Method for treating a pulmonary hypertension condition
a pulmonary hypertension and pulmonary arterial pressure technology, applied in the direction of biocide, cardiovascular disorder, drug composition, etc., can solve the problems of difficult heart pumping blood through the lungs to be oxygenated, extreme shortness of breath of patients with pulmonary arterial hypertension, and high pulmonary arterial pressure, so as to prolong the life of the subject, improve the prognosis, and reduce the effect of baselin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods Example 1
Patients
[0341]The number of subjects enrolled was 192 at 41 investigative sites.
[0342]Men and women, 18 years of age or older, with idiopathic PAH, PAH associated with connective tissue disease (CTD), e.g., mixed CTD, CREST syndrome, systemic sclerosis (scleroderma), overlap syndrome or systemic lupus erythematosus, or PAH associated with anorexigen use or HIV infection were enrolled in this study. Subjects were to have a documented mean PAP ≧25 mmHg, PVR >3 mmHg / L / min, and PCWP or LVEDP <15 mmHg. Subjects must have been able to walk a distance of at least 150 m but no more than 450 m during 2 consecutive 6MWTs to be eligible for inclusion in the study.
Study Design and Treatment
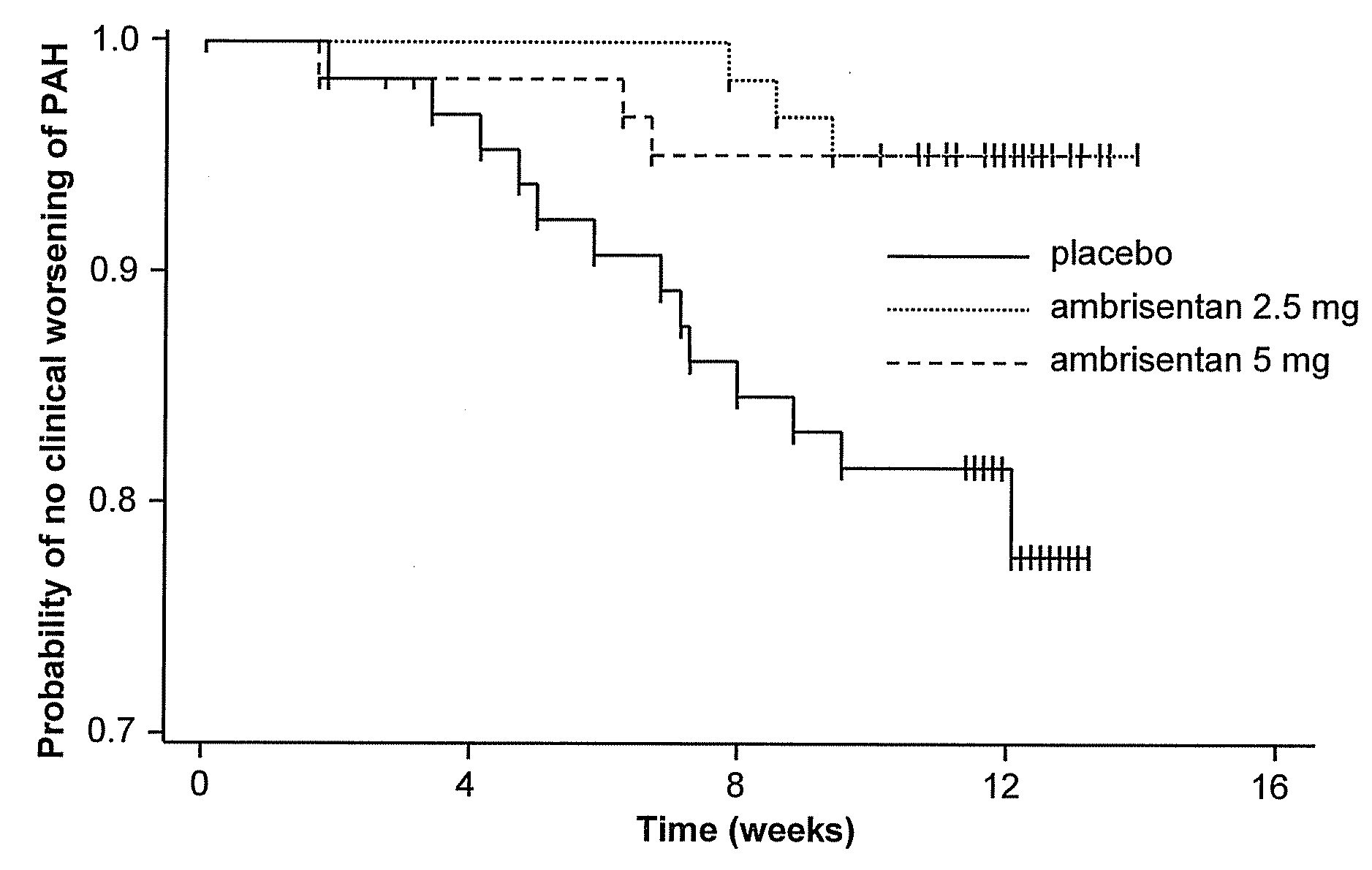
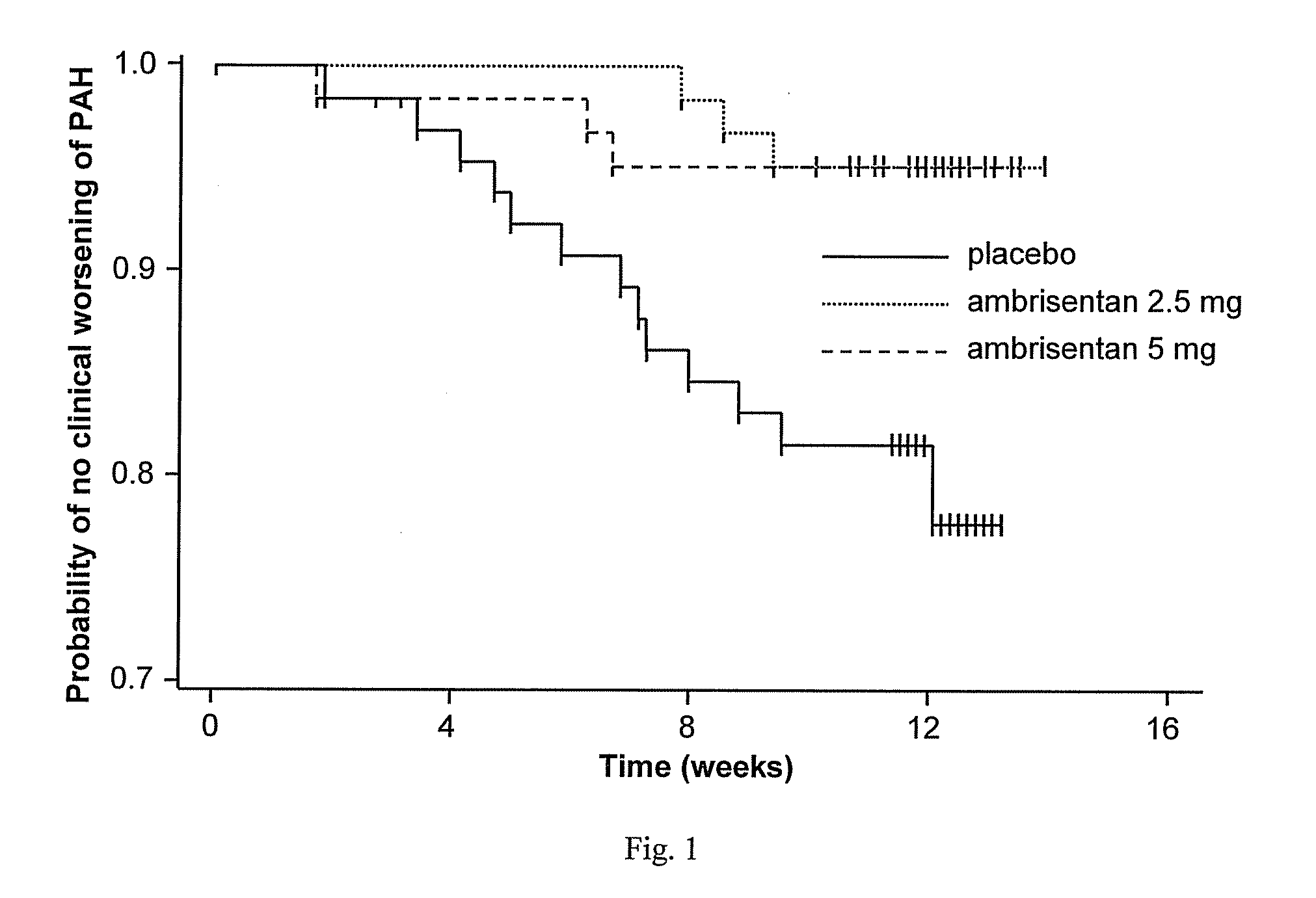
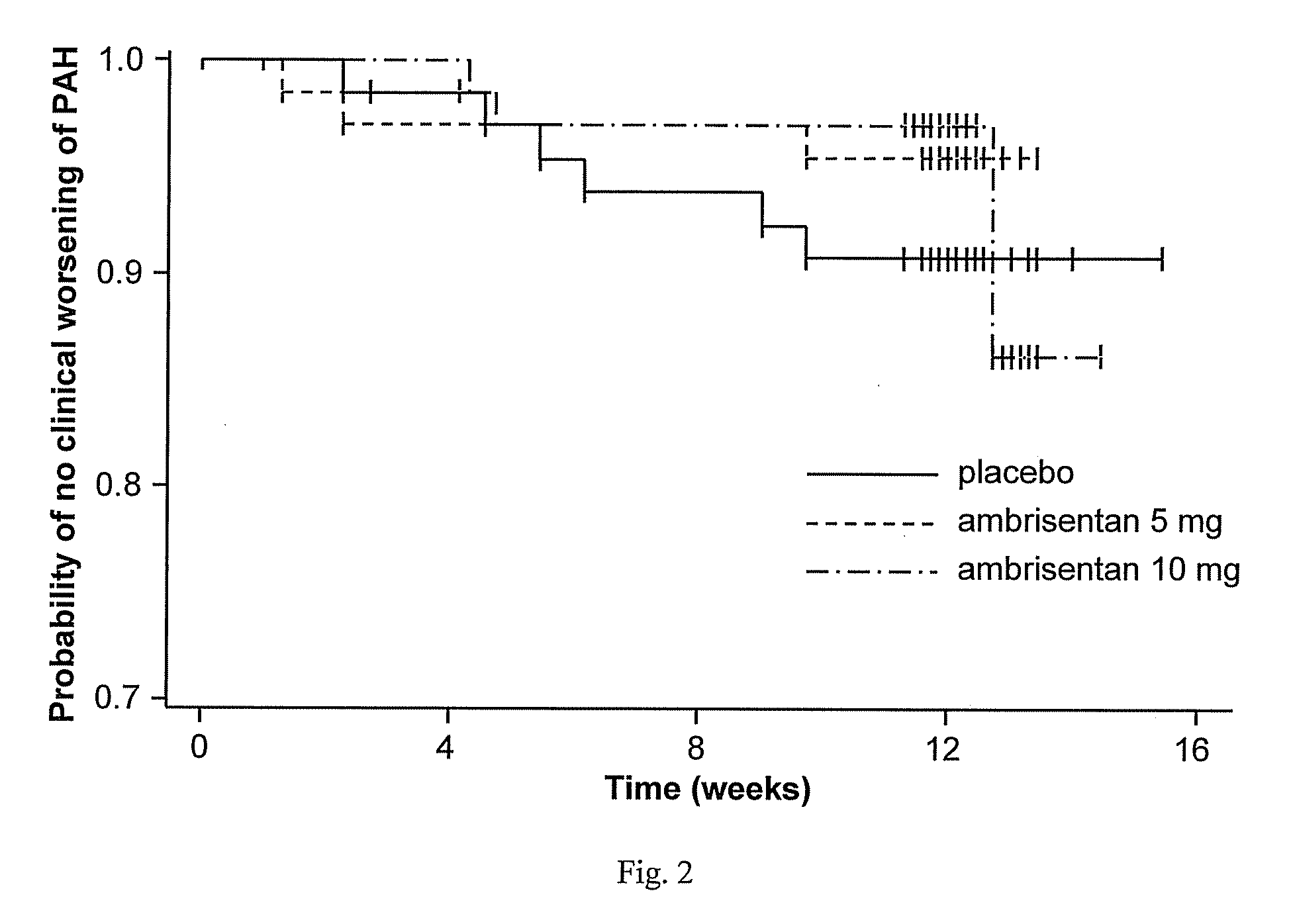
[0343]After a 2 week screening period, eligible subjects were stratified based on the underlying etiology of PAH (idiopathic or non-idiopathic) and were randomized to 1 of 3 treatment groups (placebo, 2.5 mg or 5 mg ambrisentan) in a ratio of 1:1:1. One blinded dose reduction was permitted du...
results example 1
Patients Study Population
[0382]Disposition of randomized subjects is shown in Table 1.1.
TABLE 1.1Subject disposition (% of randomized subjects)2.5 mg5 mgCombinedPlaceboambrisentanambrisentanambrisentan(N = 65)(N = 64)(N = 63)(N = 127)Randomized65(100.0)64(100.0)63(100.0)127(100.0)Completed54(83.1)58(90.6)58(92.1)116(91.3)Withdrew11(16.9)6(9.4)5(7.9)11(8.7)Reasons forwithdrawal:Adverse3(4.6)1(1.6)3(4.8)4(3.1)eventClinical1(1.5)0(0.0)0(0.0)0(0.0)status did notimprove ordeterioratedWithdrawal0(0.0)3(4.7)1(1.6)4(3.1)of consentEarly escape7(10.8)2(3.1)1(1.6)3(2.4)
[0383]A total of 192 subjects, with a mean age of 50.9 years, received at least 1 dose of study drug and were included in the ITT and safety populations. A majority of the subjects enrolled were female (74.5%) and Caucasian (84.9%). Approximately half (51.6%) of the subjects were residents of western Europe or Israel. The remainder of subjects was evenly distributed throughout eastern Europe (24.0%) and South America (24.5%).
[03...
discussion example 1
[0419]The ambrisentan treatment benefit observed by the primary and secondary endpoints of this study was robust, internally consistent, and clinically relevant.
[0420]Both doses demonstrated a statistically significant and clinically relevant improvement in 6MWD that was associated with a significant decrease in BDI. The improvement in 6MWD was nearly twice as large in the 5 mg dose group compared to the 2.5 mg dose group, and improvements in 6MWD were consistently dose-responsive for most subgroups evaluated. Furthermore, plasma BNP, a molecular marker that has been shown to decrease in patients with PAH who demonstrate improvements in 6MWD or hemodynamics, was substantially reduced with ambrisentan treatment. Ultimately, these symptomatic improvements resulted in a patient's self-assessment of an overall better quality of life, as measured by statistically significant improvements in several scales of the SF-36® health survey.
[0421]In addition to the symptomatic improvements obser...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| mean pulmonary arterial pressure | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com