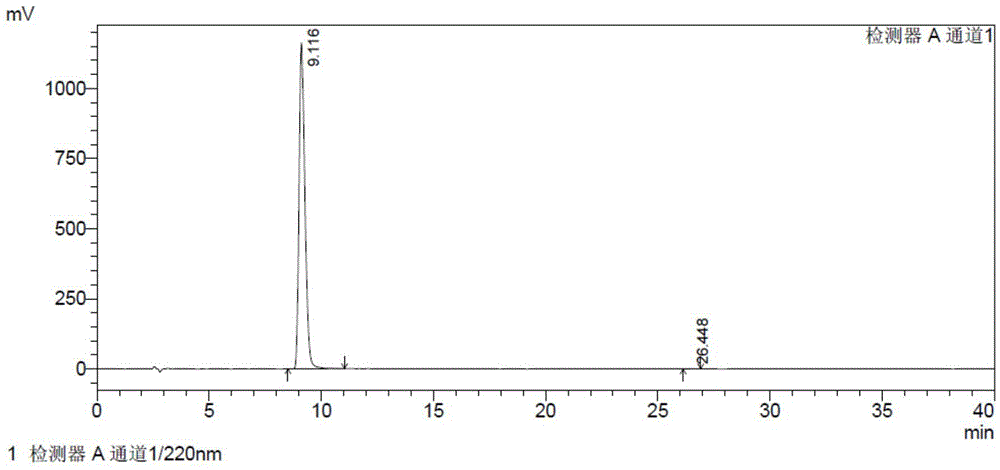
The invention provides a detection method of relative substances in raw materials and preparations of ambrisentan. The method is carried out through an HPLC method and includes following steps: (1) preparing a sample solution of the ambrisentan; and (2) feeding the sample solution into an HPLC instrument, performing gradient elution to the sample solution with a mobile phase A and a mobile phase B as a mobile phase and recording a chromatogram, wherein a stationary phase filling agent of a chromatographic column of the HPLC is octadecylsilane chemically bonded silica or octylsilane chemically bonded silica; the mobile phase A is prepared from acetonitrile and a phosphate buffer solution with a volume ratio being 30-50:50-70; and the mobile phase B is prepared from acetonitrile and a phosphate buffer solution with a volume ratio being 50-80:20-50. By means of the detection method, under the same liquid-phase condition, the relative substances in the raw materials or the preparations of the ambrisentan can be measured. The detection method is simple in operation, is high in separation degree, is strong in specificity and is accuracy and reliable in result.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for treating a pulmonary hypertension condition without companion diagnosis
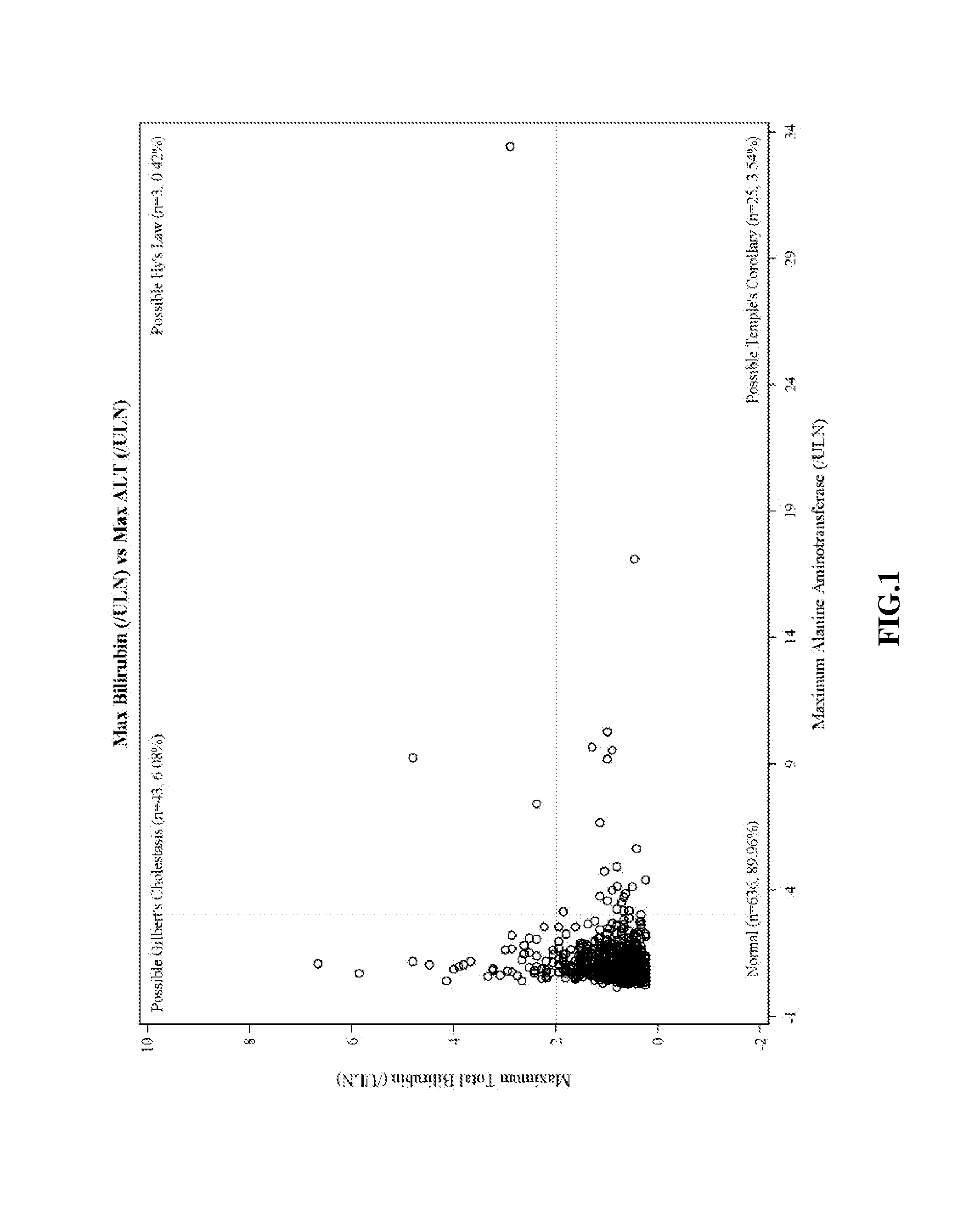
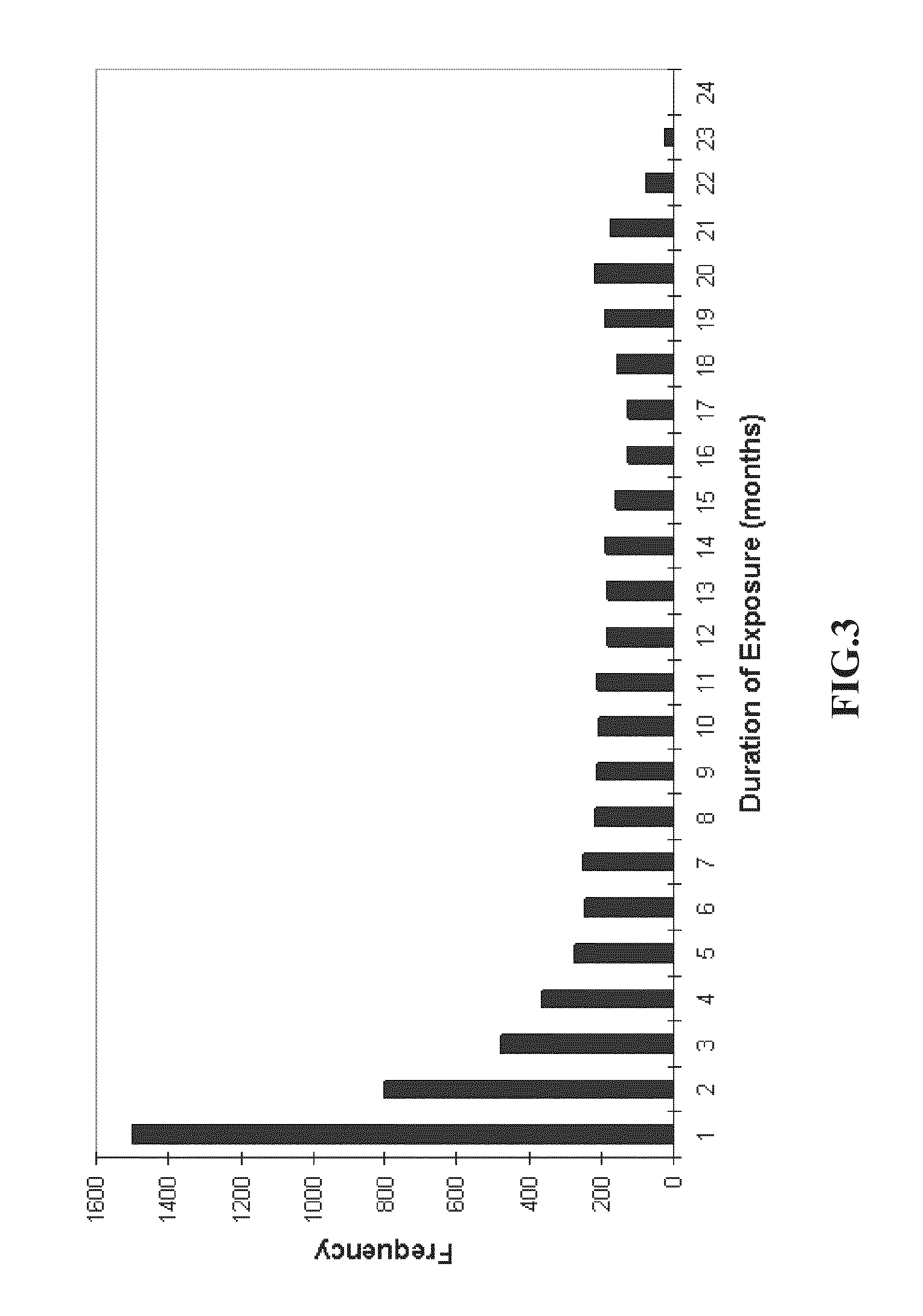
There is provided a method for treating pulmonary hypertension in a subject, comprising: administering to the subject in need thereof a daily dose of ambrisentan from 1 mg to about 15 mg, wherein the method is carried out without drug labeling instruction to monitor liver aminotransferase levels during ambrisentan treatment.
Owner:GILEAD SCI INC
Intermediate compound used for preparing Ambrisentan, preparation method thereof, and preparation of Ambrisentan
InactiveCN103420811AHigh yieldAtom utilization is highPreparation from carboxylic acid halidesOrganic compound preparationStereochemistryUtilization rate
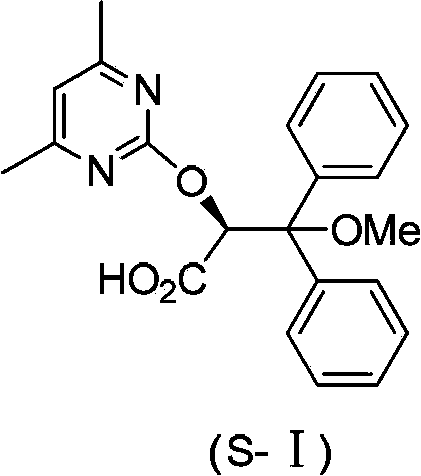
The invention discloses an intermediate IV used for preparing Ambrisentan, a preparation method thereof, and a preparation method of Ambrisentan. The intermediate compound is a compound in a S-shape, is capable of directly synthesizing Ambrisentan without separation, thus the defects in the prior art are overcome, the atom utilization rate of synthesis is improved, the cost is reduced, and the intermediate is suitable for being applied to industrial production. The invention also discloses other intermediates VI, VII, and VIII, which are used for preparing Ambrisentan, and two preparation methods of Ambrisentan.
Owner:SHANGHAI INST OF PHARMA IND
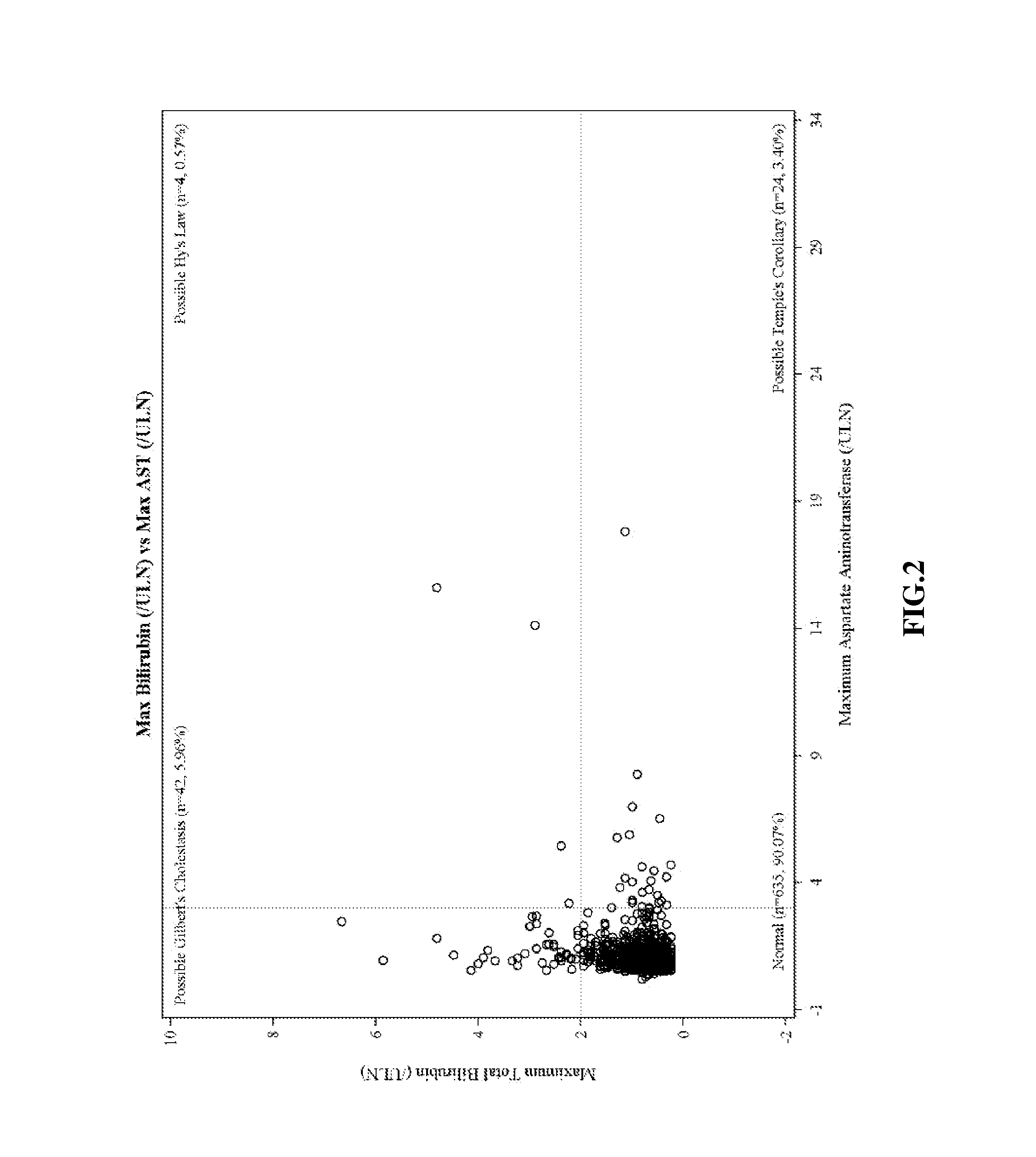
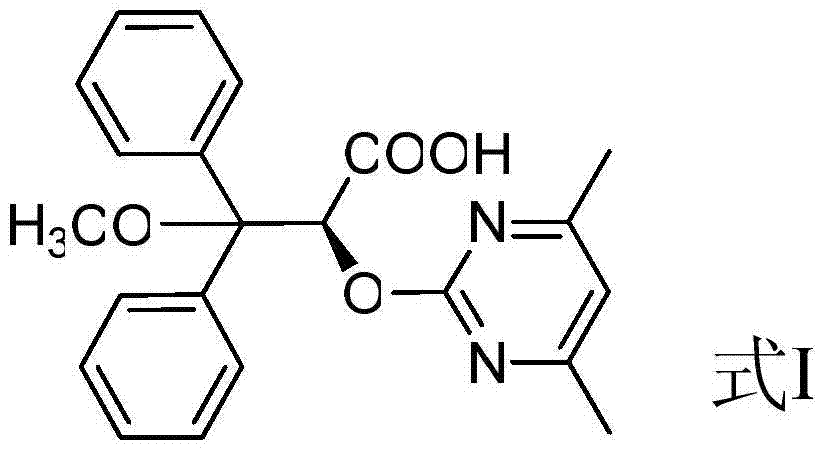
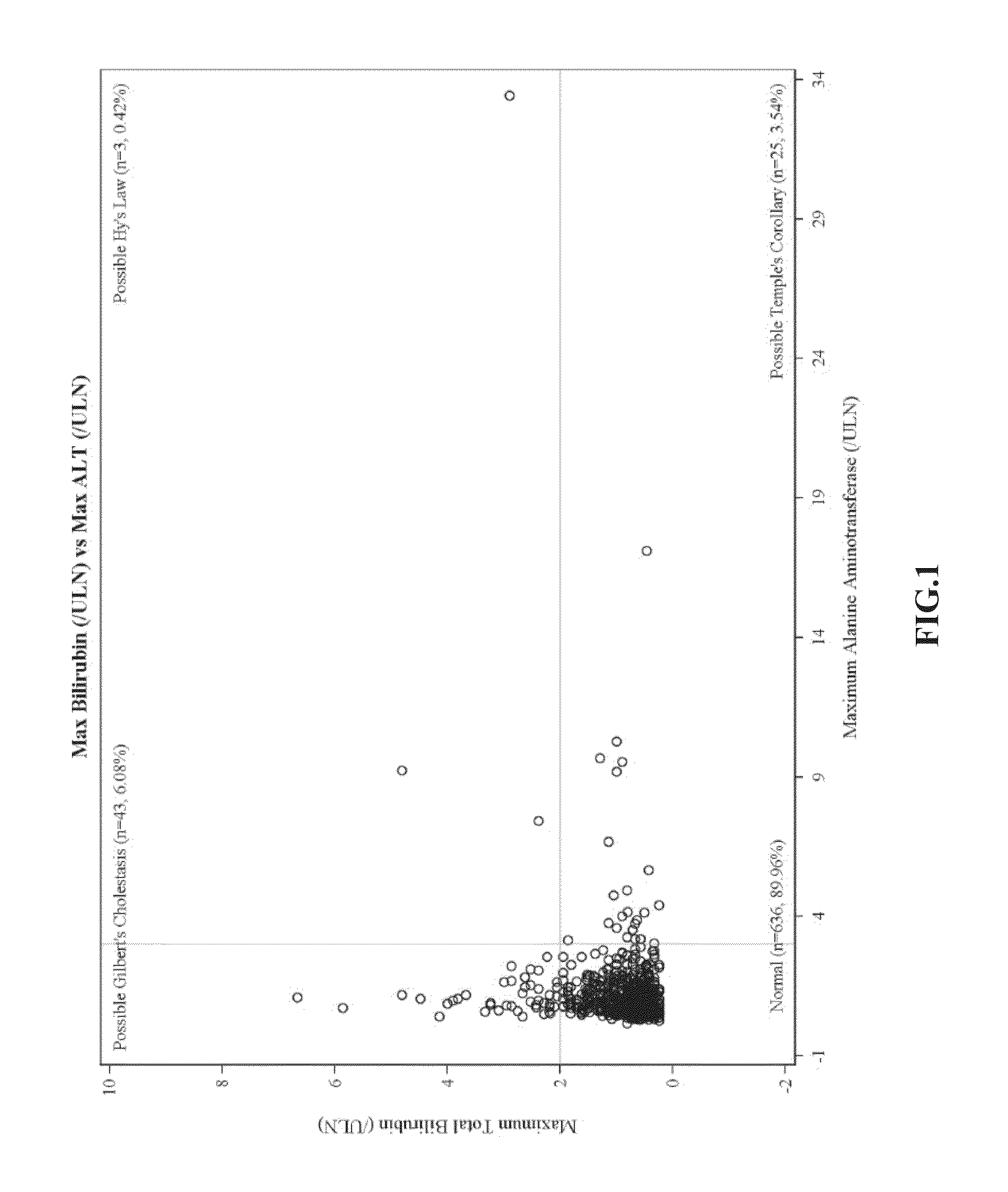
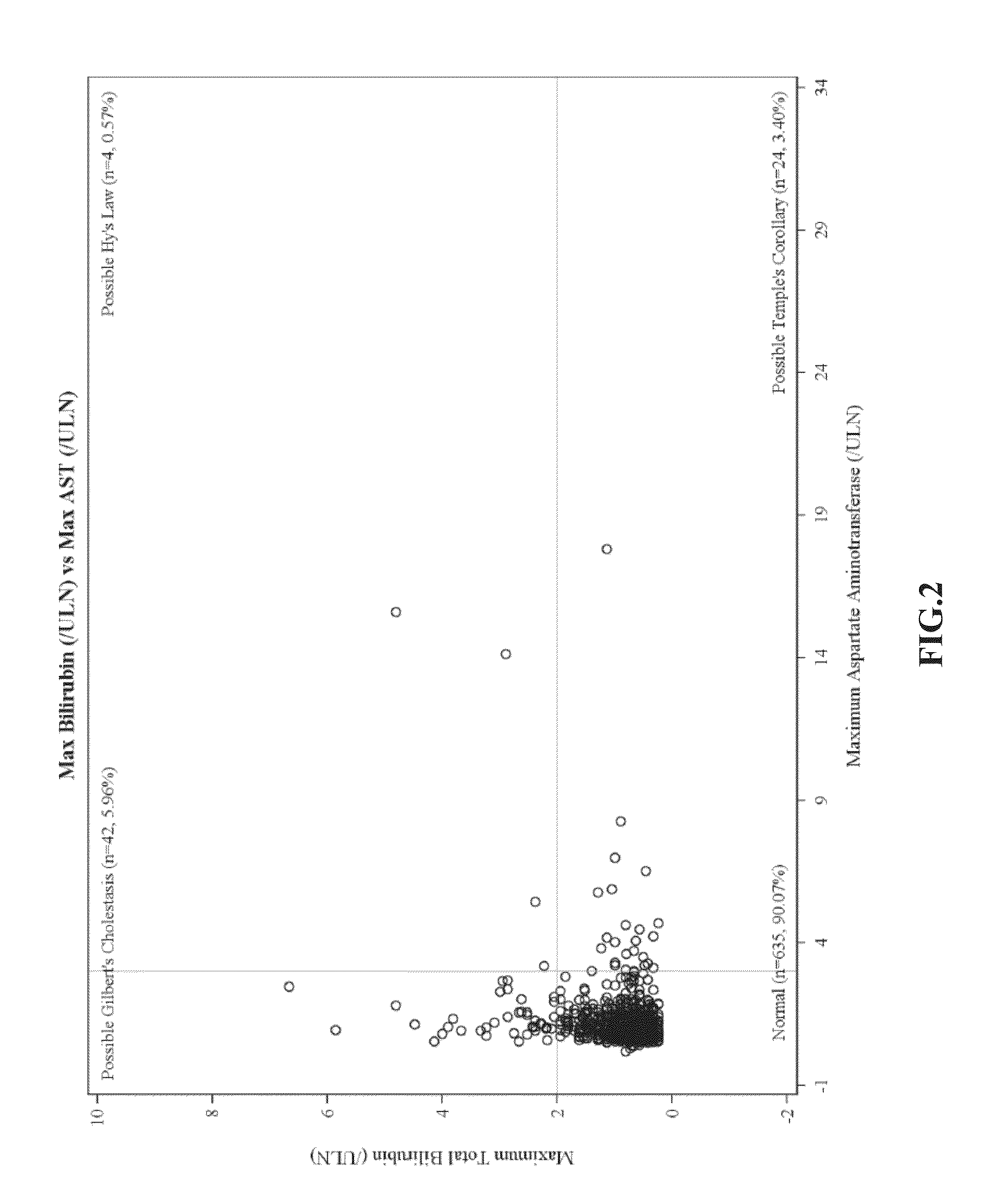
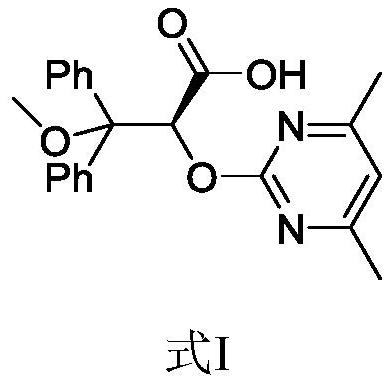
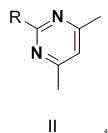
Ambrisentan degradation product and preparation method thereof
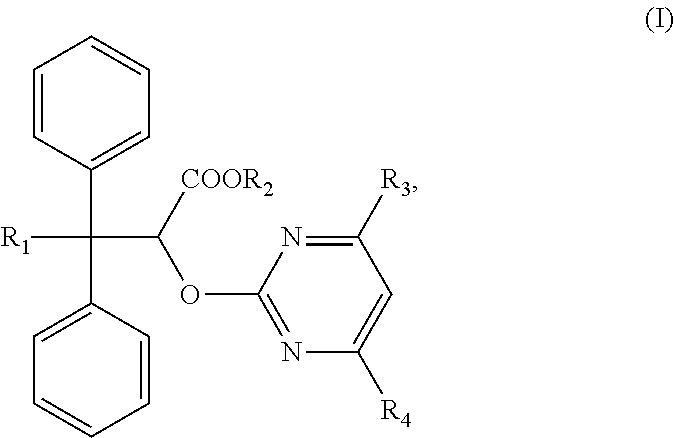
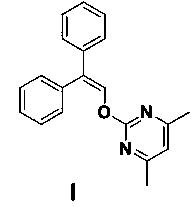
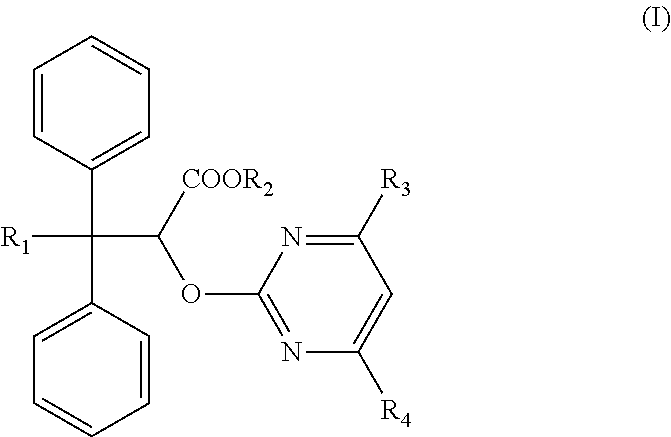
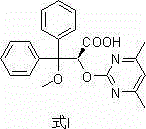
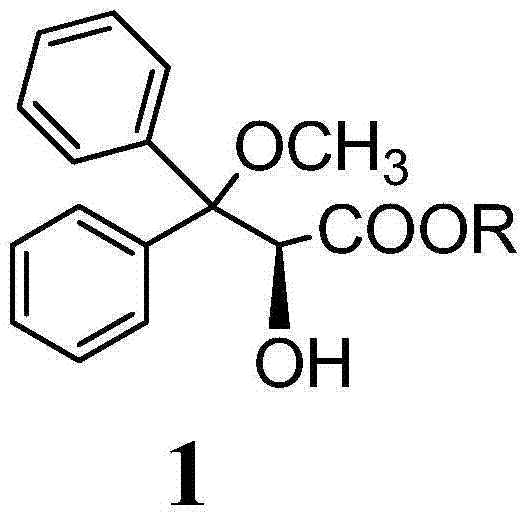
The invention discloses an ambrisentan degradation product and a preparation method thereof. The ambrisentan degradation product is represented by formula I, is one of main impurities of ambrisentan raw materials or preparations thereof and the formula I can be used for analyzing the purity of ambrisentan, and can also be used for controlling the quality of ambrisentan. The preparation method of the product of the formula I is characterized in that a substitution reaction of 2,2-diphenyl acetaldehyde and a compound represented by formula II is carried out under the action of sodium hydride and other alkaline compounds.
Owner:SHANGHAI MEIYUE BIOTECH DEV +1
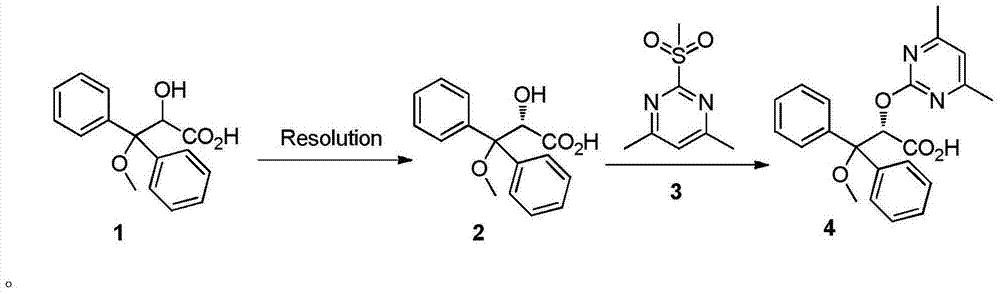
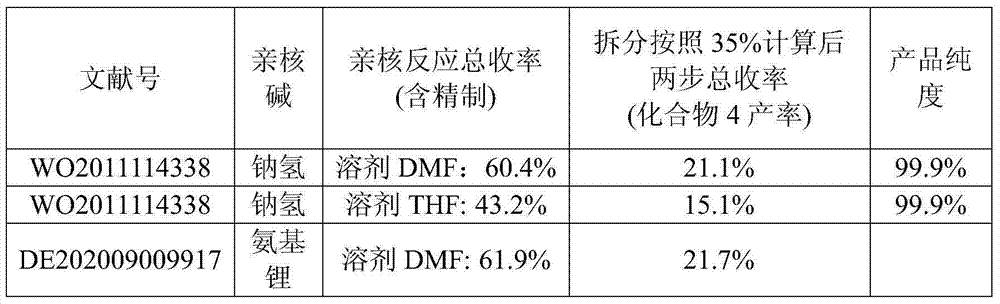
Synthetic method of ambrisentan
The invention relates to a synthetic method of ambrisentan and belongs to the technical field of medicine chemistry. The method comprises the steps of taking a racemoid 1 as a reactant, performing L-proline methyl ester hydrochloride resolution on a compound 1 to obtain a crude product of compound 2, performing nucleophilic reaction on the crude product of the compound 2 and a compound 3 to obtain compound 4. After an enantiomer of the compound 2 of compound 1 is filtered our by resolution, methyl tertiary butyl ether phase obtained by extracting hydrochloric acid free filtrate is used for drying a solvent through distillation to obtain the crude product of the compound 2 which is directly subjected to nucleophilic reaction with the compound 3, a qualified compound 4 is obtained by a subsequent process purifying treatment (comprising decoloration and salifying), the process operation is simplified, process stability is enhanced, loss of the compound 2 caused by crystallization of methylbenzene or crystallization for multiple times is avoided, and cost of process materials is greatly reduced. Lithium amide and sodium-hydrogen are innovatively substituted by lithium hydrate, safety is higher, cost is lower, lithium hydrate is proper in alkalinity, and a reaction system has few impurity points and is suitable for industrialized amplification production.
Owner:宁波人健化学制药有限公司
Method for treating pulmonary arterial hypertension in a patient not having idiopathic pulmonary fibrosis
There is provided a method of treating pulmonary hypertension in a patient in need thereof, said method comprising: administering a therapeutically effective amount of ambrisentan to the patient with pulmonary arterial hypertension, wherein the patient has been determined not to have idiopathic pulmonary fibrosis.
Owner:GILEAD SCI INC
Ambrisentan oral tablets and preparation method thereof
InactiveCN110025587ASimple production processEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionLactose
The invention provides ambrisentan oral tablets and a preparation method thereof. The ambrisentan tablets contain the following ingredients in parts by weight of 1 part of ambrisentan, 8-25 parts of lactose, 3-10 parts of microcrystalline cellulose, 0.3-1.0 part of croscarmellose sodium and 0.05-0.5 part of magnesium stearate. The prepared ambrisentan tablets are smooth in surface, good in contentuniformity, high in dissolution rate, small in dissolution differences between batches, high in in vivo biological availability, simple in preparation technology, easy to operate, low in cost and good in repeatability, and preparation quality and medication safety can be notably elevated.
Owner:CHANGZHOU HANSOH PHARM CO LTD
Process for the preparation of endothelin receptor antagonists
InactiveUS8404840B2Organic chemistryCardiovascular disorderEndothelin receptor antagonistBenzophenone
Owner:MSN LAB PTE LTD
Improved method used for preparing ambrisentan
The invention belongs to the field of chemical synthesis, and discloses an improved method used for preparing ambrisentan. According to the improved method, 2-hydroxy-3-methoxy-3,3-diphenylpropionic ester and 4,6-dimethyl-2-methylsulfonylpyrimidine are subjected to condensation, and one-pot hydrolysis in tetrahydrofuran solvent so as to obtain ambrisentan racemate; and ambrisentan is obtained via resolution with (S)-1-phenylethylamine. The improved method is simple in operation, and high in yield, and is suitable for industrial production.
Owner:山东瑞银生物工程有限公司
Topical ophthalmic formulations of endothelin receptor antagonists
ActiveUS20180110728A1Organic active ingredientsSenses disorderDiabetes mellitusEndothelin receptor antagonist
The present invention relates to a topical ophthalmic formulation comprising at least one antagonist of the endothelin receptor, preferably selected from sitaxentan, ambrisentan, atrasentran, bosentan, macitentan and tezosentan, or a mixture thereof, more preferably bosentan. It also relates to the use of a topical ophthalmic formulation comprising at least one antagonist of the endothelin receptor, preferably selected from sitaxentan, ambrisentan, atrasentran, bosentan, macitentan and tezosentan, or a mixture thereof, more preferably bosentan, as active ingredient for preventing and / or treating the retinal neurodegeneration induced by diabetes and / or aging.
Owner:RETINSET SL
Preparation method of ambrisentan tablet
PendingCN110876731ASimple production processEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsBioavailabilityMedicinal chemistry
The invention provides a preparation method of a ambrisentan tablet, the method is simple in preparation process, easy to operate, low in cost, good in reproducibility and suitable for industrial large-scale production, and the obtained ambrisentan tablet is smooth in surface, good in content uniformity, high in dissolution rate, small in dissolution difference among batches, and high in in-vivo bioavailability.
Owner:CHANGZHOU HANSOH PHARM CO LTD
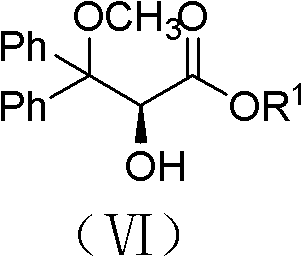
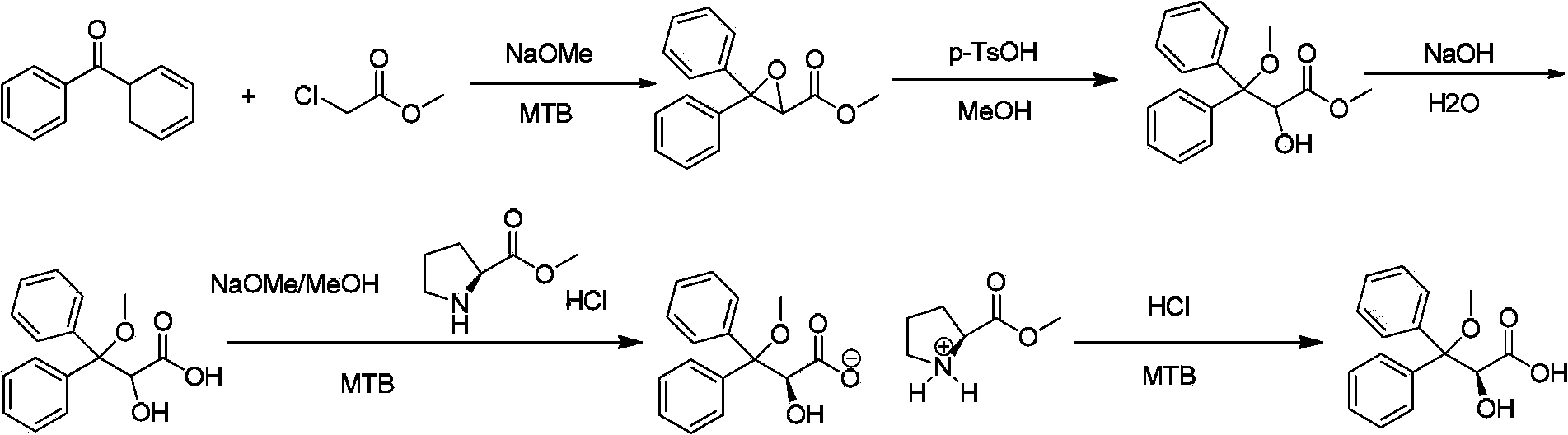
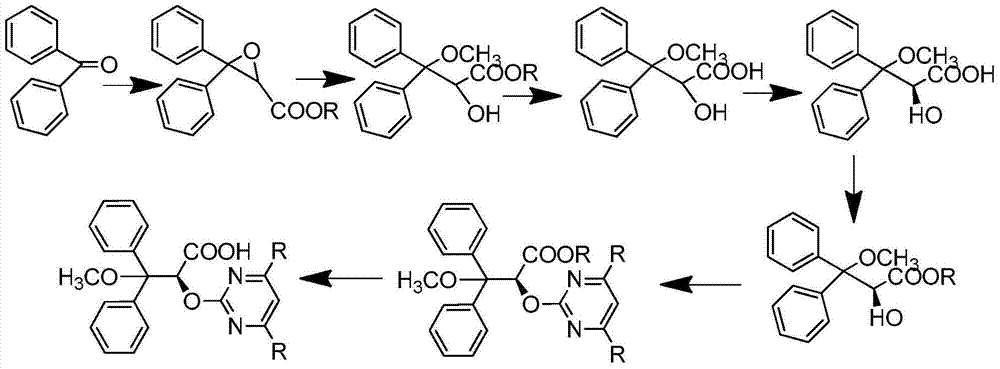
S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid and preparation method thereof
ActiveCN105801404AHigh purityShort synthesis cyclePreparation from carboxylic acid saltsAcid catalyzedSolvent
The invention provides S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid and a preparation method thereof. The preparation method comprises 1, dissolving 2, 3-epoxy-3, 3-dibenzylpropionate in methanol, carrying out acid-catalyzed ring-opening, alkali metal hydroxide hydrolysis and reaction solution condensation, adding water into the condensed solution, filtering the solution after solids are separated, and carrying out solvent washing and purification to obtain refined 2-hydroxy-3-methoxy-3, 3-dibenzylpropionate, and 2, carrying out a reaction process on the refined 2-hydroxy-3-methoxy-3, 3-dibenzylpropionate and methyl L-prolinate, carrying out splitting to obtain methyl S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid-L-prolinate, adding an acid into the methyl S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid-L-prolinate so that the methyl S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid-L-prolinate is free and carrying out extraction, drying, condensation and recrystallization to obtain S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid. The preparation method has simple processes, can produce high purity S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid, can guarantee ambrisentan reaching the standard, can shorten a synthesis period, is free of an acid binding agent in a splitting stage and reduces a cost.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
Purifying method of small-particle-size ambrisentan
InactiveCN109320463AReduce pollutionImprove working environmentOrganic chemistryAcetic acidWorking environment
The invention relates to a purifying method of small-particle-size ambrisentan, and belongs to the technical field of raw material medicine preparation. According to the technical scheme, firstly, anambrisentan crude product is dissolved in ethyl acetate, then temperature is reduced to -5 to 5 DEG C, and under stirring, 50-60% ethanol water with volume 1.5-3 times of that of the solvent in the first step is added in a flowing mode; stirring speed is 230-270 rpm, the speed of adding the ethanol water in a flowing mode is 8-15 ml / min, and after flowing adding is completed, stirring is conductedcontinuously for 4-5 hours; filtering is conducted, 50-60% ethanol water is used for washing filter cake, and drying is conducted. The invention provides a preparation method of the small-particle-size ambrisentan. A raw material medicine which can be used directly is provided for an ambrisentan preparation, environmental pollution is reduced, and a well working environment is provided for preparation workers.
Owner:WEIHAI GUANBIAO INFORMATION TECH
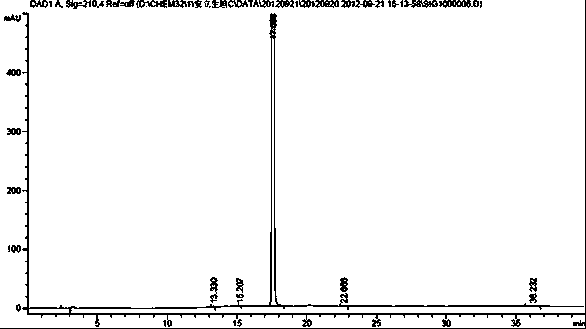
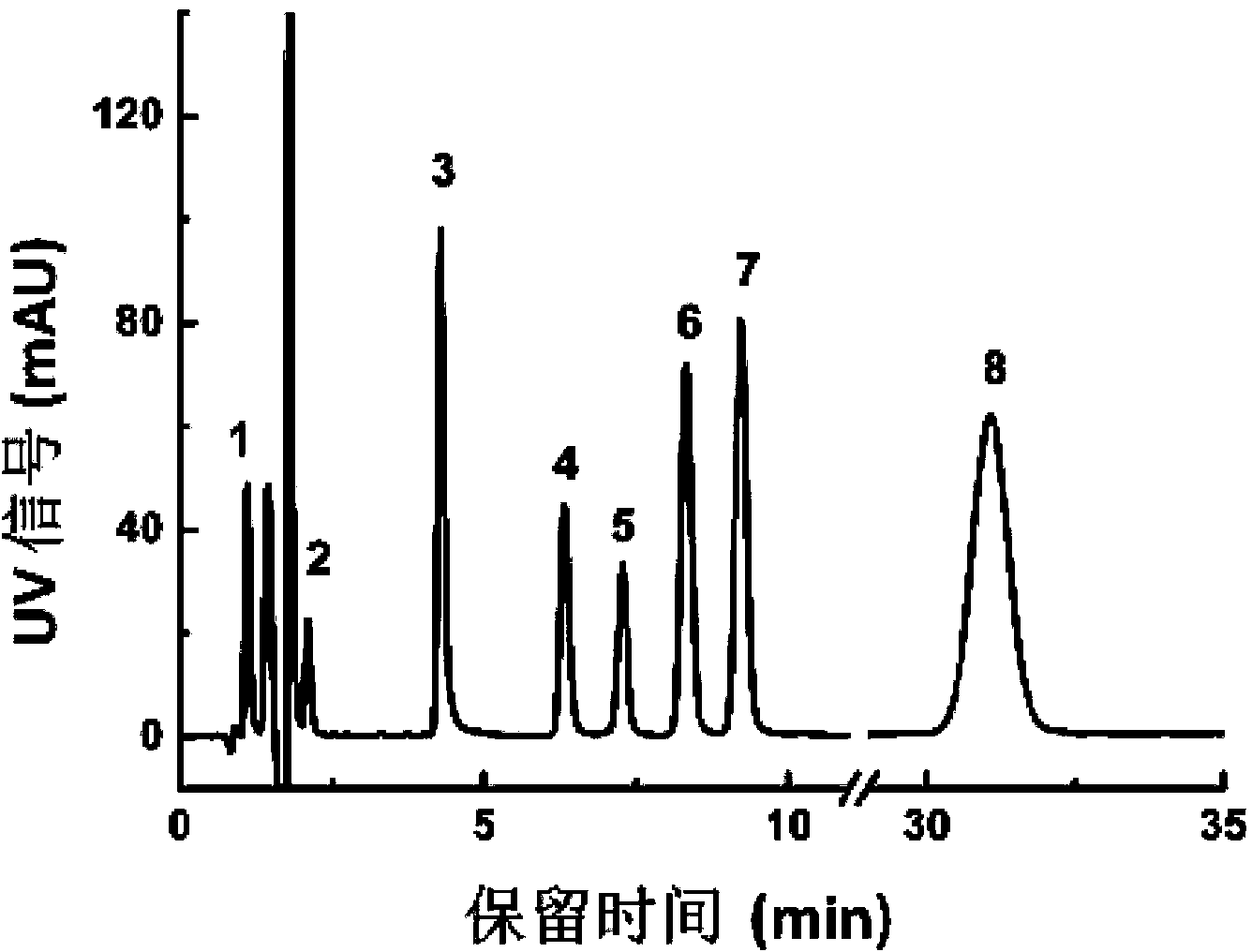
Method for separating and detecting ambrisentan and related substances thereof
The invention belongs to the field of pharmaceutical analysis, and relates to a method of using isocratic elution high performance liquid chromatography to separate ambrisentan and seven related substances thereof. The main technology characteristics comprise taking a C8 or C18 chromatographic column as a stationary phase, taking an acetonitrile / water / glacial acetic acid mixture as a mobile phase, optimizing the flow velocity and the column temperature, and setting the detection wave length to be 190-230 nm, so as to establish a rapid excellent separation system which is suitable for simultaneously separating ambrisentan and seven related substances thereof. The system employs an isocratic elution method and helps to reduce consumption of separation apparatuses, the experiment separation baseline is stationary, the noise is small, and the separation result reappearance is good. The method is wide in separation object, high in practicality, low in cost, friendly to environment, simple in experiment operation and controllable in separation conditions, is a novel quality control method for ambrisentan, also is a new method for detection analysis on seven related substances of ambrisentan, and has wide application.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Purifying method of small-particle-size ambrisentan
InactiveCN109320464AReduce pollutionImprove working environmentOrganic chemistryAcetic acidWorking environment
The invention relates to a purifying method of small-particle-size ambrisentan, and belongs to the technical field of raw material medicine preparation. According to the technical scheme, firstly, anambrisentan crude product is dissolved in ethyl acetate, then temperature is reduced to -5 to 5 DEG C, and under stirring, 50-60% ethanol water with volume 1.5-3 times of that of the solvent in the first step is added in a flowing mode; stirring speed is 230-270 rpm, the speed of adding the ethanol water in a flowing mode is 8-15 ml / min, and after flowing adding is completed, stirring is conductedcontinuously for 4-5 hours; filtering is conducted, 50-60% ethanol water is used for washing filter cake, and drying is conducted. The invention provides a preparation method of the small-particle-size ambrisentan. A raw material medicine which can be used directly is provided for an ambrisentan preparation, environmental pollution is reduced, and a well working environment is provided for preparation workers.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Process for the preparation of ambrisentan and novel intermediates thereof
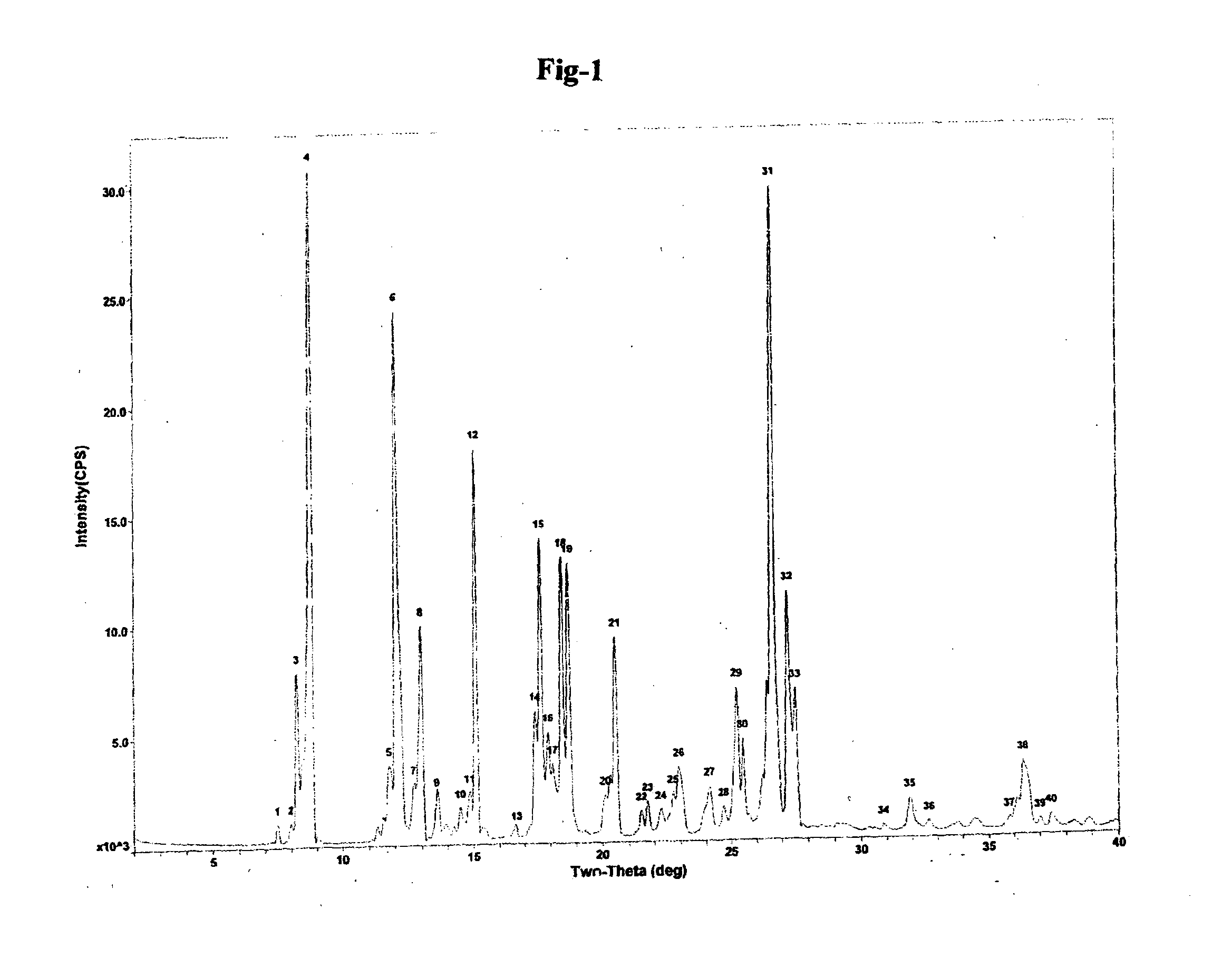
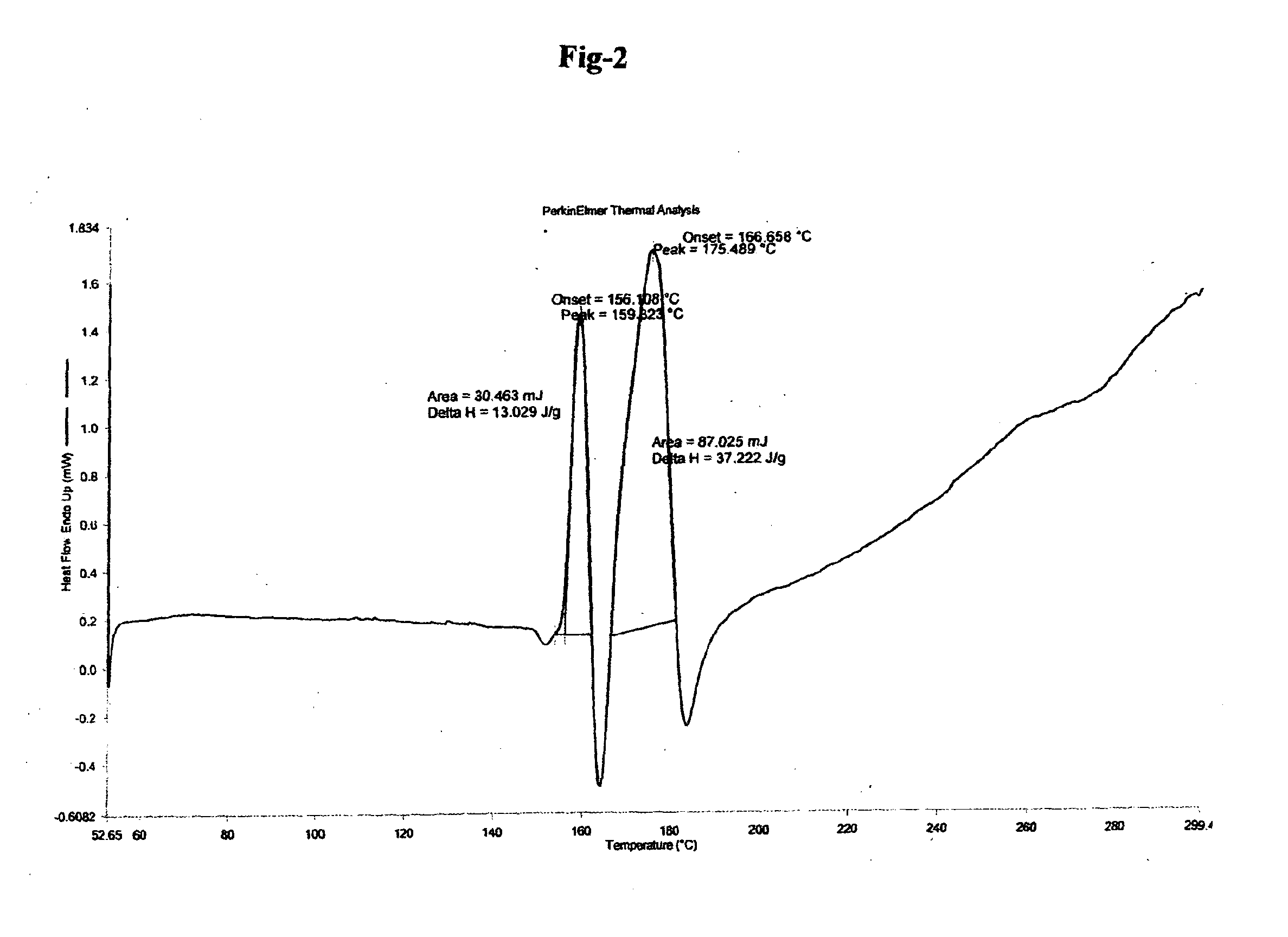
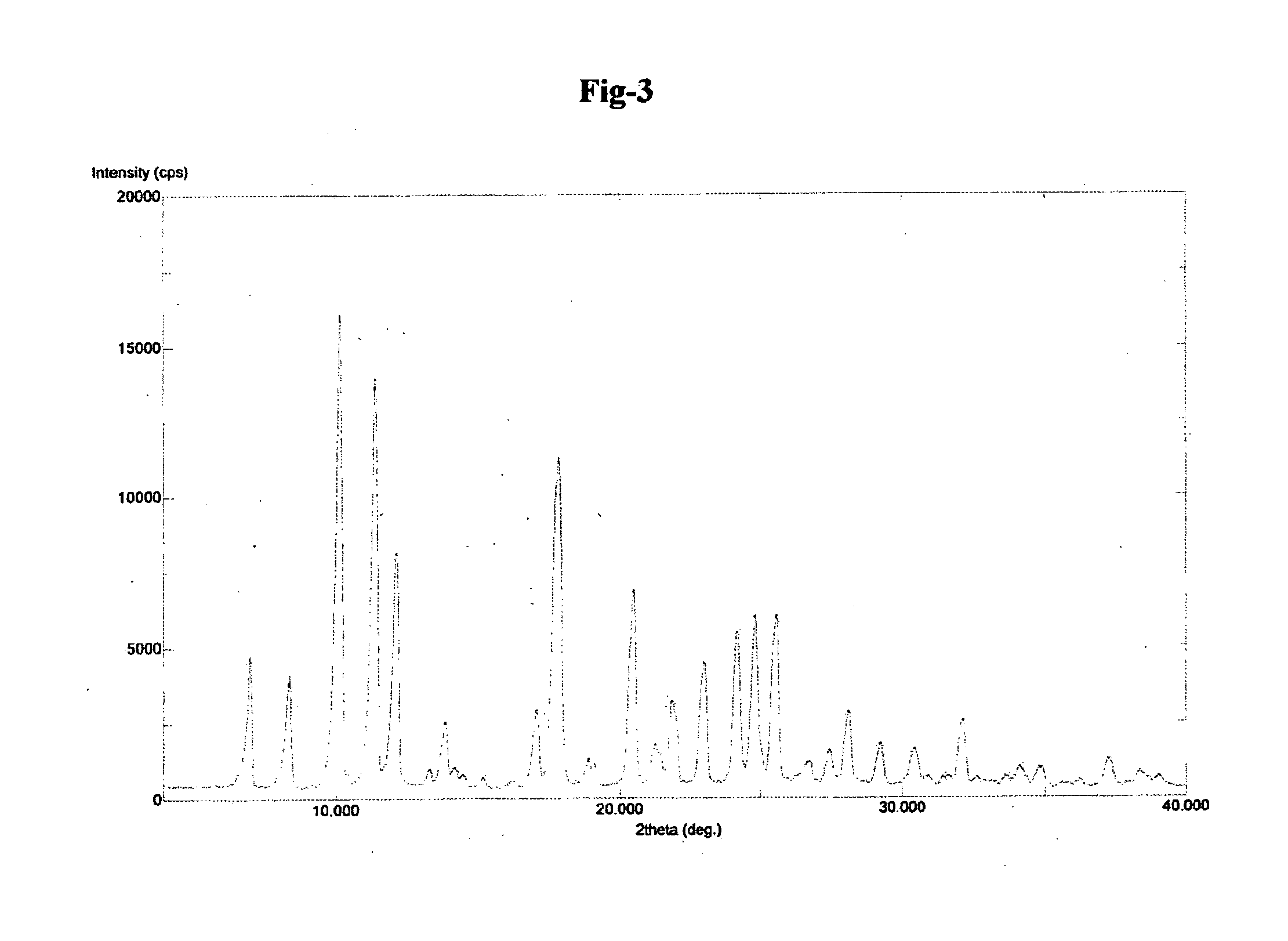
The invention relates to improved processes for the preparation of ambrisentan. The invention also relates to a novel intermediate useful in the preparation of ambrisentan and a process for the preparation of the intermediate. The invention also relates to new polymorphic form of ambrisentan. In particular, it relates to a polymorphic form, designated as Form I of ambrisentan and a process for the preparation of the Form I.
Owner:CADILA HEALTHCARE LTD
Method for treating a pulmonary hypertension condition without companion diagnosis
There is provided a method for treating pulmonary hypertension in a subject, comprising: administering to the subject in need thereof a daily dose of ambrisentan from 1 mg to about 15 mg, wherein the method is carried out without drug labeling instruction to monitor liver aminotransferase levels during ambrisentan treatment.
Owner:GILEAD SCI INC
Preparation method for ambrisentan intermediate compound
InactiveCN103755569ARaw materials are easy to getMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationReagentGrain yield
The invention discloses a preparation method for an ambrisentan intermediate compound. The preparation method comprises the following step: adding a resolving agent into a compound 10 under an acidic condition to carry out a chiral ring-opening reaction to obtain the ambrisentan intermediate compound. According to the preparation method, used raw materials are easy to obtain and the activity and the selectivity almost have no losses after a plurality of times of reactions; the raw materials can be used for a plurality of times; reaction conditions are moderate and steps are simple; the raw materials do not need to react under an ultralow temperature condition and a flammable and combustible reagent is not used, so that the ambrisentan intermediate compound is suitable for large-scale industrial production and the safety is high; the reaction yield is high and the cost is low. The preparation method is used for synthesizing ambrisentan and has a wide application prospect.
Owner:上海皓骏医药科技有限公司
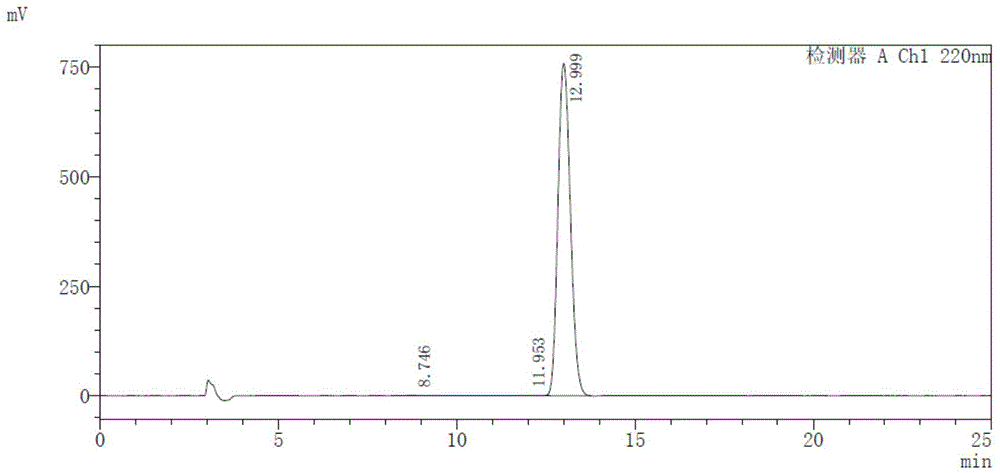
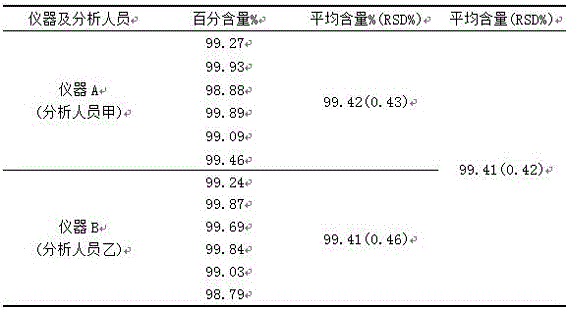
Method for measuring ambrisentan content through high performance liquid chromatography
The invention discloses a method for measuring the ambrisentan content through a high performance liquid chromatography. The chromatographic conditions are as follows: the octadecylsilane bonded silica gel is selected as a filler, the column length is 250 mm, the detection wavelength is 220 nm, the column temperature is 35 DEG C, and the flow velocity is 1.0 ml / min; elution is conducted according to gradient by adoption of a reversed-phase high performance liquid chromatography, the mobile phases are a phosphate buffered solution and a polar organic solvent, the phosphate buffered solution is the A phase, the polar organic solvent is the B phase, and an organic solvent of the B phase in the mobile phases is acetonitrile. According to the method, the reversed-phase high performance liquid chromatography is adopted, a test solution and a contrast solution are prepared, 20 [mu]l of the test solution and the contrast solution are measured respectively to be injected into a chromatographic instrument, a chromatogram is recorded, the peak area is calculated according to an external standard method, and drying calculation is conducted so as to realize measurement of the ambrisentan content. The method fills up the vacancy that no ambrisentan content measurement and analysis method exists in the prior art, can meet the requirements on research, development and production, and can effectively conduct relatively strict and effective control on the ambrisentan content.
Owner:JIANGSU SINOBIOPHARMA
Method for treating a pulmonary hypertension condition without companion diagnosis
Owner:GILEAD SCI INC
A kind of preparation method of ambrisentan
ActiveCN109705042BHigh purityEfficient removalOrganic chemistryBulk chemical productionMethanol waterDrugs synthesis
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Ambrisentan preparation method
ActiveCN108250151AConvenient sourceEasy to disassemble industriallyOptically-active compound separationOrganic racemisationPropanoic acidOrganic solvent
The invention discloses an ambrisentan preparation method. The method uses a resolving agent with better fat solublility (S)-(-)-chlorphenyl ethylamine a nd (R / S)-2-hydroxy-3-methoxy-3,3-diphenyl propionic acid to react, a chiral product further reacts with sodium alcoholate to obtain (2S)-2-hydroxy-3-methoxy-3,3-diphenyl sodium propionate, then an amine metal salt with an organic group R2NNa is taken as the alkali to have a nucleophilic substitution reaction with 4,6-dimethyl-2-methylsulfonyl pyrimidine, water is added to quench the reaction, an organic solvent is used to extract fat solubleimpurities like organic amine, and the ambrisentan is obtained after the acidizing. The dangerousness of the method is low, the post-processing is simple, the process is environmentally friendly, theoperation is easy, the product purity and the yield are high, and the method is quite suitable for industrial production.
Owner:济南周行医药科技有限公司
A method for detecting related substances of ambrisentan
The invention relates to the field of analytical chemistry, and in particular relates to a detection method of ambrisentan related substances. The detection method comprises the following steps: preparing a solution for test products by using a to-be-detected ambrisentan product, diluting the solution for the test products for 100 times to be used as a reference solution, then respectively performing HPLC (High Performance Liquid Chromatography) detection by using a trifluoroacetic acid aqueous solution as a mobile phase A and a trifluoroacetic acid acetonitrile solution as a mobile phase B, and measuring the content of the related substances according to a self-contrasted method of main components added with correction factors. The detection method disclosed by the invention adopts an acetonitrile-water-trifluoroacetic acid gradient elution system, and ensures that a chromatographic peak of ambrisentan has higher separation degree with other related substance peaks, and the chromatographic peak of ambrisentan is relatively high in peak type symmetry, so that the detection method is beneficial to detection on the related substances, has relatively high system suitability, also shows incomparable advantages in specificity, quantitation limit, detection limit, linear range and repeatability, and has relatively high precision degree.
Owner:JIANGSU KANION PHARMA CO LTD
A kind of ambrisentan degradation product and preparation method thereof
The invention discloses an ambrisentan degradation product and a preparation method thereof. The ambrisentan degradation product is represented by formula I, is one of main impurities of ambrisentan raw materials or preparations thereof and the formula I can be used for analyzing the purity of ambrisentan, and can also be used for controlling the quality of ambrisentan. The preparation method of the product of the formula I is characterized in that a substitution reaction of 2,2-diphenyl acetaldehyde and a compound represented by formula II is carried out under the action of sodium hydride and other alkaline compounds.
Owner:SHANGHAI MEIYUE BIOTECH DEV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com