Star-like oxazolidine latent curing agent and preparation method as well as use thereof
A technology of latent curing agent and oxazolidine, which is applied in the preparation and application field of star-shaped oxazolidine latent curing agent, which can solve the problems of difficult mixing, low crosslinking density, uneven defoaming, etc., and meet the requirements of synthesis equipment Not high, improved tensile strength, improved heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
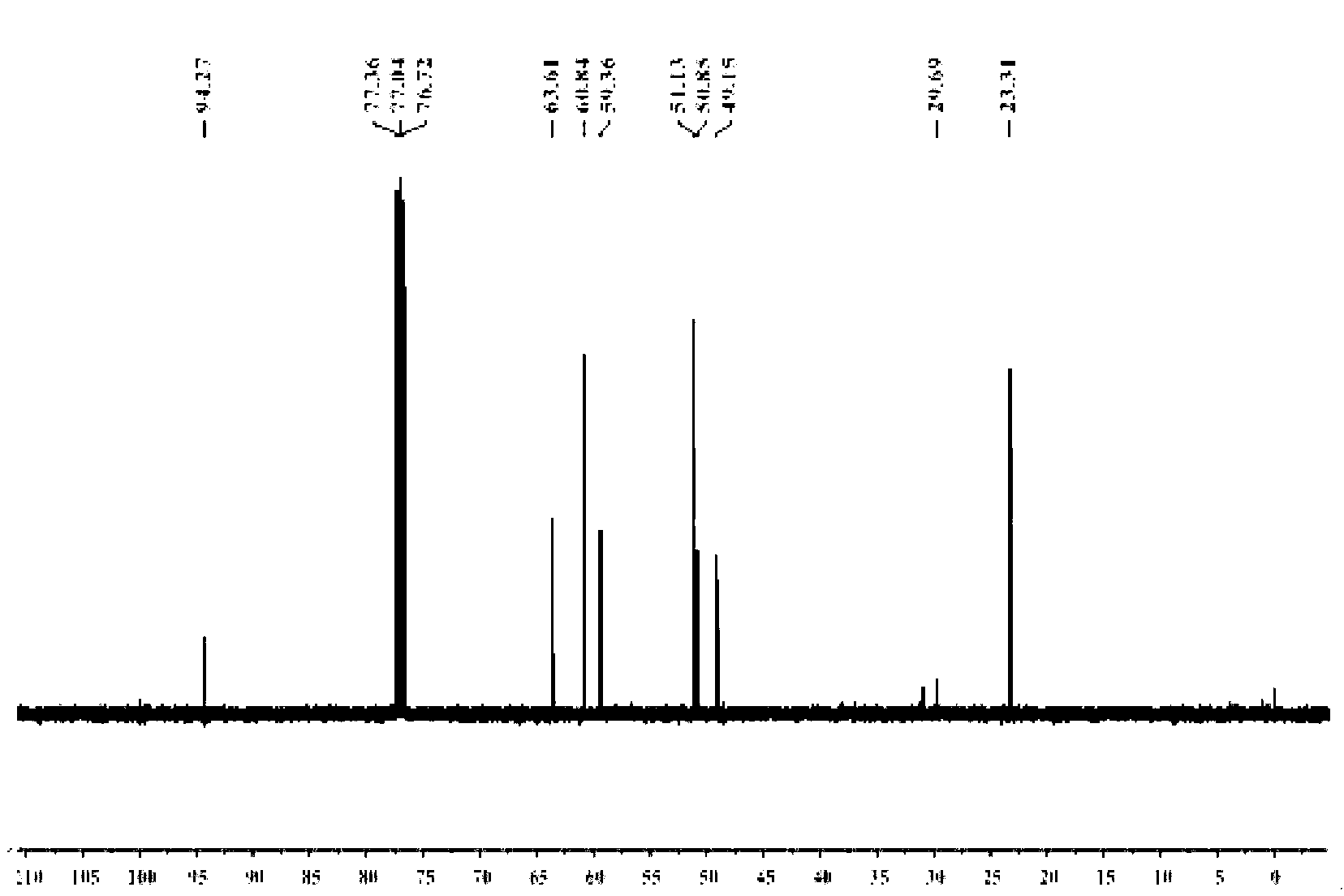
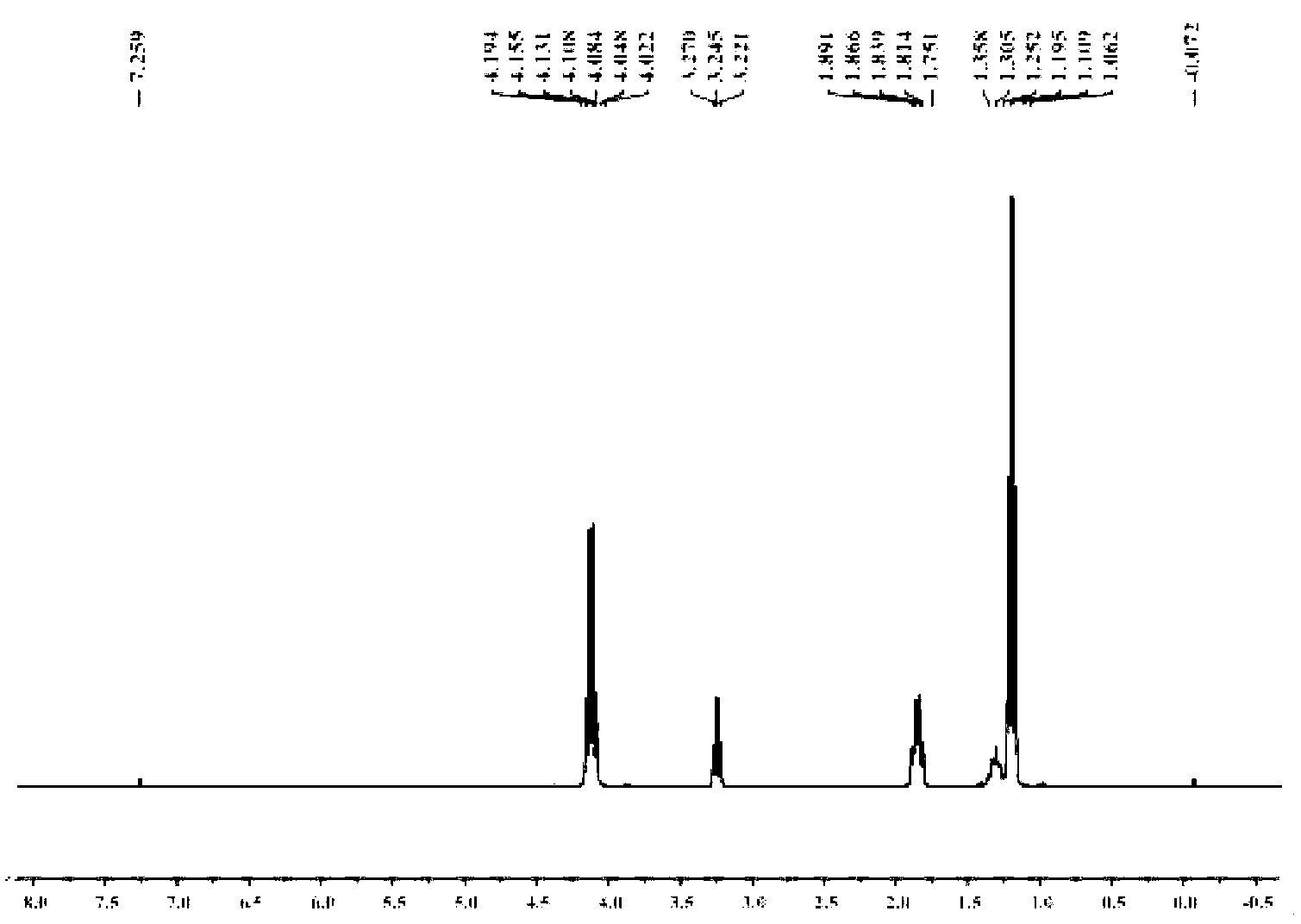
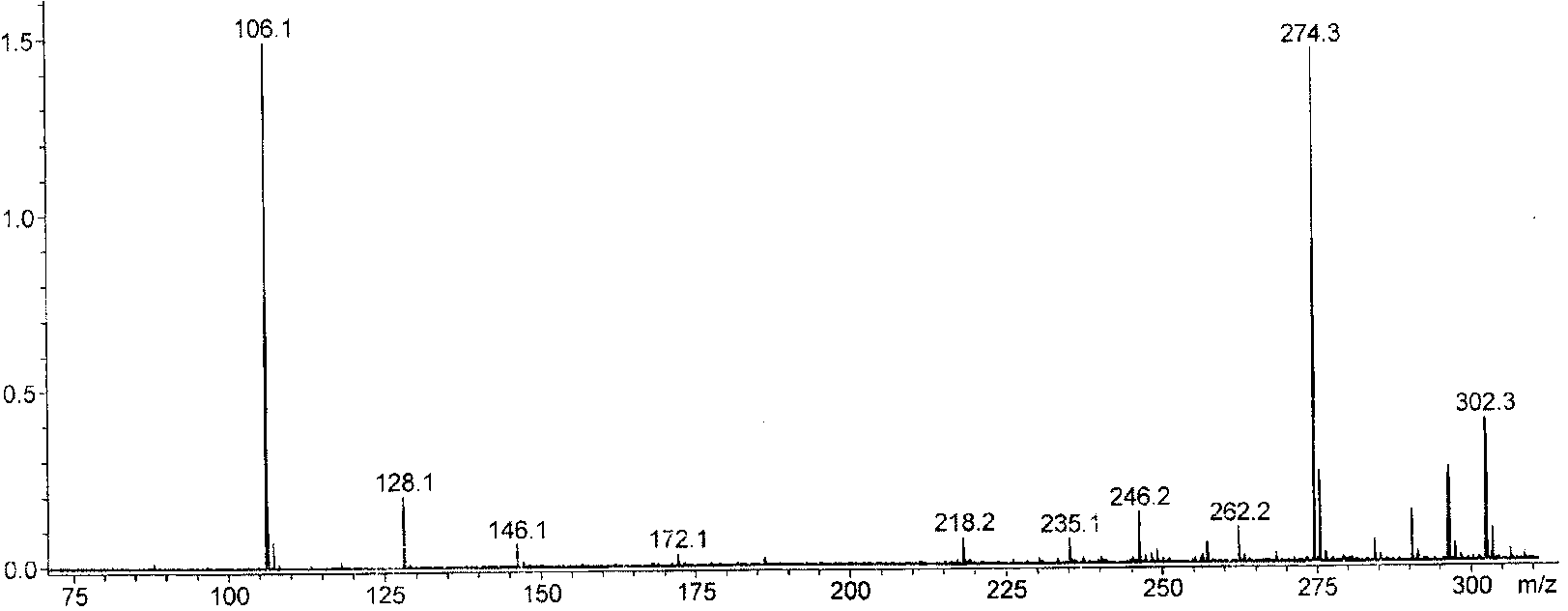
[0054] 1. Add 20.5g of diethanolamine and 50mL of toluene into the reaction flask, stir evenly, heat to 35°C, add 20g of acetone dropwise, continue the reaction for 1h, heat and reflux to divide the water, and stop the reaction when the water output reaches or approaches the theoretical value. After the reaction liquid is lowered to room temperature, heat and distill the toluene for reuse; the concentrated liquid is fractionated under reduced pressure, and the fraction at 65°C is collected at 100 Pa to obtain light yellow liquid 2,2-dimethyl-N-hydroxyethyl-1, 3-Oxazolidine (OX) 15.3 g.
[0055] 2. Add 10.5g of anhydrous diethyl malonate, 5.7g of 1,3-dibromopropane, 100mg of tetraethylammonium bromide, 3g of potassium carbonate powder, and 20mL of toluene into the reaction flask, stir evenly, and reflux the reaction 8h, the reaction solution was cooled to room temperature, distilled water was added to the reaction flask, tetraethylammonium bromide and potassium carbonate were r...
Embodiment 2
[0058] 1. Add 78.8g of diethanolamine and 150mL of toluene into the reaction flask, stir evenly, heat to 40°C, add 76.5g of acetone dropwise, continue the reaction for 1.2h, heat and reflux to divide the water, and stop the reaction when the water output reaches or approaches the theoretical value , when the reaction solution is lowered to room temperature, then heated to distill toluene and reused; the concentrated solution was fractionated under reduced pressure, and the fraction at 65°C was collected at 100 Pa to obtain light yellow liquid 2,2-dimethyl-N-hydroxyethyl-1 , 3-Oxazolidine (OX) 54.4g.
[0059] 2. Add 50.5g of anhydrous diethyl malonate, 25.5g of 1,3-dibromopropane, 200mg of tetraethylammonium bromide, 30g of potassium carbonate powder, and 120mL of toluene into the reaction flask, stir well, and reflux for 9h , the reaction solution was cooled to room temperature, distilled water was added to the reaction flask, tetraethylammonium bromide and potassium carbonate...
Embodiment 3
[0062] 1. Add 150.0g of diethanolamine and 300mL of toluene into the reaction flask, stir evenly, heat to 45°C, add 180.0g of acetone dropwise, continue the reaction for 1.5h, heat and reflux to divide the water, and stop the reaction when the water output reaches or approaches the theoretical value , when the reaction solution is lowered to room temperature, then heated to distill toluene and reused; the concentrated solution was fractionated under reduced pressure, and the fraction at 65°C was collected at 100 Pa to obtain light yellow liquid 2,2-dimethyl-N-hydroxyethyl-1 , 3-Oxazolidine (OX) 118.5g.
[0063] 2. Add 100g of anhydrous diethyl malonate, 50g of 1,3-dibromopropane, 400mg of tetraethylammonium bromide, 60g of potassium carbonate powder, and 200mL of toluene into the reaction flask, stir evenly, and reflux for 11 hours. Cool the reaction solution to room temperature, add distilled water to the reaction bottle, remove tetraethylammonium bromide and potassium carbon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com