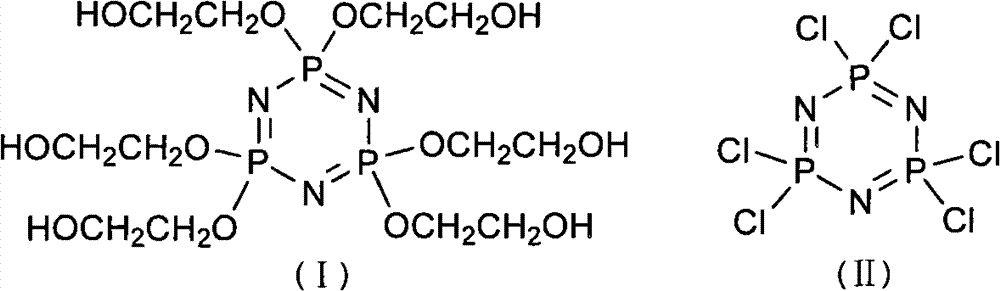
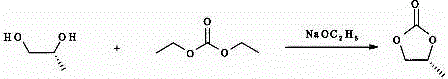
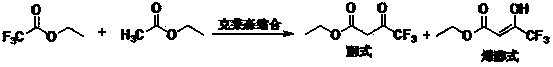Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
417 results about "Sodium ethoxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium ethoxide (also is the organic compound with the formula C₂H₅ONa) is a white to yellowish powder that dissolves in polar solvents such as ethanol. It is commonly used as a strong base.
Poly-beta-peptides from functionalized beta-lactam monomers and antibacterial compositions containing same
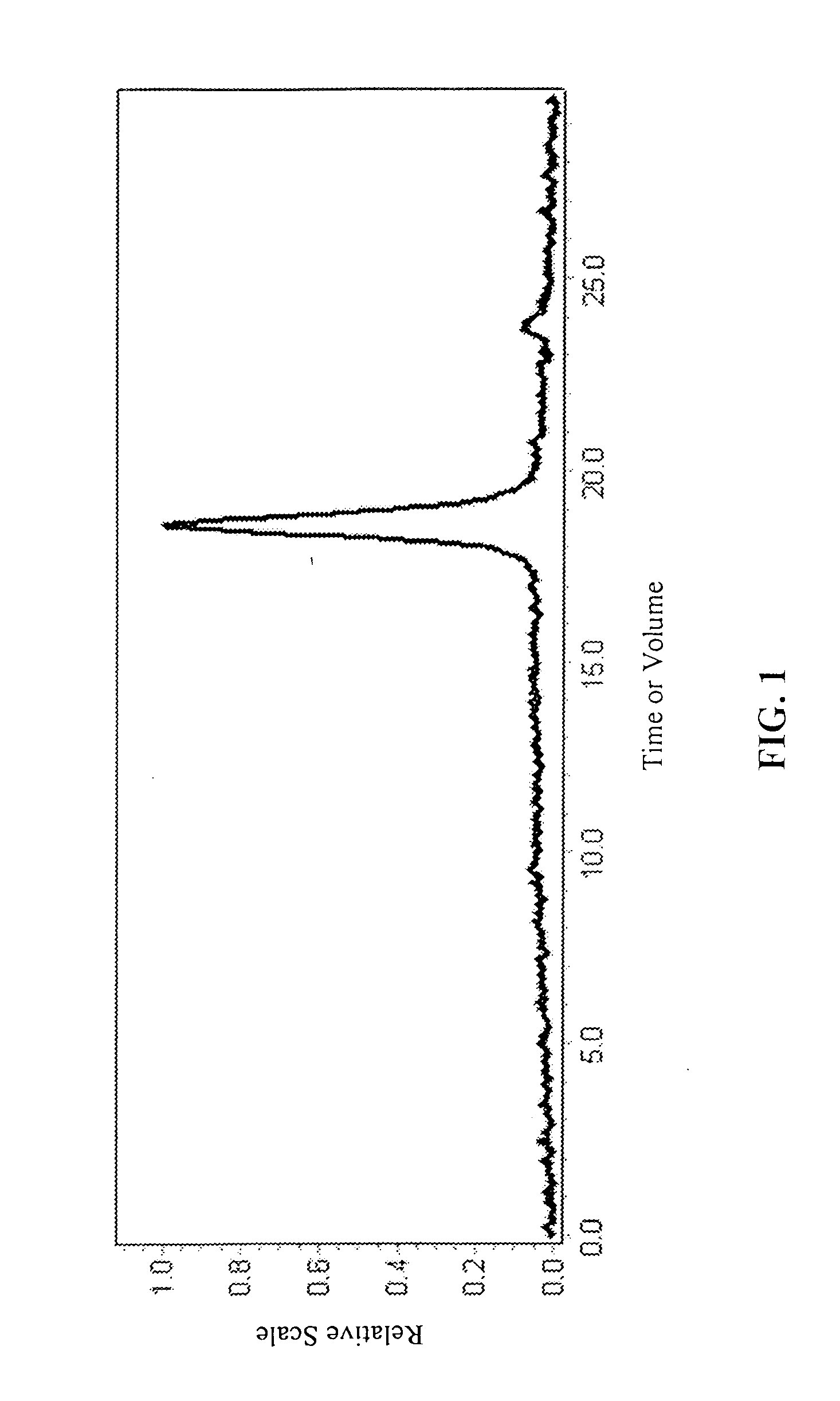
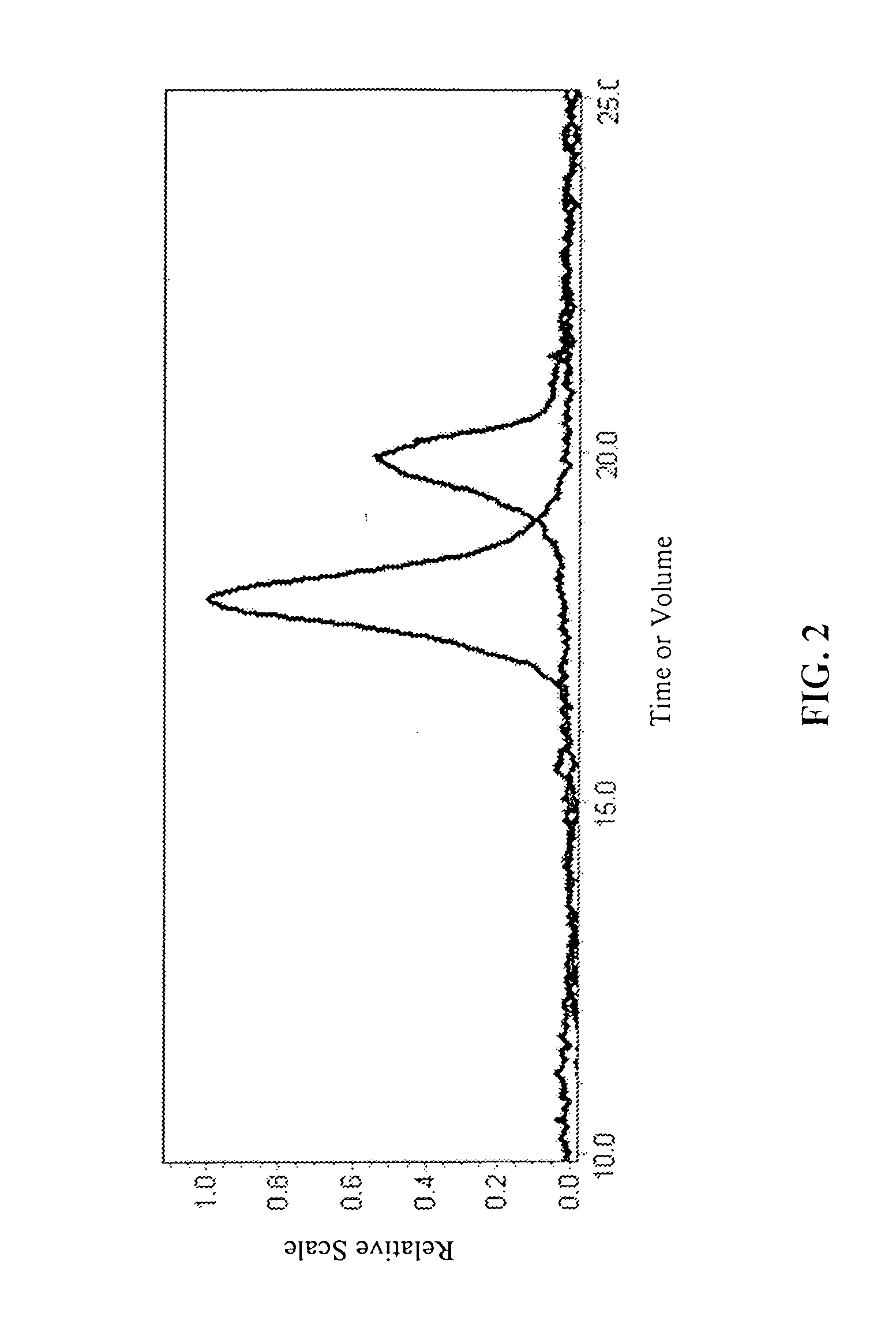
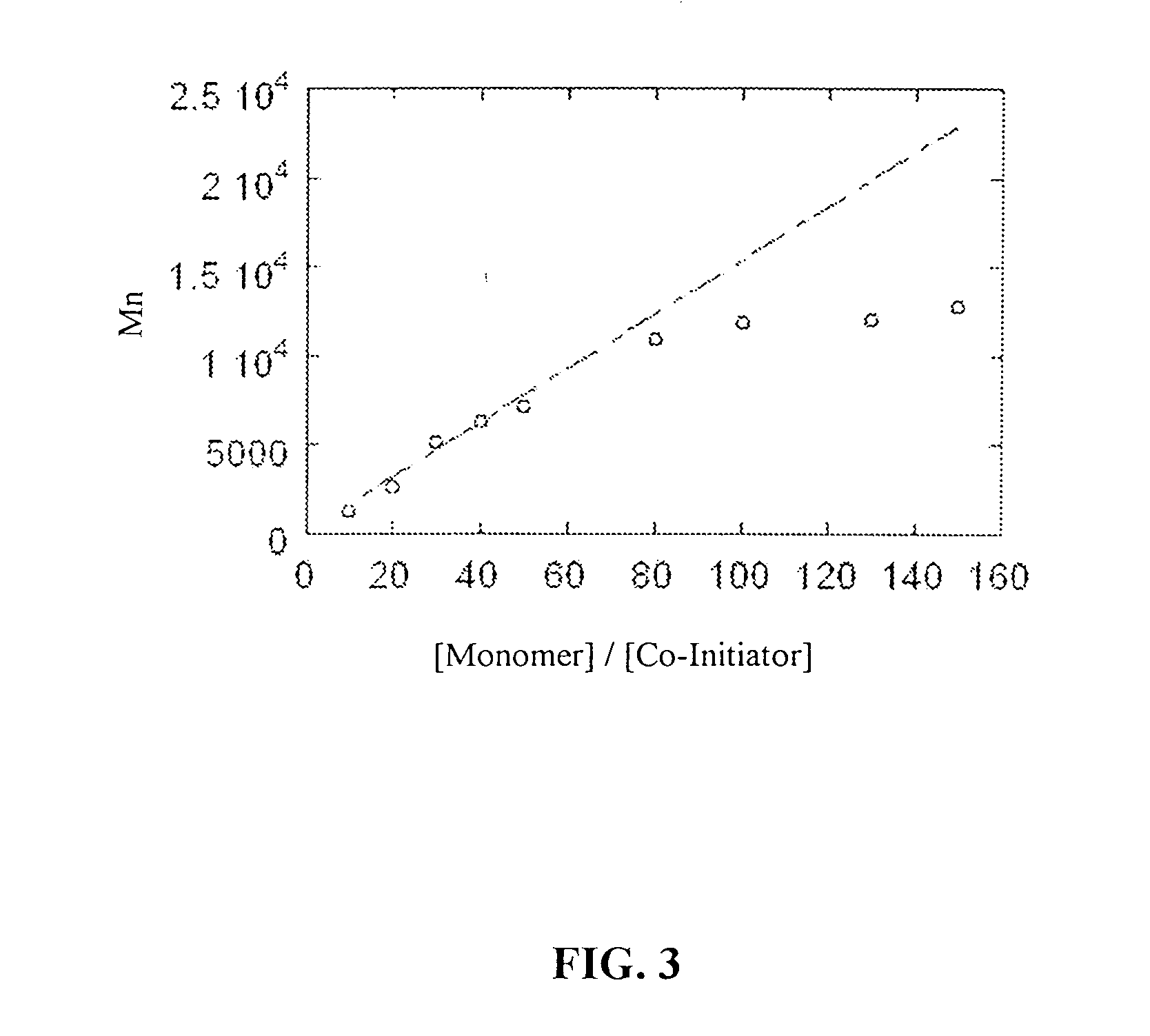
InactiveUS20070087404A1Control over polymerization conditionLarge molecular weightAntibacterial agentsPeptide/protein ingredientsMonomerTetrahydrofuran
Disclosed is a method of making β-polypeptides. The method includes polymerizing β-lactam-containing monomers in the presence of a base initiator and a co-initiator which is not a metal-containing molecule to yield the product β-polypeptides. Specifically disclosed are methods wherein the base initiator is potassium t-butoxide, lithium bis(trimethylsilyl)amide (LiN(TMS)2), potassium bis(trimethyl-silyl)amide, and sodium ethoxide, and the reaction is carried out in a solvent such as chloroform, dichloromethane, dimethylsulfoxide, or tetrahydrofuran.
Owner:WISCONSIN ALUMNI RES FOUND
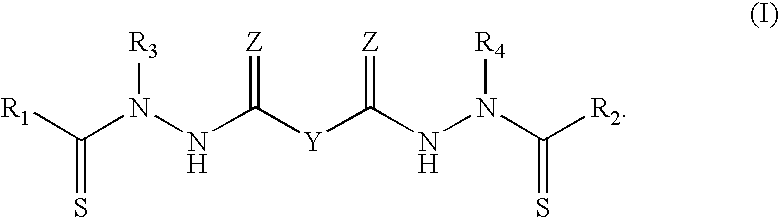
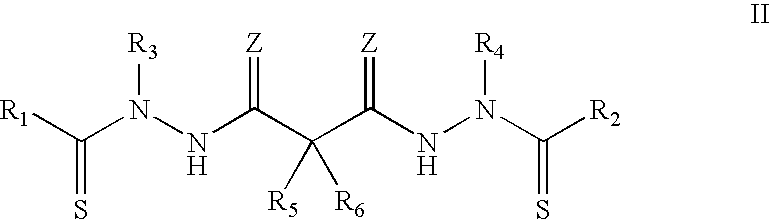
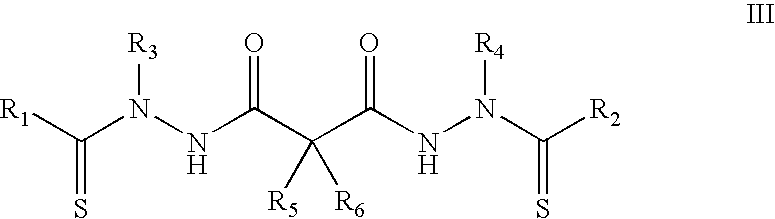
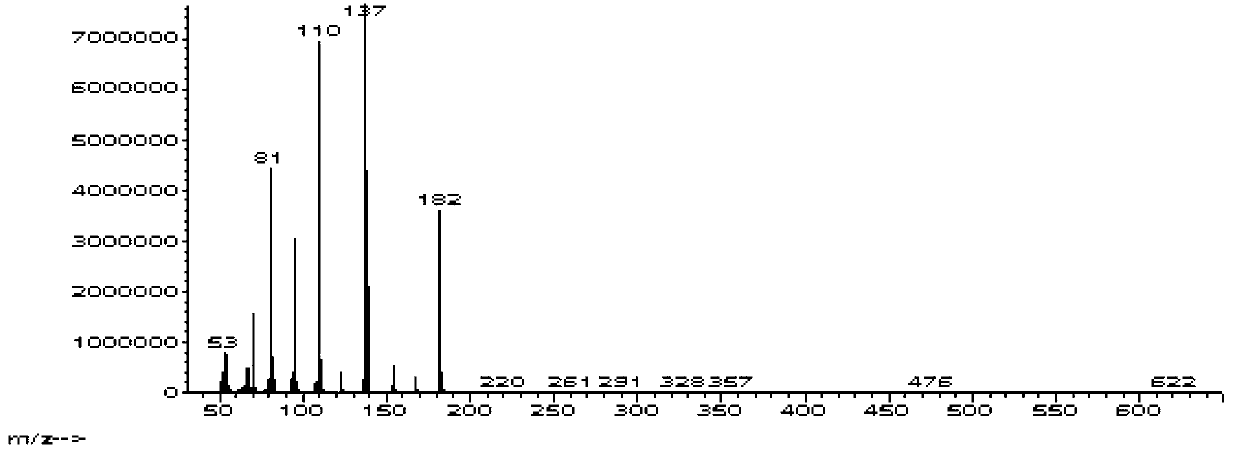
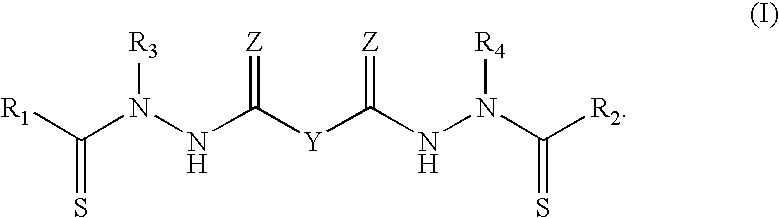
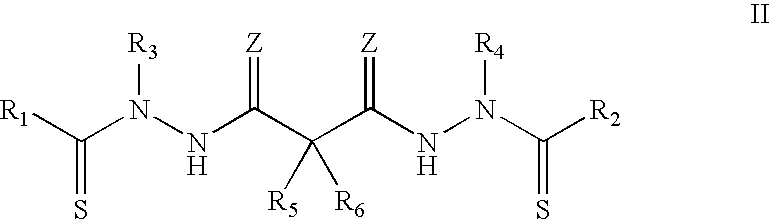
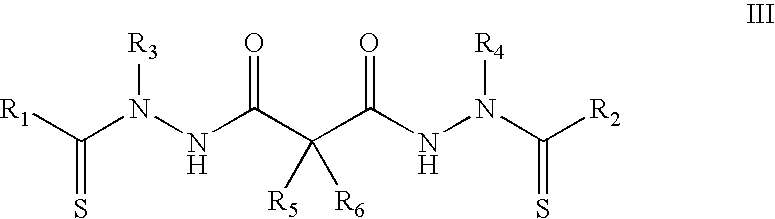
Synthesis of bis(thio-hydrazide amide) salts
InactiveUS20060270873A1Easy to disassembleLower levelOrganic chemistryOrganic compound preparationThio-Potassium hydroxide
A method of preparing a bis(thio-hydrazide amide) disalt includes the steps of combining a neutral bis(thio-hydrazide amide), an organic solvent and a base to form a bis(thio-hydrazide amide) solution; and combining the solution and methyl tert-butyl ether, thereby precipitating a disalt of the bis(thio-hydrazide amide). In some embodiments, a method of preparing a bis(thio-hydrazide amide) disalt includes the steps of combining a neutral bis(thio-hydrazide amide) and an organic solvent selected from methanol, ethanol, acetone, and methyl ethyl ketone to make a mixture; adding at least two equivalents of a base selected from sodium hydroxide, potassium hydroxide, sodium methoxide, potassium methoxide, sodium ethoxide and potassium ethoxide to the mixture, thereby forming a solution; and combining the solution and methyl tert-butyl ether to precipitate the disalt of the bis(thio-hydrazide amide). The disclosed methods do not require lyophylization and the solvents used in the process can be more readily removed to low levels consistent with pharmaceutically acceptable preparation.
Owner:SYNTA PHARMA CORP
Solid basic catalyst, preparation method of solid basic catalyst and application of solid basic catalyst in ester exchange reaction
InactiveCN102698811AImprove stabilityImprove catalytic performanceOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from organic carbonatesOrganic baseSilicon oxide
The invention discloses a solid basic catalyst, a preparation method of the solid basic catalyst and an application of the solid basic catalyst in ester exchange reaction. The solid basic catalyst catalyzes the ester exchange reaction by the reaction of metal organic compound and hydroxyl on a carrier under the moderate conditions to synthesize dimethyl carbonate. The solid basic catalyst provided by the invention consists of metal organic alkali and the carrier. The metal organic alkali is linked to the carrier in bond-forming manner; the metal organic alkali is one or more of lithium methoxide, lithium ethoxide, lithium isopropoxide, lithium n-butoxide, lithium tert-butoxide, sodium methoxide, sodium ethoxide, sodium isopropoxide, sodium n-butoxide, sodium tert-butoxide, potassium methoxide, potassium ethoxide, potassium isopropoxide, potassium n-butoxide and potassium tert-butoxide; the carrier is one or more of silicon oxide, aluminium oxide, titanium oxide, zirconia, mesoporous silicon oxide synthesized by the template method, mesoporous aluminium oxide synthesized by the template method, mesoporous titanium oxide synthesized by the template method, and mesoporous zirconia synthesized by the template method.
Owner:NANJING UNIV OF TECH
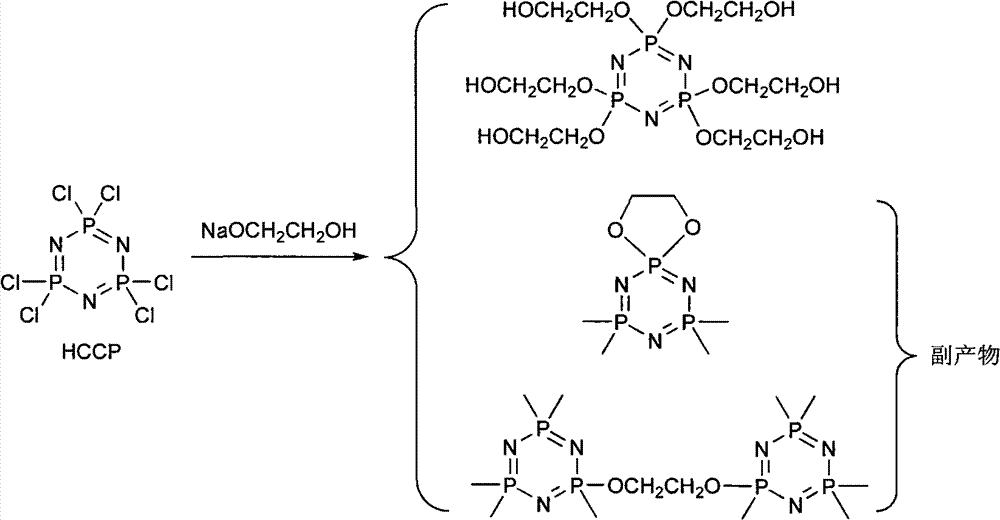
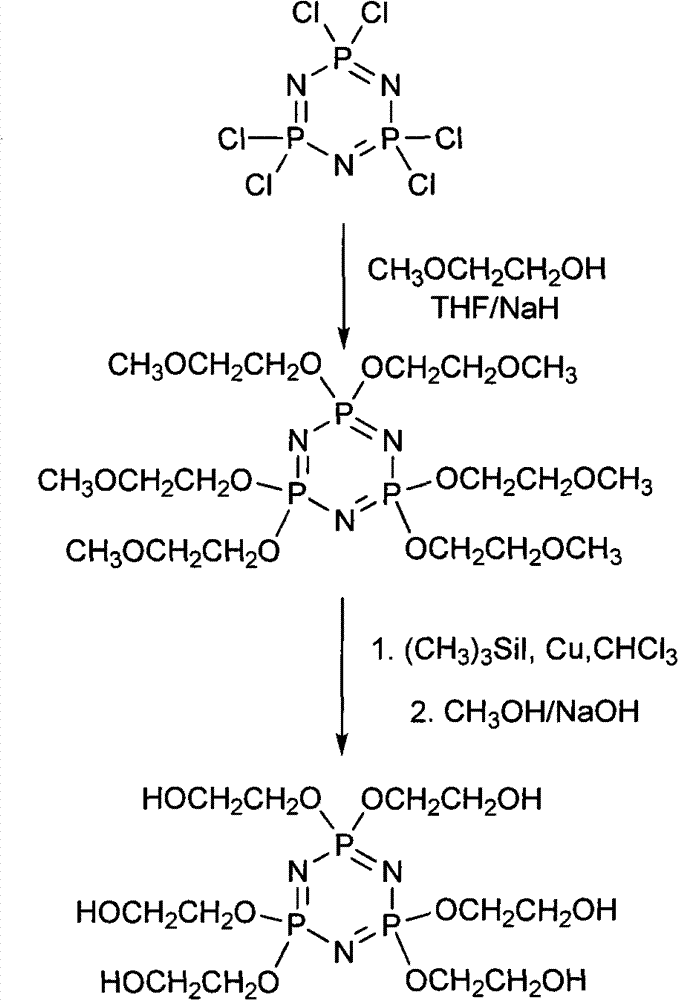
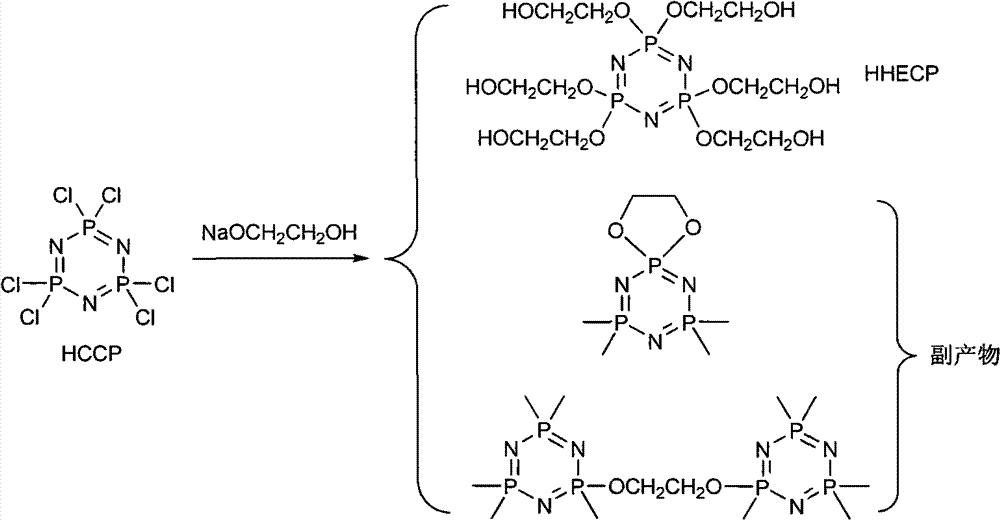
Synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene
InactiveCN102766167ASimple post-processingHigh reaction yieldGroup 5/15 element organic compoundsDistillationSodium hydride
The invention discloses a synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The method includes the following steps: (1) enabling methyl cellosolve and sodium hydride to react to obtain methyl sodium ethoxide, adding a tetrahydrofuran solution of hexachlorocyclotriphosphazene, reacting for 30min-45min at the temperature of 20 DEG C-35 DEG C, filtering, removing solvent by steaming, extracting, washing and conducting reduced pressure distillation to obtain 6(4-methoxyl ethyoxyl) cyclotriphophazene; (2) enabling the 6(4-methoxyl ethyoxyl) cyclotriphophazene and iodotrimethylsilane to react for 30h-40h at the temperature of 25 DEG C-35 DEG C under the condition of stirring, adding a methanol solution of sodium hydroxide, reacting for 6h-8h at the temperature of 15-30 DEG C under the condition of stirring, filtering, and conducting reduced pressure removing of the solvent by steaming to obtain the 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The synthetic method of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene is high in reacting yield and is mainly used for synthesis of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene.
Owner:XIAN MODERN CHEM RES INST
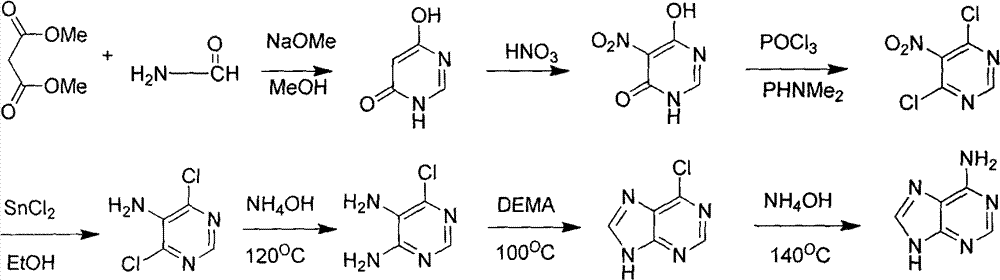
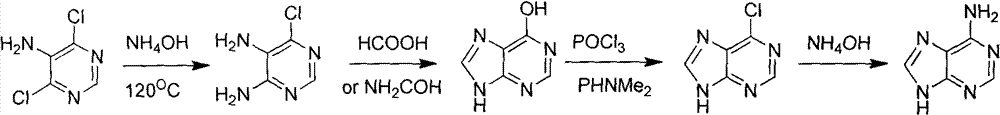
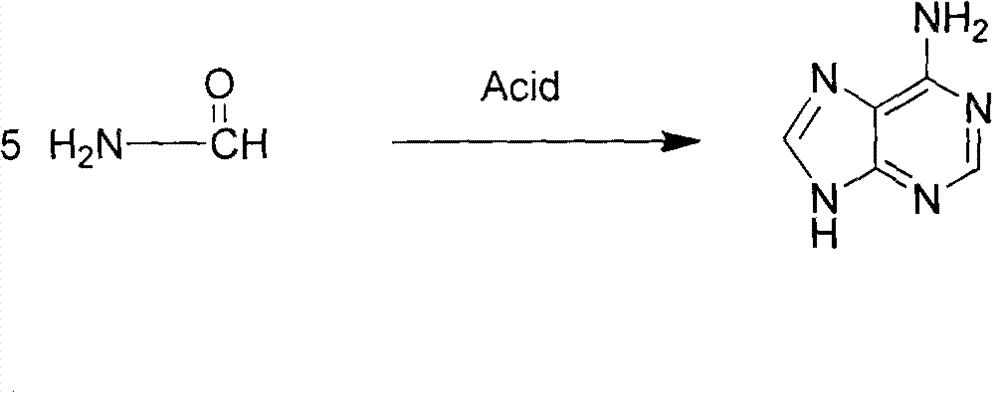
Novel chemical synthesis method for adenine
InactiveCN102887899AShort route stepsIncrease productivityOrganic chemistryChemical synthesisAcetic anhydride
The invention discloses a method for synthesizing adenine represented by a formula (I), wherein the method comprises the following steps of making formamide react with diethyl malonate in an ethanol solution of sodium ethoxide to obtain a raw material 4,6-dihydroxypyrimidine represented by a formula (VI); nitrifying the 4,6-dihydroxypyrimidine to obtain 4,6-dihydroxy-5-nitropyrimidine represented by a formula (V); carrying out chlorination reaction on the 4,6-dihydroxy-5-nitropyrimidine (V) to obtain 4,6-dichloro-5-nitropyrimidine represented by a formula (IV), carrying out aminolysis reaction on the 4,6-dichloro-5-nitropyrimidine (IV) and an saturated aminoethanol solution to obtain 4,6-diamino-5-nitropyrimidine represented by a formula (III); carrying out catalytic hydrogenation on the 4,6-diamino-5-nitropyrimidine (III), and reducing a nitro group to obtain 4,5,6-triaminopyrimidine represented by a formula (II); making the 4,5,6-triaminopyrimidine (II) react with ethyl orthoformate in acetic anhydride to obtain the adenine represented by the formula (I). The method provided by the invention has the advantages of cheap and easily-obtained raw materials, mild reaction conditions, single product, high total yield, low production cost and easiness in industrial production.
Owner:YANGZHOU UNIV
Synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene
InactiveCN102766168ASimple post-processingHigh reaction yieldGroup 5/15 element organic compoundsDistillationSodium hydride
The invention discloses a synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The method includes: (1) enabling methyl cellosolve and sodium hydride to react to obtain methyl sodium ethoxide, adding a tetrahydrofuran solution of hexachlorocyclotriphosphazene, reacting for 30min-45min at the temperature of 20-35 DEG C, filtering, removing solvent by steaming, extracting, washing and conducting reduced pressure distillation to obtain 6(4-methoxyl ethyoxyl) cyclotriphophazene; (2) enabling the 6(4-methoxyl ethyoxyl) cyclotriphophazene and boron tribromide to react for 2h-5h at the temperature of 0 DEG C-20 DEG C under the condition of stirring, after the reaction, adding water to conduct cancellation reaction, standing to separate a water phase, and conducting reduced pressure distillation to remove water to obtain the 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The synthetic method of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene is high in reacting yield and is mainly used for synthesis of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene.
Owner:XIAN MODERN CHEM RES INST
Continuous 3-methyl-3-buten-1-ol production method
ActiveCN105693470APromote depolymerizationGenerate efficientlyOrganic compound preparationHydroxy compound preparationDepolymerizationMethyl group
The invention discloses a continuous 3-methyl-3-buten-1-ol production method.The method includes that paraformaldehyde is put in corresponding alcoholic solution with sodium methylate or sodium ethoxide mass concentration being 1-5% to form methanal hemiacetal solution by depolymerization and condensation; isobutene and methanal hemiacetal are put in a tubular reactor to generate the target product 3-methyl-3-buten-1-ol by one-step reaction under the action of catalysts; the product generated in the reaction stage is cooled and then fed into an isobutene recovery tower, a light component removal tower and a product refining tower sequentially.By the continuous 3-methyl-3-buten-1-ol production method, the defects of low heat and mass transfer efficiency, high reaction pressure, more side reactions, low production efficiency and the like in an intermittent tank process are overcome, and continuous 3-methyl-3-buten-1-ol production with the isobutene and the methanal hemiacetal serving as raw materials is realized.
Owner:JIANGSU SOBUTE NEW MATERIALS +1
Process for the preparation of sucralose by the chlorination of sugar with triphosgene (BTC)
In one embodiment of the invention a method to prepare sucralose-6-acylate through chlorinating sucrose-6-acylater by BTC in the process of sucralose preparation is disclosed. In this embodiment a Vilsmeier reagent is firstly prepared below 0° C. by dissolving BTC in DMF or in component solvent, containing DMF, toluene, dichloroethane, chloroform and carbon tetrachloride. Consequently, sucrose-6-ester was chlorinated by Vilsmeier reagent. BTC can also be dissolved in one or several organic solvent such as toluene, dichloroethane, chloroform and carbon tetrachloride, and added to a DMF solution of sucrose-6-acylate for chlorination. Sucralose was prepared through de-esterifying the obtained sucralosed 6-ester using sodium methoxide / methanol or sodium ethoxide / ethanol.
Owner:MAMTEK INT
Method for preparing low-chlorine bleach lac
InactiveCN101157828AMeet the requirements of low chlorine bleached shellacNatural resin purificationFood preparationFiltrationBleach
A preparation method of less chlorine bleaching lac: first, lac grains are put into sodium carbonate solution under the temperature of 60 DEG C to 100 DEG C and are completely dissolved. And then the solution is cooled below 70 DEG C and waxiness and insoluble content in the lac are filtrated; the filtrated solution under the temperature of 20 DEG C to 40 DEG C is added with sodium hypochlorite to blanch, then the temperature is increased to 70 DEG C to 100 DEG C. Next, sodium ethoxide is put into the solution to react. After the solution being cooled to room temperature or below 30 DEG C, sodium hypochlorite solution is dropped into the solution to blanch. Then the solution is filtrated with filtration aids such as white mud, diatomite and so on; the filtrated solution is added with dilute sulphuric acid or dilute hydrochloric acid to precipitate. The precipitation is dried by heat air after being washed and solid / liquid separation. In the end, the less chlorine bleaching lac which is white or slight yellow powder can be gotten. The product can satisfy the demand for food industry and pharmacy. The chlorine content in the product is less than 0.3 percent (wt percent). The time for thermal polymerization under the temperature of 170 DEG C plus or minus 1 DEG C amounts to above 5 minutes. The storage period is more than 2 years.
Owner:KUNMING UNIV OF SCI & TECH
Method for synthesizing tolfenpyrad
The invention provides a method for synthesizing tolfenpyrad, relates to a preparation method of the tolfenpyrad, and the method is used for solving the problems such as tedious process and poor product purity of an existing tolfenpyrad synthesis method. The method comprises the following steps of: 1, synthesizing ethyl propionyl pyruvate; 2, synthesizing ethyl 3-ethyl-5-pyrazolecarboxylate; 3, synthesizing ethyl 1-methyl-3-ethyl-5-pyrazolecarboxylate; 4, synthesizing ethyl 1-methyl-3-ethyl-4-chloro-5-pyrazolecarboxylate; 5, synthesizing 4-(4-methyl phenoxy) cyanophenyl; 6, synthesizing 4-(4-methyl phenoxy) benzylamine; and 7, synthesizing the tolfenpyrad. Since sodium ethoxide is replaced by sodium hydroxide, the method provided by the invention has the characteristics of short reaction time, no generation of isomer and high purity of the product; and the obtained product has high purity and does not need re-crystallization.
Owner:HEILONGJIANG UNIV
Synthesis of bis(thio-hydrazide amide) salts
InactiveUS7709683B2Easy to disassembleLower levelHydrazine preparationOrganic compound preparationThio-Potassium hydroxide
Owner:SYNTA PHARMA CORP
Solar energy mono-crystalline silicon piece flocking solution and application method thereof
InactiveCN103205815AEvenly distributedImprove passivation effectAfter-treatment detailsPolyethylene glycolCarboxylic salt
The invention provides a solar energy mono-crystalline silicon piece flocking solution and an application method of the solar energy mono-crystalline silicon piece flocking solution. The flocking solution comprises the following components: 9.6 to 12.5g / L of NaOH, 50 to 100mL / L of ethanol, 0.25 to 2g / L of sodium ethoxide, 1 to 2g / L of vitamin, and one of 0.5 to 2.5g / L of at least one of potassium perfluoro alkyl ether carboxylate FC-5, polyoxyethylene ether, polyethylene glycol acid ester, polyol ester or lauroyl diethanol amine; and water and used as a solvent. A flocked face prepared by the method provided by the invention has good effect, pyramids of about 1-3 microns are fully and uniformly distributed on the flocked face, thus, little defect mode appears on the surface, the passivating effect of the flocked face is good, the minority carrier lifetime is prolonged, and the open-circuit voltage (Voc) (Variable Output Circuit), the short-circuit current (Isc) and a fill factor (FF) are correspondingly increased; the obtained pyramids are uniformly distributed in dimension and have no sharp edges and apex angles, therefore, the defect mode density on the surface can be greatly reduced, the passivating effect is improved, and the conversion efficiency of a solar cell can be increased.
Owner:SHANGHAI JIAO TONG UNIV
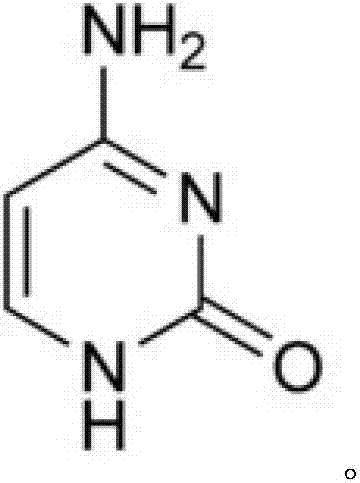
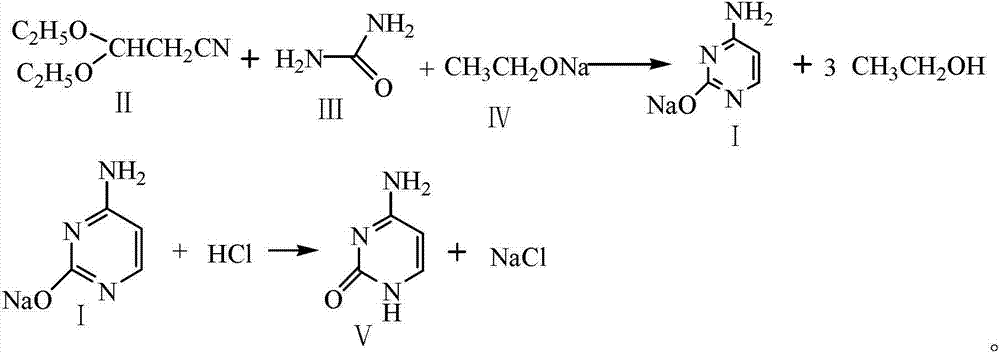
Preparation method for cytosine
The invention discloses a preparation method for cytosine. The preparation method comprises the following steps of: (1) mixing sodium ethoxide, dimethylbenzene, and carbamide, stirring the mixture at a temperature of 85 DEG C to 95 DEG C for 0.3-0.5 hours, dropping 3,3-diethoxy propionitrile, controlling the temperature between 100 DEG C and 105 DEG C, after finishing dropping, back-flow reacting for 8-10 hours, concentrating the mixture to be dried after finishing the reaction, adding distilled water to dissolve condensate, standing for stratification, dropping hydrochloric acid on a water layer and uniformly mixing, adjusting a pH value to equal to 7.0-7.5, cooling, crystallizing and centrifuging to obtain a crude product; and (2) adding the crude product in water and activated carbon, decoloring at the temperature of 70 DEG C to 80 DEG C, filtering and removing the activated carbon, cooling and crystallizing filter liquid, centrifuging to obtain a wet end product, and drying to obtain the cytosine. According to the preparation method, a catalyst difficult to obtain is prevented from using, the adopted raw materials are basic raw materials, the raw materials are cheap and easy to obtain, a subsequent treatment is simple, the cost is low, the yield is high, and the preparation method is suitable for the industrial production.
Owner:SHANGYU HUALUN CHEM
Preparation method of secondary alcohol ethoxylate
ActiveCN107021875AEther separation/purificationEther preparation from oxiranesLithium hydroxidePotassium hydroxide
The invention relates to a preparation method of secondary alcohol ethoxylate. According to an adopted technical scheme, the method includes the steps of: in the presence of an acidic catalyst, reacting secondary alcohol with ethylene oxide to obtain a secondary alcohol ethoxylate crude product; mixing a first treatment agent with the crude product to obtain an intermediate material I; mixing a second treatment agent with the intermediate material I to obtain an intermediate material II; filtering the intermediate material II to obtain filtrate; mixing the filtrate with water, conducting standing layering above the turbidity point of secondary alcohol ethoxylate to obtain an oil phase; and removing impurity water from the oil phase, thus obtaining a refined secondary alcohol ethoxylate product. Specifically, the first treatment agent is selected from at least one of lithium hydroxide, calcium hydroxide, calcium oxide, calcium carbonate, barium hydroxide, barium oxide, magnesium oxide, magnesium hydroxide, strontium hydroxide and strontium oxide; and the second treatment agent is selected from at least one of potassium hydroxide, sodium hydroxide, sodium methoxide, potassium methoxide, sodium ethoxide and sodium carbonate.
Owner:SHANGHAI DUOLUN CHEM
Production process of tetramethyldivinyldisiloxane
ActiveCN101792459AHarm reductionRelieve pressureSilicon organic compoundsEnvironmental engineeringDimethyldiethoxysilane
The invention relates to a production process of an organic material and discloses a production process of tetramethyldivinyldisiloxane. On the basis of the production process of the tetramethyldivinyldisiloxane in the prior art, a technical process of dripping dimethyldichlorosilane is adopted before a predistillation step, the dripped dimethyldichlorosilane reacts with sodium ethylate generated in a reaction to form dimethyldiethoxysilane and sodium chloride mainly, the sodium ethylate content of waste residues is reduced or zero, so the damages caused by sodium ethylate to human bodies and the environment are reduced, the risks in storage and transportation process are reduced, the production cost is reduced and the environmental protection burden is relieved.
Owner:浙江佳汇新材料有限公司
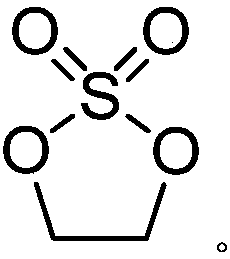
Preparation method of ethylene sulfate
ActiveCN108610324AEliminate potential safety hazardsEasy to makeOrganic chemistrySodium methoxideLithium hydroxide
The invention discloses a preparation method of ethylene sulfate. The method comprises the steps of: reacting ethylene glycol with diethyl sulfate in the presence of an alkaline catalyst to produce ethylene sulfate, wherein the alkaline catalyst is one of or a combination of several of lithium hydroxide, sodium methoxide, sodium ethoxide, potassium carbonate, sodium carbonate and lithium carbonate. The preparation method provided by the invention can reduce environmental pollution, is more environmental friendly, and has the advantages of simple operation, safety, ideal yield and the like.
Owner:SUZHOU HUAYI NEW ENERGY TECH CO LTD
Foam combination flooding produced liquid demulsifier and preparation method thereof
ActiveCN102925204AEffective demulsificationEffective treatment of demulsificationDewatering/demulsification with chemical meansDemulsifierPotassium hydroxide
The invention discloses a foam flooding produced liquid demulsifier which is a non-ionic polyether demulsifier and is characterized in that the demulsifier is prepared by three steps: phenolic resin serving as an initiator firstly reacts with epoxypropane in the presence of an alkaline compound serving as a catalyst to obtain a propoxylated polyether compound; the propoxylated polyether compound performs addition reaction with ethylene oxide under the effect of an alkaline catalyst to form an intermediate; and the intermediate reacts with epoxypropane, wherein in the reaction formula of each step, the alkaline compound is sodium hydroxide, potassium hydroxide or sodium ethoxide; in the three steps of reaction for preparing the demulsifier, the mass ratio of the phenolic resin to alkaline compound to epoxypropane in the first step of reaction is (20-30):(0.05-0.15):(50-90); the mass ratio of the propoxylated polyether compound to alkaline compound to ethylene oxide in the second step of reaction is (20-35):(0.1-0.45):(70-160); and the mass ratio of the intermediate to alkaline compound to epoxypropane in the third step of reaction is (20-30):(0.05-0.15):(50-90).
Owner:CHINA PETROLEUM & CHEM CORP +2
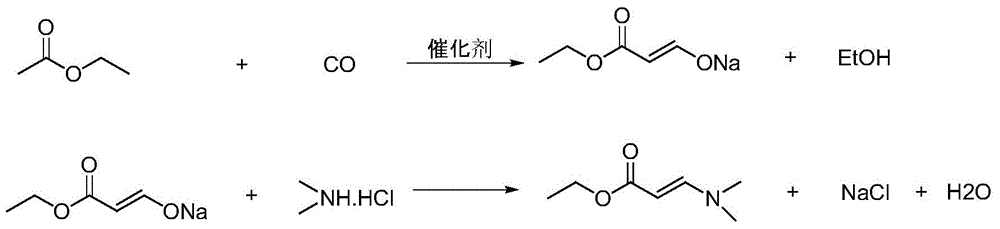
N,N-dimethylamino ethyl acrylate preparation method
InactiveCN105646257AReduce contentImprove solubilityOrganic compound preparationPreparation by carbon monoxide or formate reactionSodium methoxideSolvent
The present invention relates to a N,N-dimethylamino ethyl acrylate preparation method, which comprises that (1) ethyl acetate and carbon monoxide are subjected to a reaction in a mixed solvent medium of ethyl acetate and ethanol under the effect of a catalyst to obtain a sodium salt of formyl ethyl acetate, wherein the catalyst is sodium methoxide or sodium ethoxide; and (2) the sodium salt of formyl ethyl acetate is added to a mixed solvent of ethyl acetate and ethanol, and then a reaction is performed with a dimethylamine salt to prepare the N,N-dimethylamino ethyl acrylate. According to the present invention, the use of the expensive and high-toxicity raw material is avoided, the process is simple, the operation is easy, the contents of various impurities are minimized through the two-step method, and the conversion rate of the main reaction is improved; and by adjusting the ratio of the mixed solvent, the viscosity of the reaction system is reduced, such that the process is easy to enlarge, and is suitable for large-scale industrial production.
Owner:NANJING ZHUOYE PHARMA CO LTD
Novel synthetic method of (S)-3-morpholinyl carboxylic acid
ActiveCN102617503AMild reaction conditionsSpecific responseOrganic chemistryCarboxylic acidTert butyl
The invention discloses a synthetic method of (S)-3-morpholinyl carboxylic acid, which comprises the following steps: (1) taking L-serine as a raw material to synthesize L-serine tert-butyl ester; (2) dissolving L-serine tert-butyl ester in dichloromethane, adding a dichloromethane solution of chloroacetyl chloride drop by drop to obtain N-chloroacetyl-L-serine tert-butyl ester; (3) dissolving N-chloroacetyl-L-serine tert-butyl ester in a toluene solution, adding the toluene solution of sodium ethoxide drop by drop to obtain (S)-5-oxo 3-morpholinyl carboxylic acid tert-butyl ester; (4) dissolving (S)-5-oxo 3-morpholinyl carboxylic acid tert-butyl ester in methanol, successively adding aluminum trichloride and sodium borohydride to carry out a reaction to obtain the (S)-3-morpholinyl carboxylic acid tert-butyl ester; (5) dissolving (S)-3-morpholinyl carboxylic acid tert-butyl ester in methanol, adding a methanol solution of hydrogen chloride for reacting to obtain the (S)-3-morpholinyl carboxylic acid. The method of the invention has the advantages of mild reaction condition, easily available raw material and less three waste, and is suitable for industrial production.
Owner:上海常丰生物医药科技有限公司
Oligomeric quaternary ammonium salt bactericide and preparation method thereof
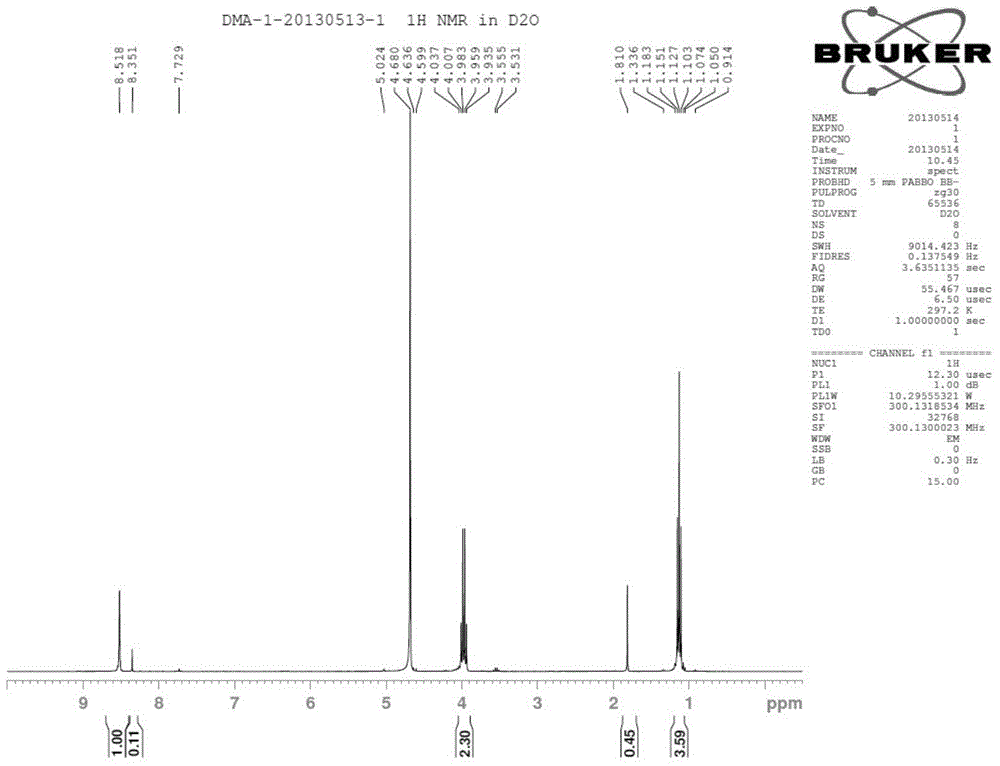
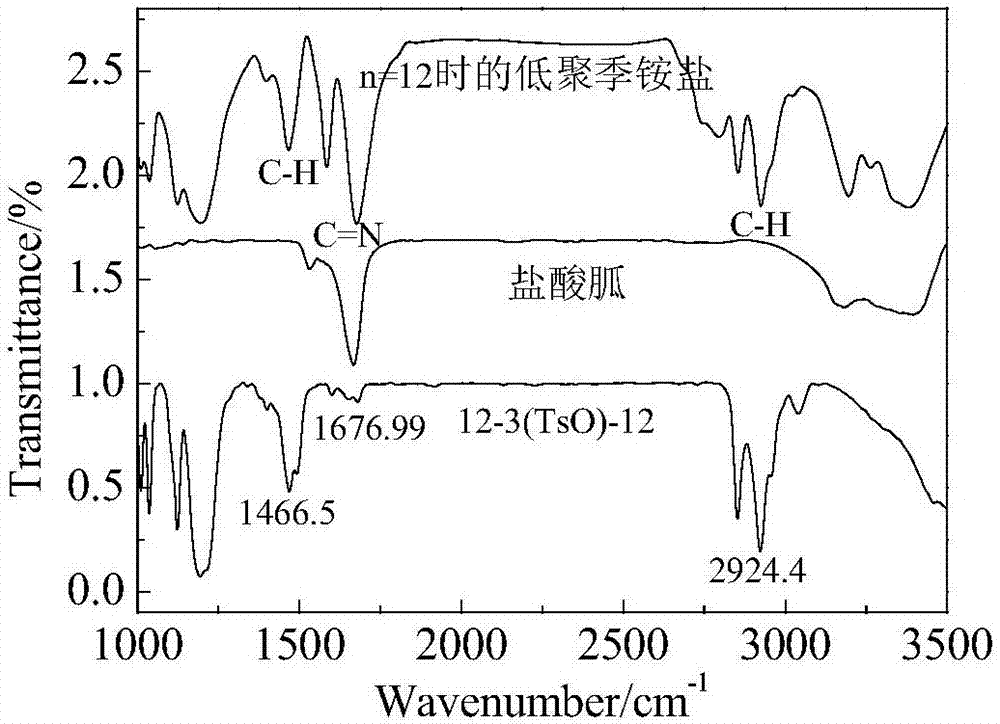
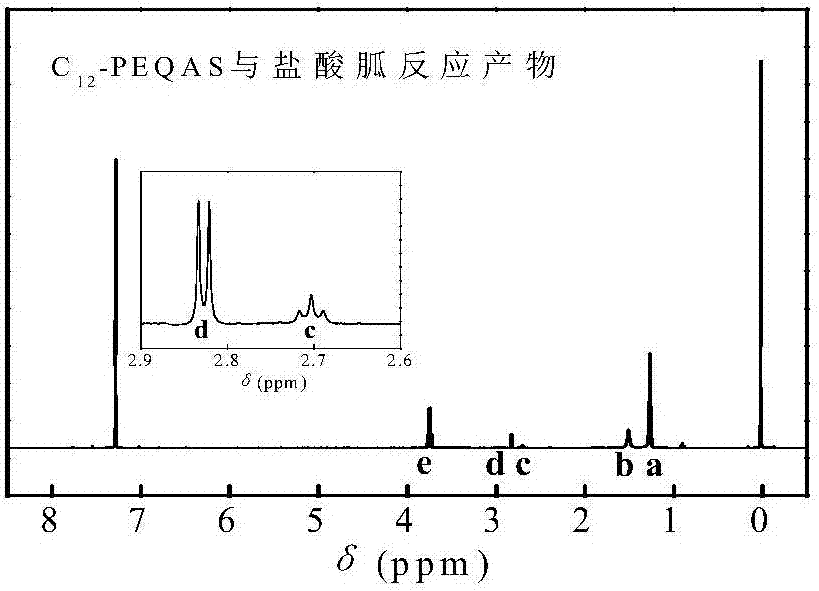
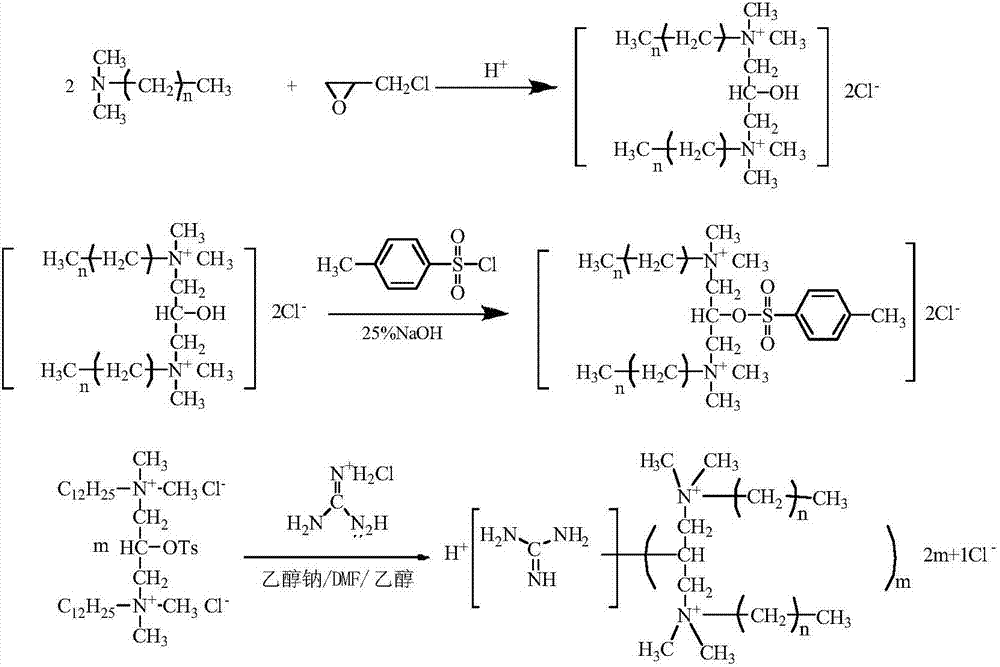
InactiveCN107156167AQuick deathSpeed up the leakBiocideOrganic compound preparationSolubilityQuaternary ammonium cation
The invention relates to an oligomeric quaternary ammonium salt bactericide and a preparation method thereof. Ester p-toluenesulfonate-based gemini quaternary ammonium salt, guanidine hydrochloride and sodium alkoxide are added into solvent, stirred and heated to 120 DEG C to 180 DEG C, heating is stopped after 10 to 48 hours of reaction, so that a crude product is obtained, and after post-treatment, the oligomeric quaternary ammonium salt bactericide is obtained; and the substance amount ratio of the ester p-toluenesulfonate-based gemini quaternary ammonium salt, the guanidine hydrochloride and the sodium alkoxide is (0.008 to 0.040):0.008:0.008. The gemini quaternary ammonium salt is introduced to a plurality of active sites, eight active sites are contained in the structure of the oligomeric quaternary ammonium salt bactericide, the oligomeric quaternary ammonium salt bactericide can show good water solubility and surface activity in aqueous solution, and an important role is played in the process of oligomeric quaternary ammonium salt preparation. A bacteria killing test on three types of bacteria, i.e. sulfate reducing bacteria, iron bacteria and saprophytic bacteria, indicates that the oligomeric quaternary ammonium salt bactericide has a good bactericidal effect.
Owner:SHAANXI UNIV OF SCI & TECH
Industrialization production technology for tenofovir disoproxil fumarate
InactiveCN105859781AHigh yieldHigh purityGroup 5/15 element organic compoundsDiethyl phosphateDiethyl methylphosphonate
An industrial production process of tenofovir, the production process steps are as follows: add R-1,2-propylene glycol, diethyl carbonate and sodium ethylate into the reaction kettle; add absolute ethanol and diphosphite Ethyl ester, stirring, after the stirring is completed, put in paraformaldehyde and triethylamine, after the reaction is complete, add anhydrous sodium sulfate, dry, filter, and distill out, the distilled product is diethyl p-toluenesulfonyloxymethylphosphonate Fine product; add adenine, R-propylene carbonate, DMF and NaOH in the reaction kettle, after the reaction is complete, add magnesium tert-butoxide, drop diethyl p-toluenesulfonyl phosphate, after the reaction is complete, add acetic acid, Concentrate under reduced pressure, add hydrochloric acid, filter, filter out the solid and dry under normal pressure to obtain PMPA fine product. The method has the advantages of high yield, high product purity and low impurity content, and can be fully industrialized.
Owner:JINGMEN SHUAIBANG CHEM SCI & TECHCO
Iron mineral inhibitor and production method thereof
ActiveCN106824550AImprove acceleration performanceHigh selectivityFlotationSodium acetateSodium chloroacetate
The invention discloses a production method of an iron mineral inhibitor. The production method comprises the following steps: step I, enabling a mixture of following components in parts by weight to react for 1 to 4 hours at 60 to 90 DEC to obtain an additive (1): 100 parts of corn starch, cassava starch or wheat starch, 0.5 to 4.0 parts of sodium ethoxide or sodium hydroxide and 5 to 15 parts of sodium chloroacetate; step II, enabling a mixture of following components in parts by weight to react for 3 to 6 hours at 50 to 80 DEG C to obtain an additive (2): 100 parts of the additive (1) obtained in the step I, 5 to 15 parts of ethylene oxide, and 0.2 to 1.0 part of sodium carbonate or sodium acetate; and step III, enabling a mixture of the following components in parts by weight to react for 1 to 3 hours at 30 to 60 DEG C to obtain an inhibitor finished product: 100 parts of the additive (2) obtained in the step II, 1 to 12 parts of sodium periodate or hydrogen peroxide, and 0.2 to 1.0 part of ferrous sulfate or cupric sulfate.
Owner:天津天宝翔科技有限公司
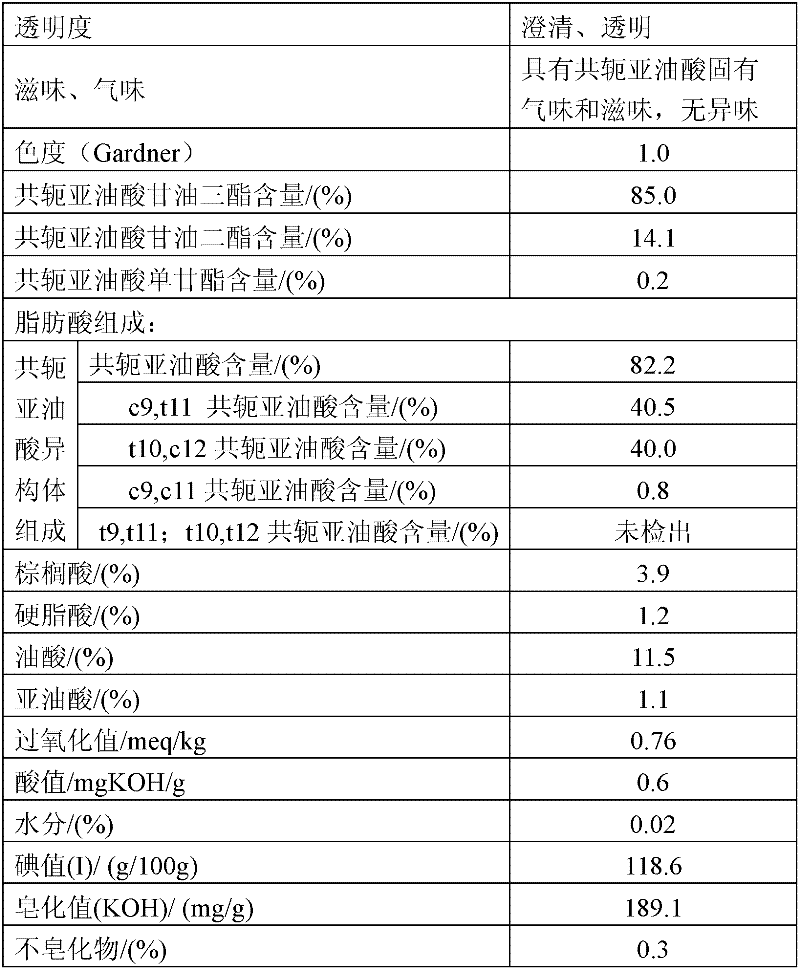
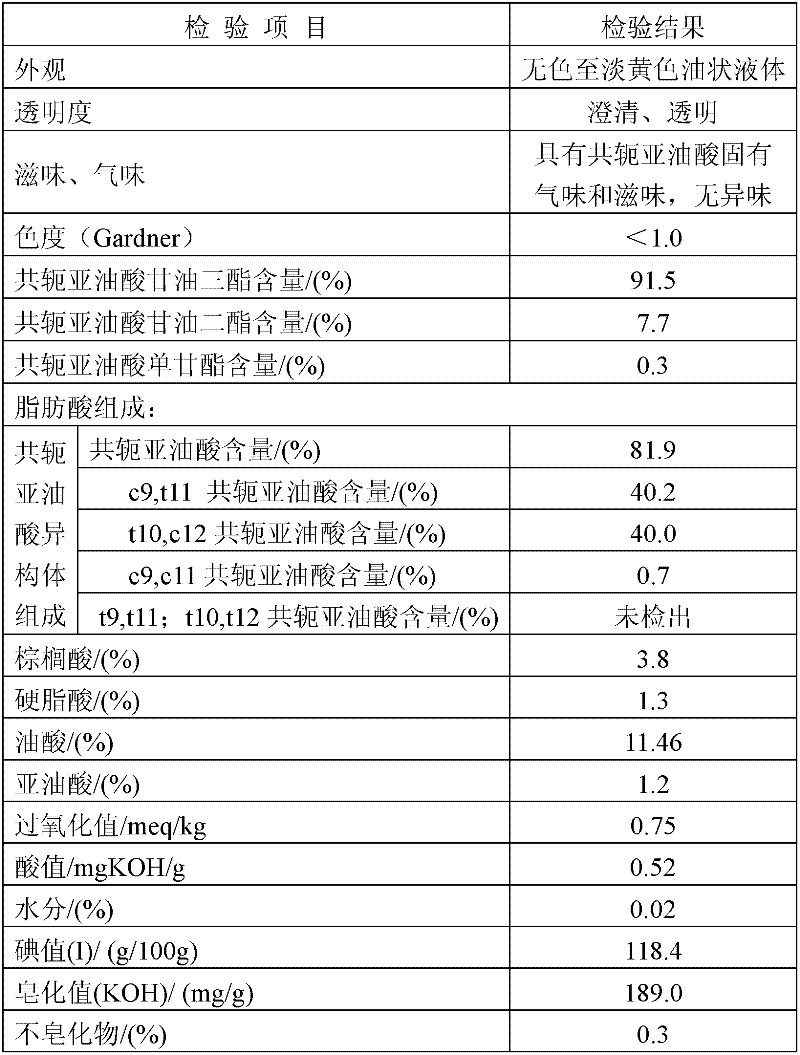
Preparation method of conjugated linoleic acid glycerides
ActiveCN102584586ANo smellNo residuePreparation by transesterificationNatural resourcePotassium ethoxide
A preparation method of conjugated linoleic acid glycerides comprises the following steps: (1) C1-4 sodium alcoholate / potassium alcoholate is taken as a catalyst, linoleic acid short-chain alcohol ester is prepared to be conjugated linoleic acid short-chain alcohol ester through inversion reaction for 1-8 hours at 90-150 DEG C, and the using amount of the catalyst is 1.5-8.0% of weight of the linoleic acid short-chain alcohol ester; (2) sodium methylate / potassium methylate and sodium ethoxide / potassium ethoxide are taken as catalysts, the conjugated linoleic acid short-chain alcohol ester and acetoglyceride with mole ratio being 3:1-3.5:1 are reacted for 3-10 hours at 100 DEG C and 170 DEG C to prepare the conjugated linoleic acid glycerides, and the using amount of the catalysts is 0.8-10% of the weight of acetoglyceride. According to the preparation method, the natural resources are utilized sufficiently, an organic solvent is not needed in the main reaction; the esterification rate of conjugated oil acid is more than 99%; the reaction time is short, and the postprocessing simple; the triglyceride content of the conjugated linoleic acid glycerides is more than 90%, and the acid value of the conjugated linoleic acid glycerides is under 1.0mgKOH / g; and the conjugated linoleic acid glycerides is free from extraneous odor, good in taste and light in color, and the optimal Gar is less than or equal to 1.0.
Owner:INNOBIO CORP LTD
Preparation method for improving dyeing depth of natural dye and reactive dye of wool
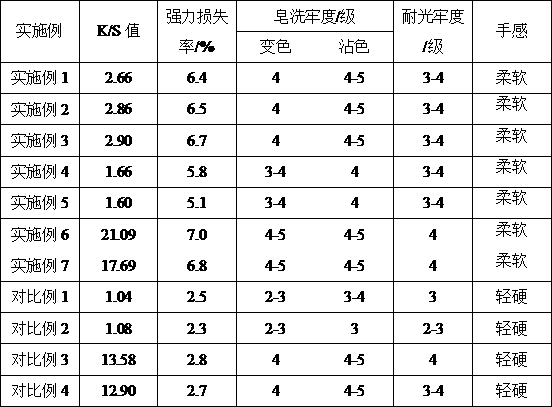
The invention discloses a preparation method for improving dyeing depth of natural dye and reactive dye of wool. The preparation method comprises the following steps: (1) performing pre-treatment on the wool, namely, soaking wool fibres in a pre-treatment liquid which is compounded by sodium citrate, sodium ethoxide and a penetrating agent, and treating for 20 to 40 minutes at 30 to 50 DEG C; (2)performing acid neutralizing and water washing, namely, neutralizing the pre-treated wool fibres with a suitable amount of acid and washing the neutralized wool fibres with water; and (3) dyeing, namely, dyeing the wool fibres by adopting the natural dye and a reactive dye for wool. According to the preparation method, after the wool fibres are treated at low temperature through a diluted pre-treatment liquid, the wool fibres are dyed through the natural dye or the reactive dye for the wool. A dyed wool product with soft hand feeling, deep colour and excellent colour fastness can be prepared;the method is simple and feasible and is low in implementation cost, and has low damage to wool strength; and energy resources are saved by low-temperature dyeing.
Owner:常州喜莱维纺织科技有限公司
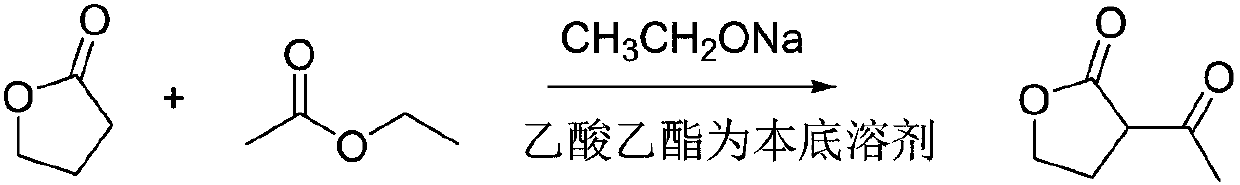
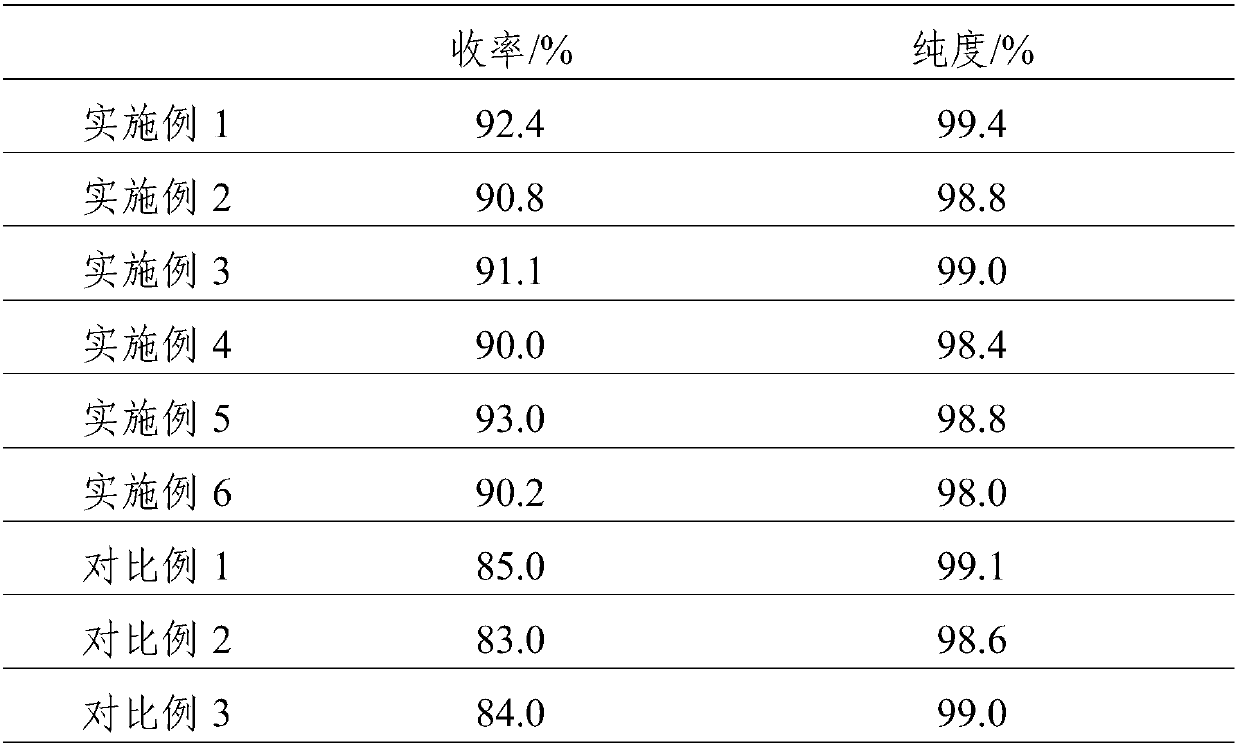
Synthesis method for alpha-acetyl gamma-butyrolactone
InactiveCN107857745AHarm reductionAvoid prone to flushingOrganic chemistrySodium ethoxideVacuum distillation
The invention relates to the field of synthesis of organic intermediates of prothioconazole, in particular to a synthesis method for alpha-acetyl gamma-butyrolactone. The synthesis method includes thefollowing steps of (1) slowly heating a reactor to 75 DEG C, and adding gamma-butyrolactone, ethyl acetate and sodium ethoxide for reflux reaction for 10 hours to obtain a sodium salt of alpha-acetylgamma-butyrolactone and a by-product ethanol; (2) distilling the product obtained in the step (1) to remove the ethanol and excess ethyl acetate, adjusting the pH of the residue to 3-4 with dilute sulfuric acid, standing for liquid separation, removing aqueous phase, and performing vacuum distillation in an organic phase at a pressure of 0.1 MPa and a temperature of 65-70 DEG C to obtain alpha-acetyl gamma-butyrolactone. The synthesis method uses a reaction base material ethyl acetate as a base solvent and uses sodium ethoxide as a condensing agent, has mild reaction conditions and improved safety, avoids pollution caused by adopting additional solvents such as benzene solvent and the like, and has good safety, simple post-treatment method, high yield of alpha-acetyl gamma-butyrolactone above 90%, and high purity of alpha-acetyl gamma-butyrolactone above 98%.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Preparation method of ethyl 4,4,4-trifluoroacetoacetate
InactiveCN103694119AThorough responseHigh yieldOrganic compound preparationCarboxylic acid esters preparationClaisen condensationTrifluoroacetic acid
The invention discloses a preparation method of ethyl 4,4,4-trifluoroacetoacetate. With an ethanol solution of sodium ethoxide as a catalyst and in the presence of an organic solvent, ethyl trifluoroacetate and ethyl acetate are subjected to a Claisen condensation reaction, and ethyl 4,4,4-trifluoroacetoacetate is synthesized. The method provided by the invention has the advantages of mild conditions, simple operation, relatively high conversion rate of raw materials, relatively high product selectivity, easy separation of the product, low energy consumption and the like, and is suitable for industrialized production. The prepared ethyl 4,4,4-trifluoroacetoacetate is an important pesticide and medicine intermediate, can be used for preparation of thifluzamide, fluacrypyrim, thiazopyr and other pesticides, and also can be used for preparation of befloxatone and other medicines.
Owner:SINOCHEM LANTIAN +1
Star-like oxazolidine latent curing agent and preparation method as well as use thereof
ActiveCN103289038AReduce the presence of air bubblesHigh tensile strengthOrganic chemistryTanning treatmentFiltrationFractionation
The invention discloses a star-like oxazolidine latent curing agent and a preparation method as well as an application of the latent curing agent. The latent curing agent is characterized by being prepared by the following steps of: adding 0.01-0.1 part of sodium ethoxide, 5-60 parts of tetraethyl 1,1,5,5-pentane-terminated tetraformate and 15-120 parts of 2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidine into a reaction kettle, uniformly mixing the above raw materials, under the protection of nitrogen, increasing the temperature to 105-115 DEG C, carrying out reflux reaction for 2.5-3.5h, after the fractionation of ethanol is finished, increasing the temperature of a reaction solution to 115-125 DEG C to fractionate residual 2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidine, when the amount of 2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidine is not changed any more, stopping heating, dropwise adding 10-50 parts of butanone solution of p-toluenesulfonic acid at the concentration of 20-40% while stirring, carrying out reaction for 25-35min, neutralizing the reaction solution using acid until the pH (Potential of Hydrogen) is 7-7.5, adding 0.05-0.1 part of calcium oxide into the neutralized reaction solution, continuing the reaction for 0.5-1.5h, carrying out hot filtration at 75 DEG C to obtain 6-80 parts of thick semisolid tetra-2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidinyl 1,1,5,5-pentane-terminated tetraformate.
Owner:ZHEJIANG GREAT CHEM SCI & TECH
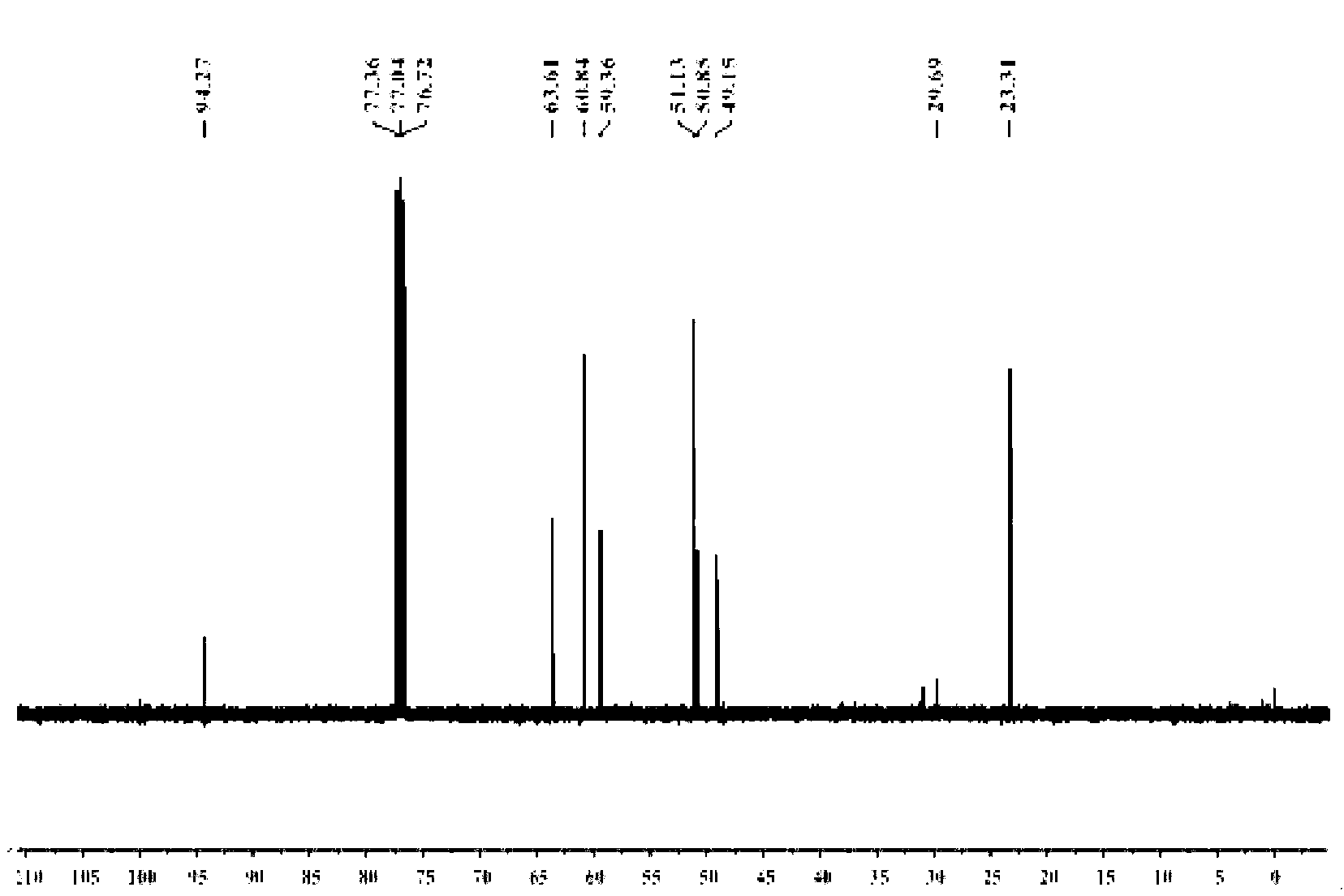
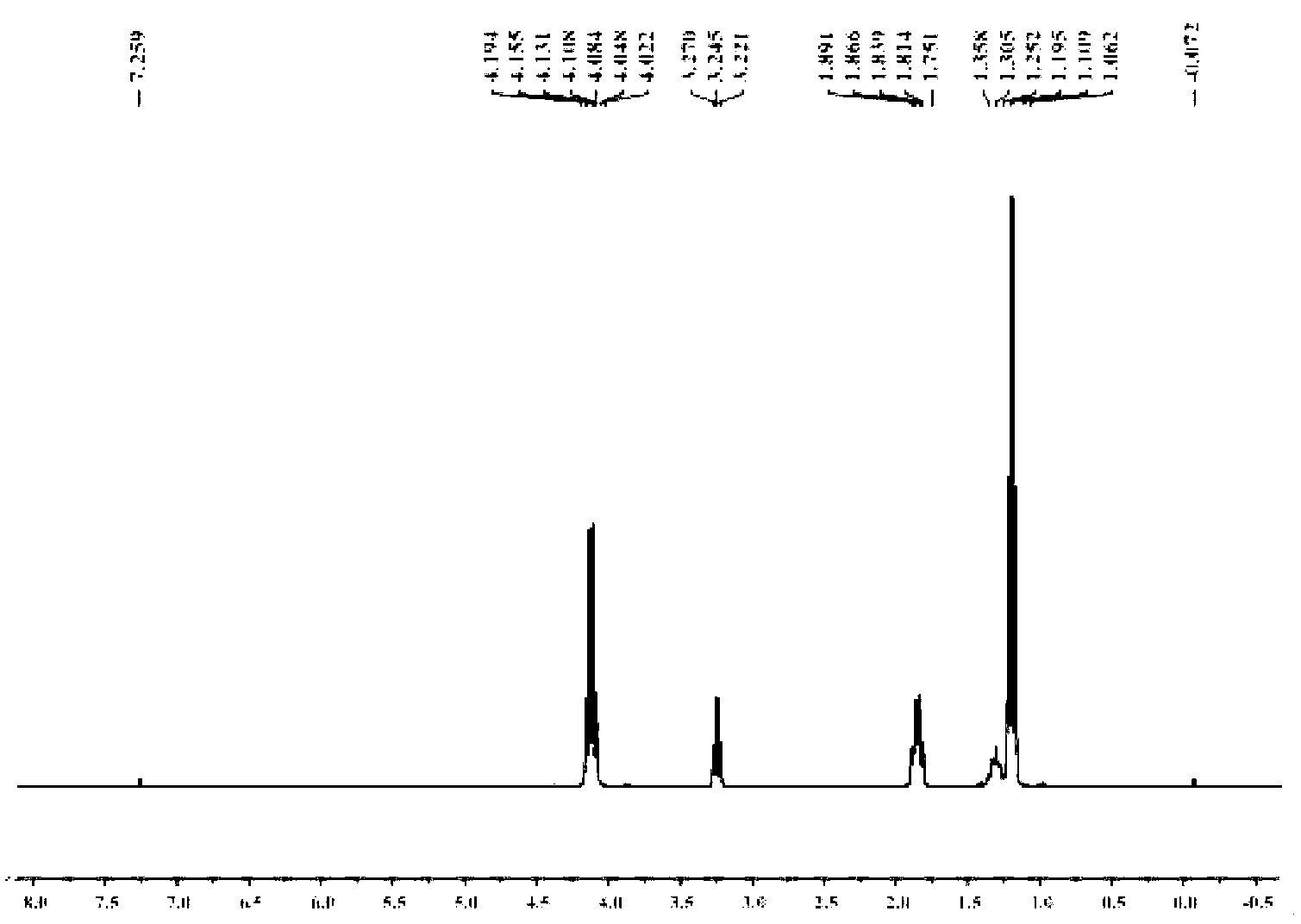
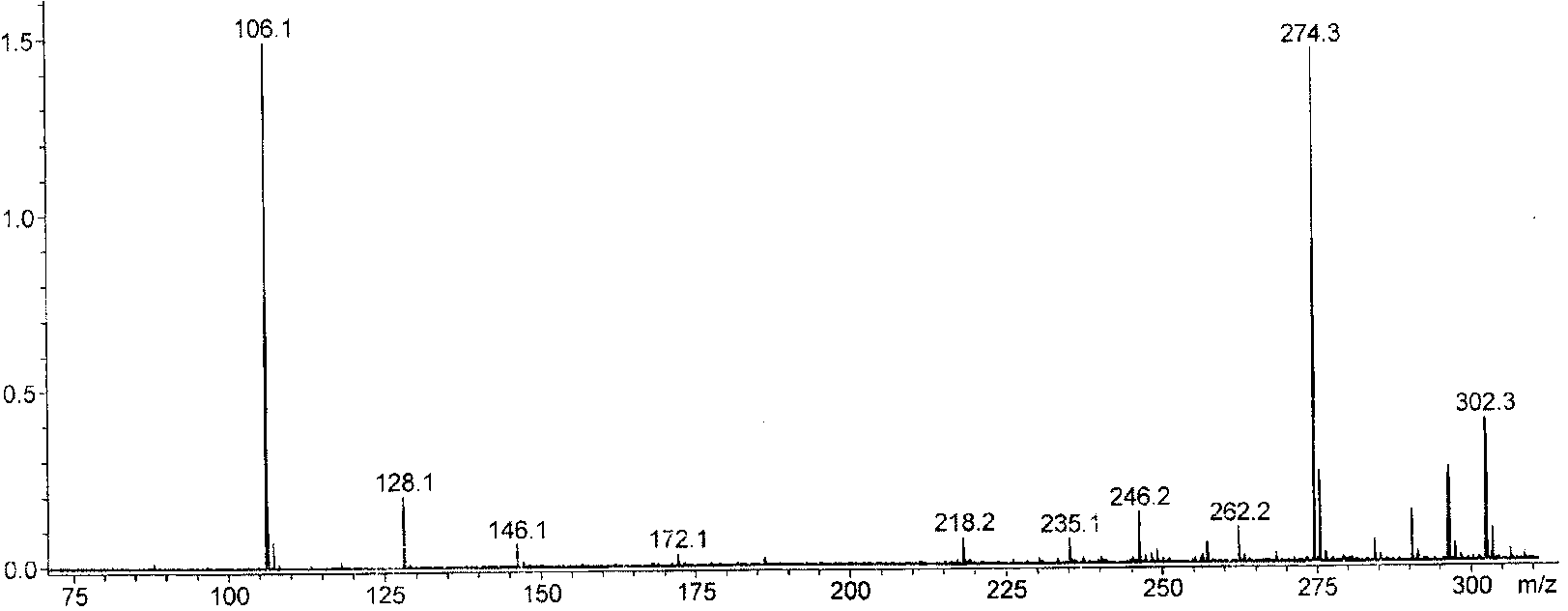
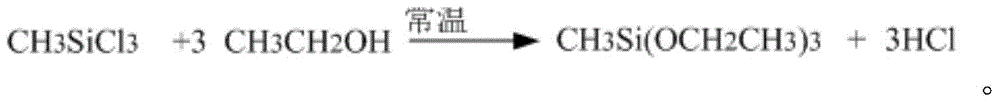
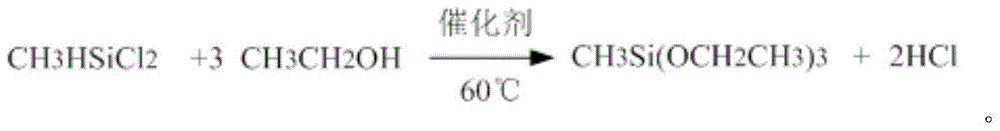
Preparation method of methyl triethoxysilane
The invention discloses a preparation method of methyl triethoxysilane. The method includes the following steps that 1, methyl dichloresilane and a catalyst are added to a reaction still and heated to flow back, and absolute ethyl alcohol is added dropwise from the bottom of the reaction still under the stirring condition; 2, after all absolute ethyl alcohol is added dropwise, the reaction temperature is controlled to be 60+ / -1 DEG C, the reaction is continued for more than 2 hours, backflow deacidification is conducted, sodium ethoxide is neutralized and rectified, and methyl triethoxysilane with the content of more than 99.0% is obtained. Methyl dichloresilane serves as the raw material to prepare methyl triethoxysilane, and the price of methyl dichloresilane is far lower than that of methyl trichlorosilane, so that production cost is remarkably reduced, meanwhile, by-product chlorine hydride can be greatly reduced, by-product treatment cost is reduced, the method is simple, easy to carry out and suitable for industrial production, and the product yield is high.
Owner:ZHEJIANG QUZHOU GUIBAO CHEM
Preparation method of topiroxostat
ActiveCN107573330AReaction raw materials are readily availableThe reaction conditions are mild and easy to controlOrganic chemistryLoop closingTopiroxostat
The invention provides a preparation method of topiroxostat. The preparation method comprises the steps that 2-cyano methyl isonicotinate is used as raw materials; hydrazinolysis is performed at -10 DEG C to -20 DEG C to obtain an intermediate; the intermediate and 4-cyanopyridine react under the conditions with sodium ethoxide and the pH being 4 to 6 to obtain the topiroxostat. The preparation method has the advantages that the 2-cyano methyl isonicotinate is used as a starting material; the 2-cyano methyl isonicotinate and hydrazine hydrate take condensation reaction at low temperature to prepare the intermediate; the intermediate and the 4-cyanopyridine are subjected to condensation and loop closing under the acid condition with sodium ethoxide to prepare the topiroxostat. The raw materials can be easily obtained; the reaction conditions are mild and are easy to control; a reagent with high toxicity is not used in the reaction process; the released toxic substances are few; the sidereaction products are few; the reaction safety is high; the pollution is small; the obtained purity is high; the preparation method is suitable for industrial production.
Owner:HEBEI UNIV OF CHINESE MEDICINE +1
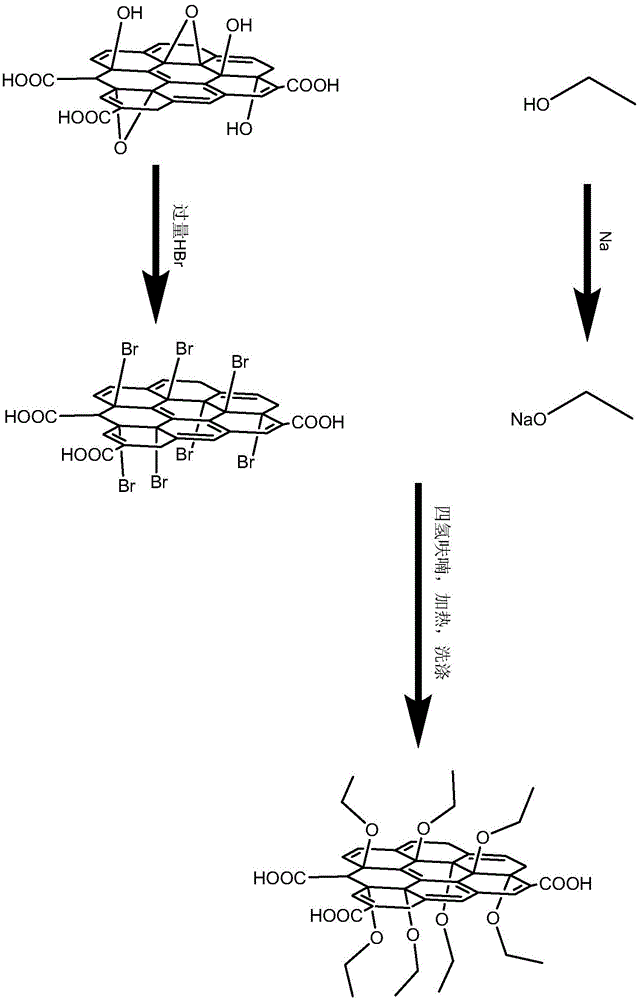
Preparation method for reinforced graphene surfactant
The invention relates to a preparation method for a reinforced graphene surfactant. The objective of the invention is to overcome the technical problem of poor compatibility between graphene oxide and organic solvents in the prior art while maintain carboxyl groups at the edge of graphene oxide and guarantee hydrophilicity of graphene oxide. The method comprises the following steps: 1, preparation of graphene oxide; 2, preparation of brominated graphene; 3, preparation of sodium ethoxide; and 4, grafting of a -OCH2CH3 group on the surface of graphene: a step of adding the brominated graphene powder prepared in the step 2 into 10 mL of tetrahydrofuran, carrying out ultrasonic treatment, then adding sodium ethoxide prepared in the step 3, continuing ultrasonic treatment, then carrying out reflux, subjecting the obtained solution to centrifugation with ethanol at first and then successively carrying out cleaning and then drying in a vacuum drying box so as to obtain a solid which is the reinforced graphene surfactant. The method has the advantages of simplicity, low cost, good repeatability, a short production period, etc. The preparation method is applied to the field of preparation of graphene surfactants.
Owner:HARBIN INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com