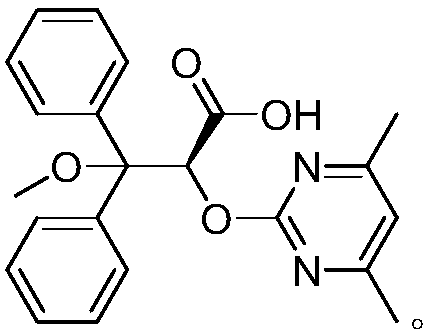
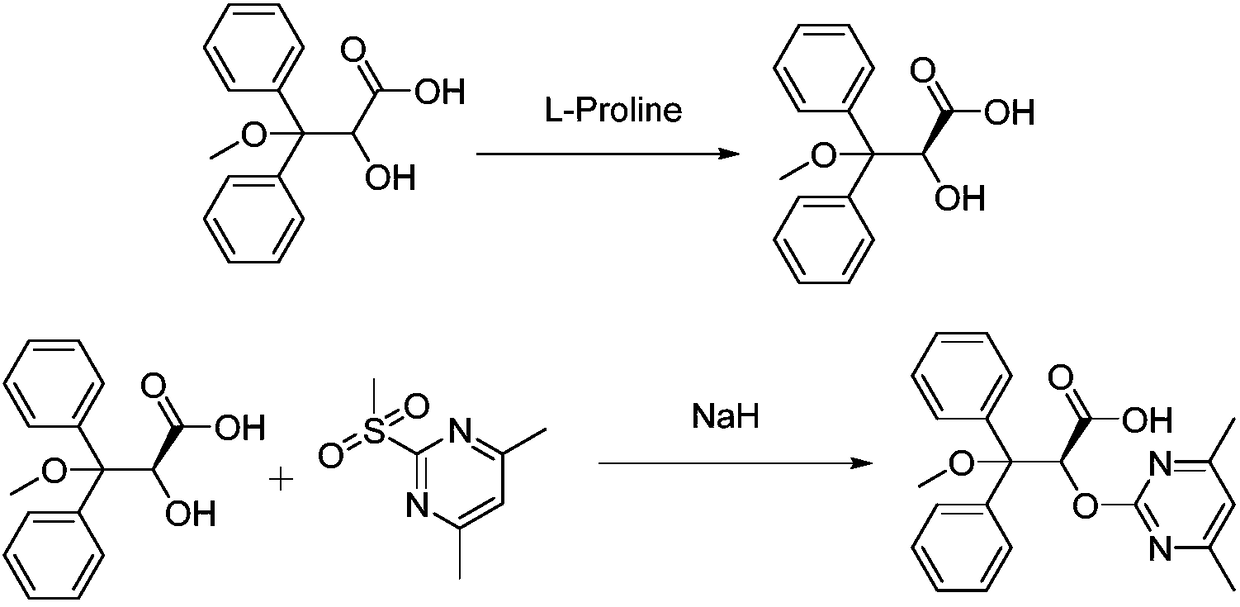
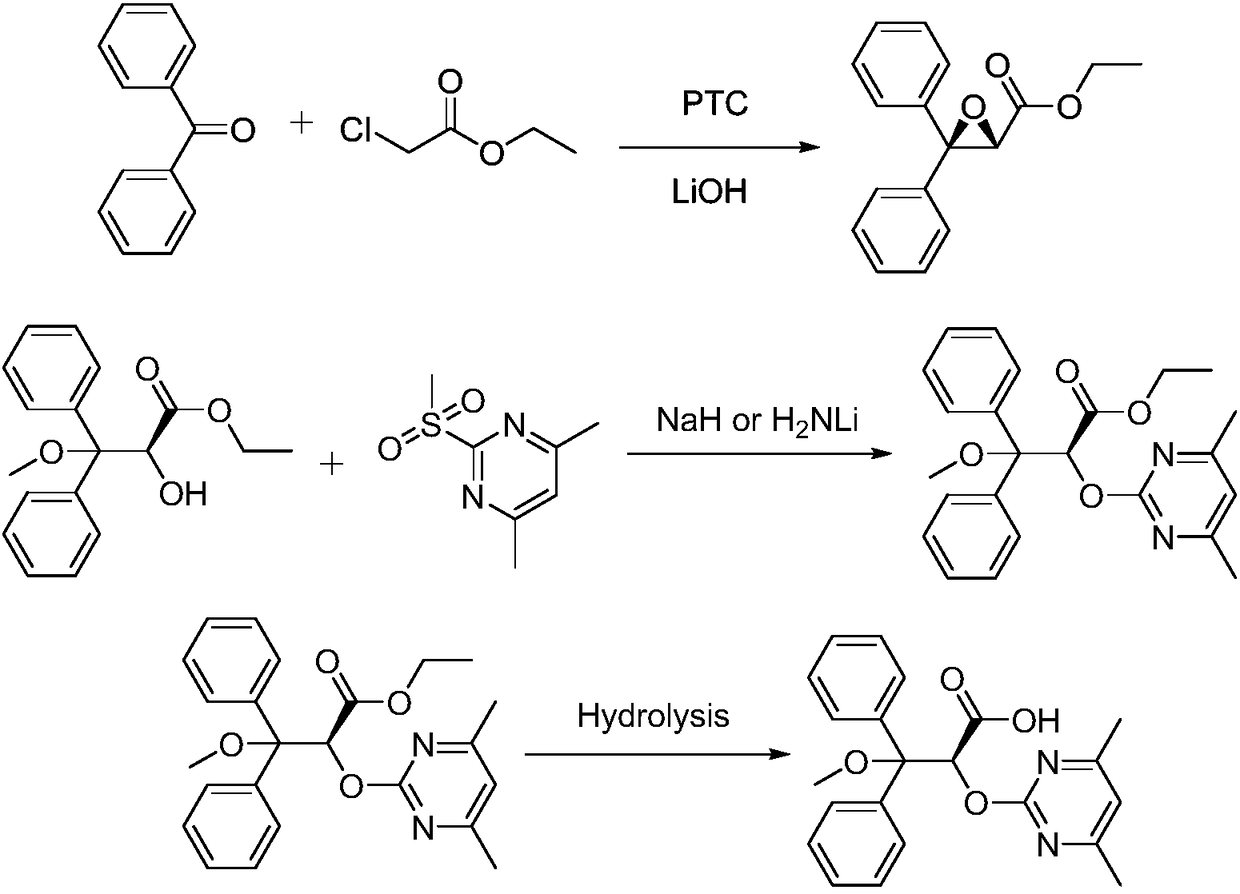
Ambrisentan preparation method
A technology of ambrisentan and hydroxyl, which is applied in the field of preparation of ambrisentan, can solve the problems of cost, production capacity and recycling, risk reduction, difficult operation, etc., and achieve easy industrial separation, risk reduction, easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Put 27.2g (R / S)-2-hydroxy-3-methoxy-3,3-diphenylpropionic acid into a 250mL reaction flask, add 136mL isopropanol, stir, add 7.8g (S )-(-)-1-(2-chlorophenyl)ethanamine, kept at 20°C and stirred for 2h, the chiral product (S)-2-hydroxy-3-methoxy-3,3-diphenylpropionic acid The (S)-(-)-2-chlorophenylethylamine salt was precipitated; suction filtration, the filter cake was washed with isopropanol, dried by suction, and set aside. (R)-2-hydroxy-3-methoxy-3,3-diphenylpropionic acid in isopropanol solution can be recycled.
[0035] The filter cake was dissolved in 65 mL of ethyl acetate, stirred, slowly added with 4.05 g of sodium methoxide, slowly cooled to 0 °C, and kept for 1 h, the product (2s)-2-hydroxy-3-methoxy-3,3-diphenyl Sodium propionate was precipitated; suction filtration, washed the filter cake with a small amount of isopropanol, and air-dried at 40-50°C to obtain 13.9 g of product, yield 45.1%, HPLC purity: 99.22%, chiral purity: 99.80%. The resolving agen...
Embodiment 2
[0038]Put 27.2g (R / S)-2-hydroxy-3-methoxy-3,3-diphenylpropionic acid into a 250mL reaction flask, add 136mL ethanol, stir, add 7.8g (S)-(-) -1-(3-Chlorophenyl)ethylamine, kept at 20°C and stirred for 3 hours, filtered with suction, washed the filter cake with ethanol, sucked dry, dissolved the filter cake in 65 mL of ethyl acetate, stirred, and slowly added 5.10 g of sodium ethoxide, Slowly lower the temperature to 5°C, keep the temperature for 2h, filter with suction, wash the filter cake with a small amount of ethanol, and blow dry at 40-50°C to obtain (2s)-2-hydroxy-3-methoxy-3,3-diphenylpropane Sodium 13.2g, yield 42.8%, HPLC purity: 99.06%, chiral purity: 99.85%.
[0039] Put 30.8g of (2s)-2-hydroxy-3-methoxy-3,3-diphenylpropionate sodium into a 500mL reaction flask, add 124mL of tetrahydrofuran, stir, control the temperature to -5-5°C, and dropwise add 2mol / L 55mL of tetrahydrofuran solution of sodium di(isopropyl)amide, temperature controlled at 0-10°C, 22.3g of tetra...
Embodiment 3
[0041] Put 27.2g (R / S)-2-hydroxy-3-methoxy-3,3-diphenylpropionic acid into a 250mL reaction flask, add 136mL methanol, stir, add 7.8g (S)-(-) -1-(4-Chlorophenyl)ethylamine, kept at 20°C and stirred for 3 hours, filtered with suction, washed the filter cake with methanol, sucked dry, dissolved the filter cake in 65 mL of ethyl acetate, stirred, and slowly added 4.05 g of sodium methoxide, Slowly lower the temperature to 2°C, keep the temperature for 2h, filter with suction, wash the filter cake with a small amount of methanol, and blow dry at 40-50°C to obtain (2s)-2-hydroxy-3-methoxy-3,3-diphenylpropane Sodium 12.9g, yield 42.0%, HPLC purity: 99.10%, chiral purity: 99.83%;
[0042] Put 30.8g of (2s)-2-hydroxy-3-methoxy-3,3-diphenylpropionate sodium into a 500mL reaction flask, add 124mL of tetrahydrofuran, stir, control the temperature to -5-5°C, and dropwise add 2mol / L 55 mL of tetrahydrofuran solution of sodium bis(ethyl)amide, temperature controlled at 0-10 °C, 22.3 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com