Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Bosentan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
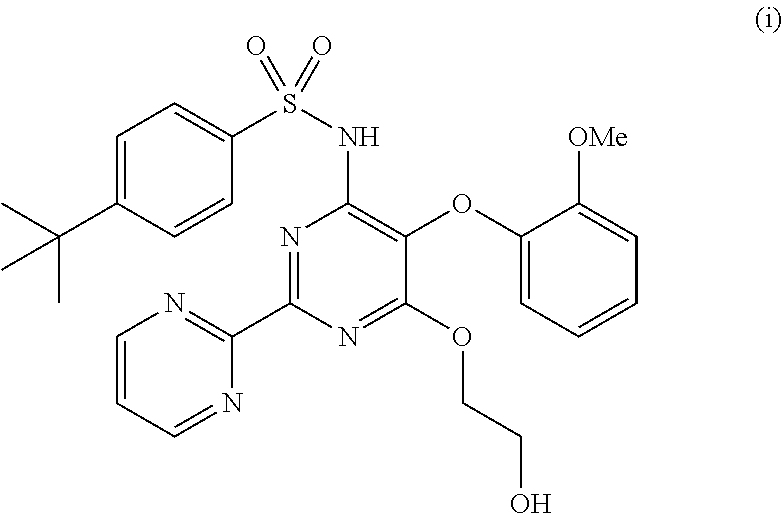
Bosentan is used to treat high blood pressure in the lungs (pulmonary arterial hypertension). This condition is thought to be caused by increased levels of a certain natural substance (endothelin-1).
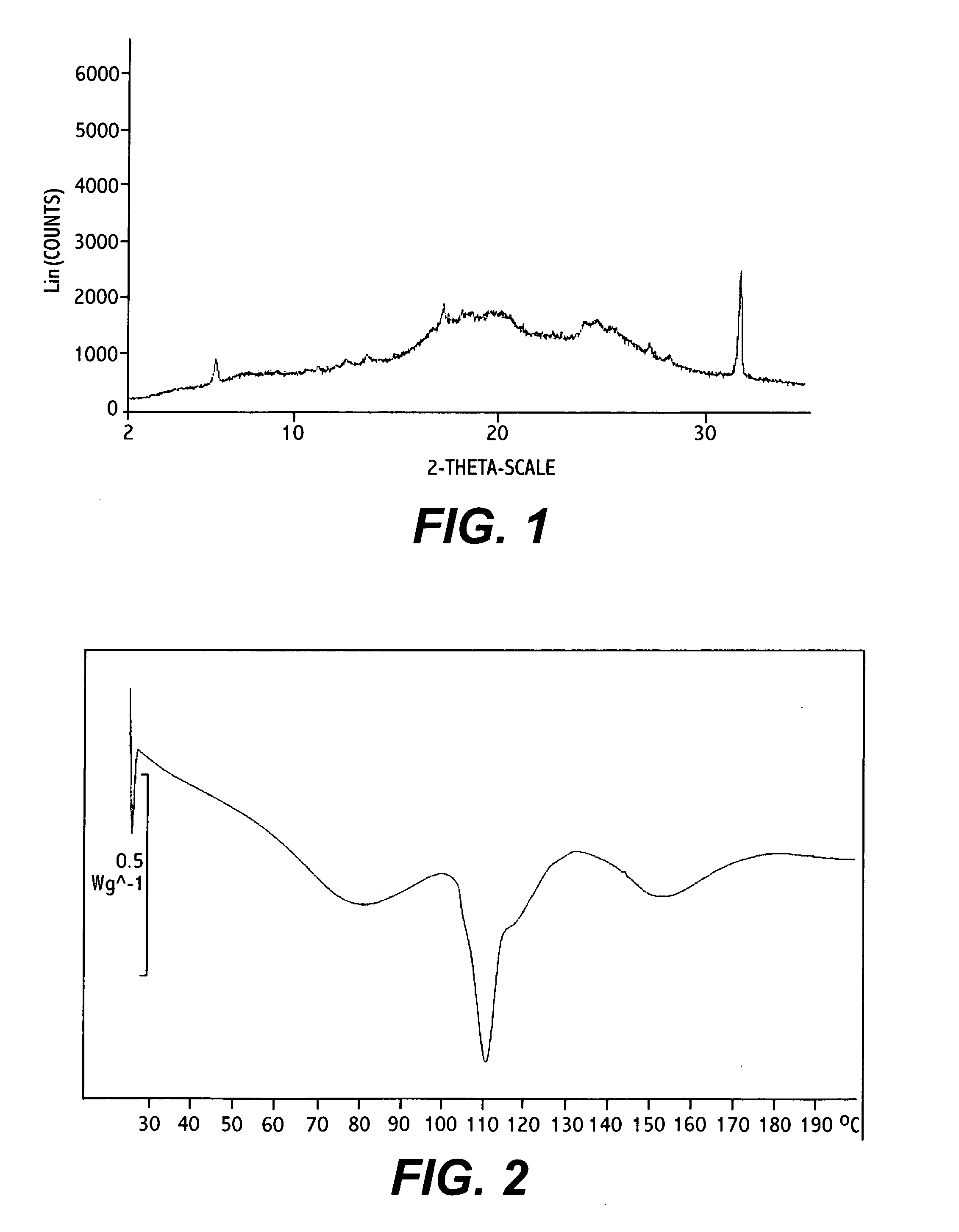
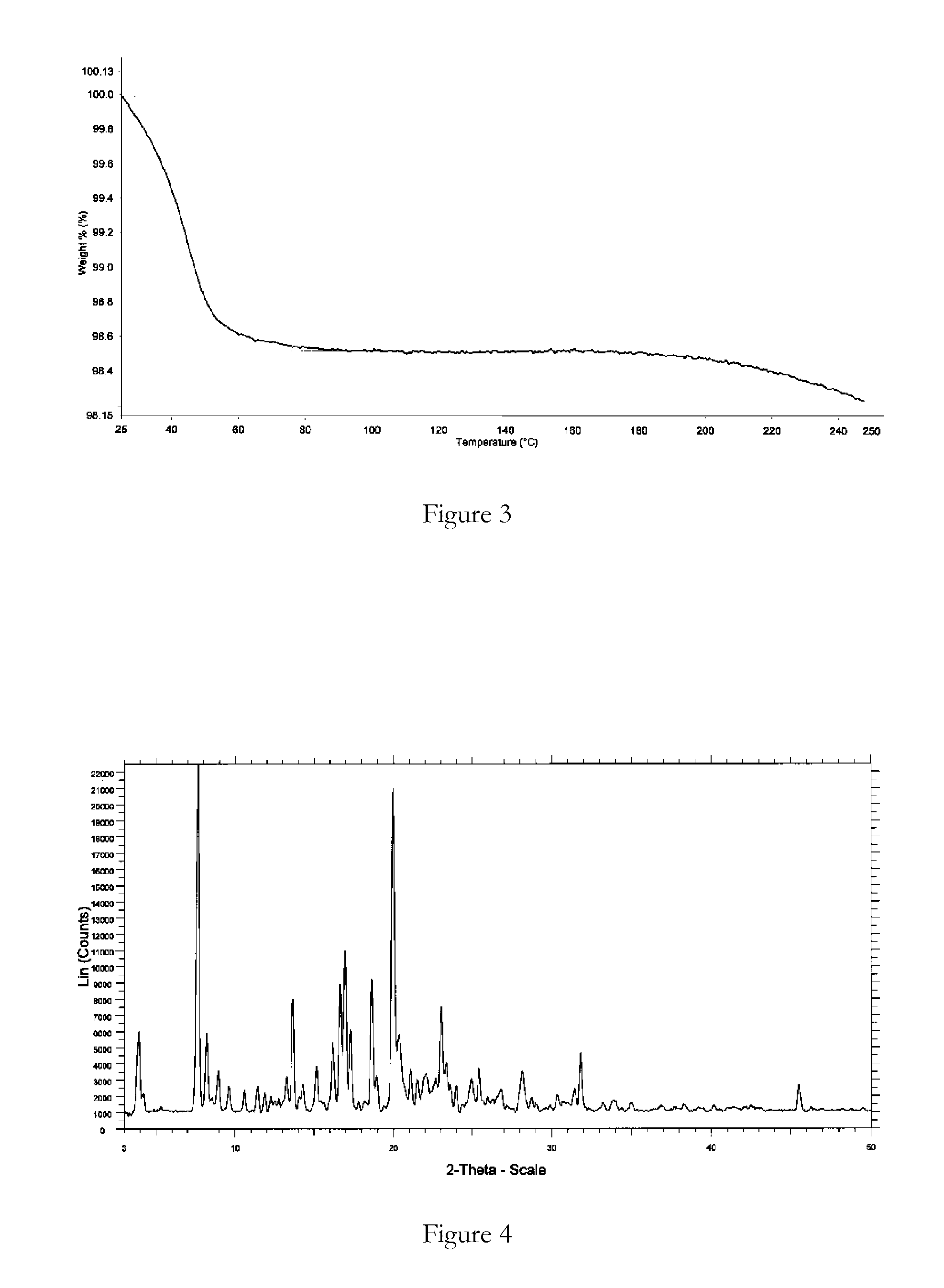
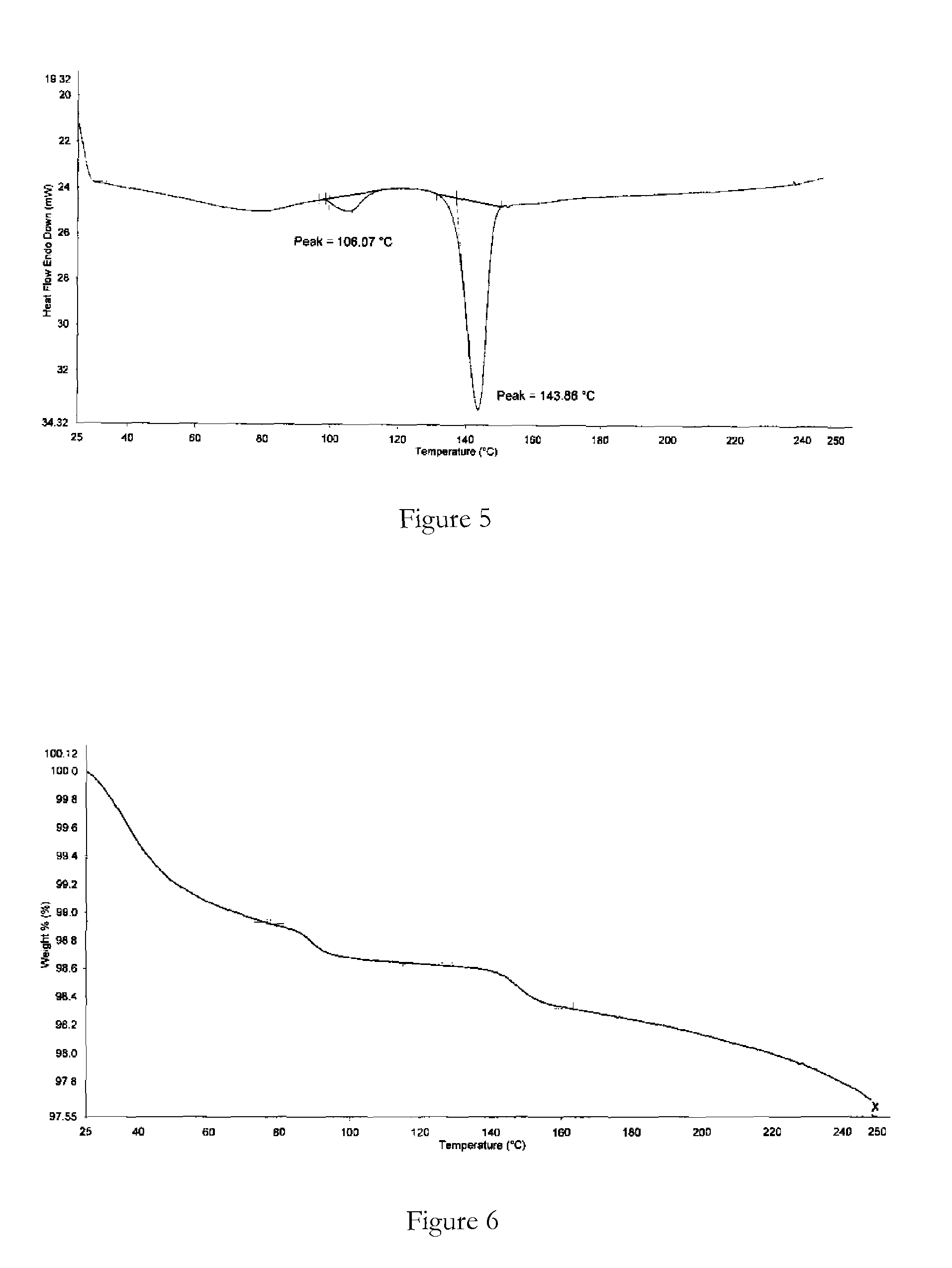
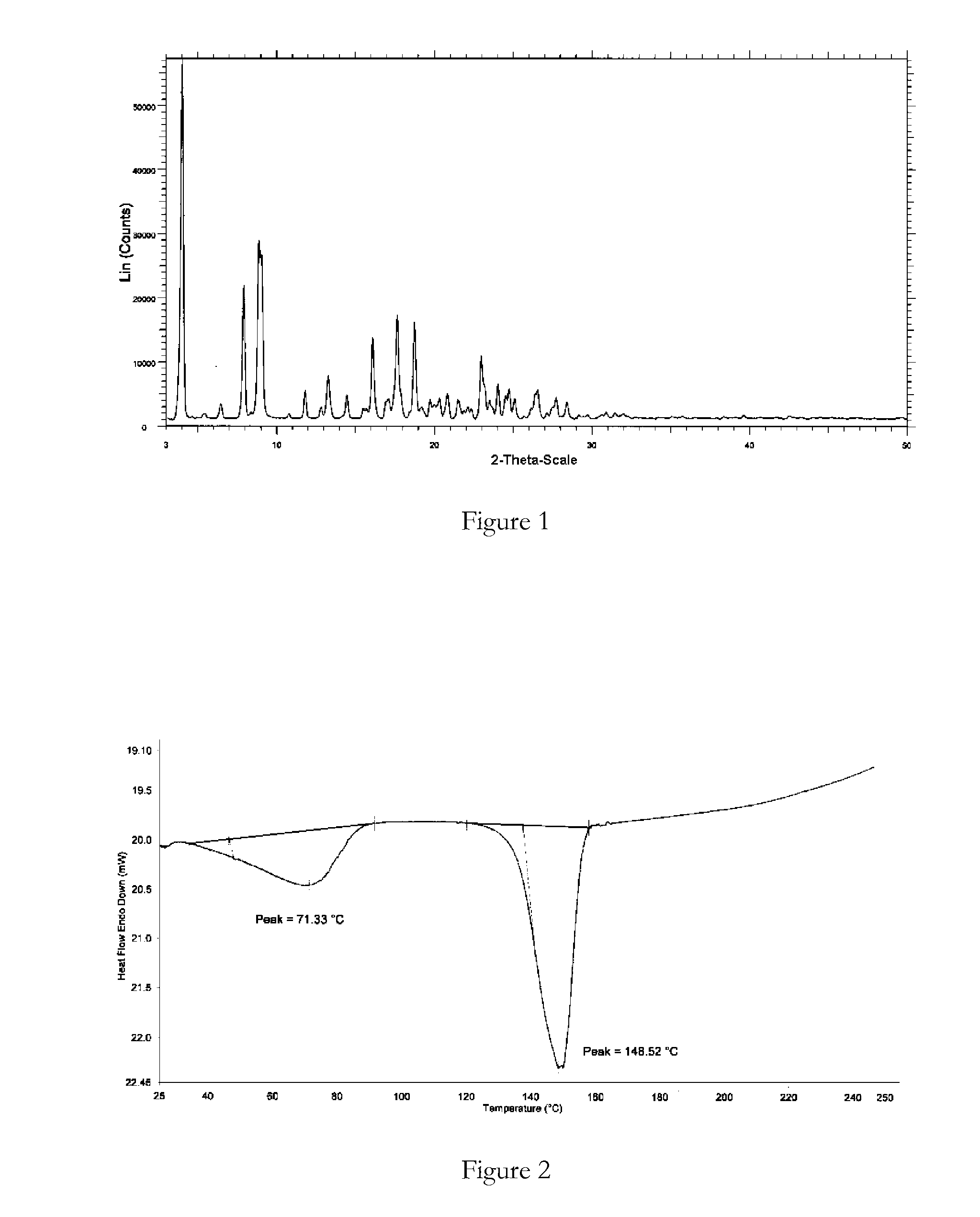
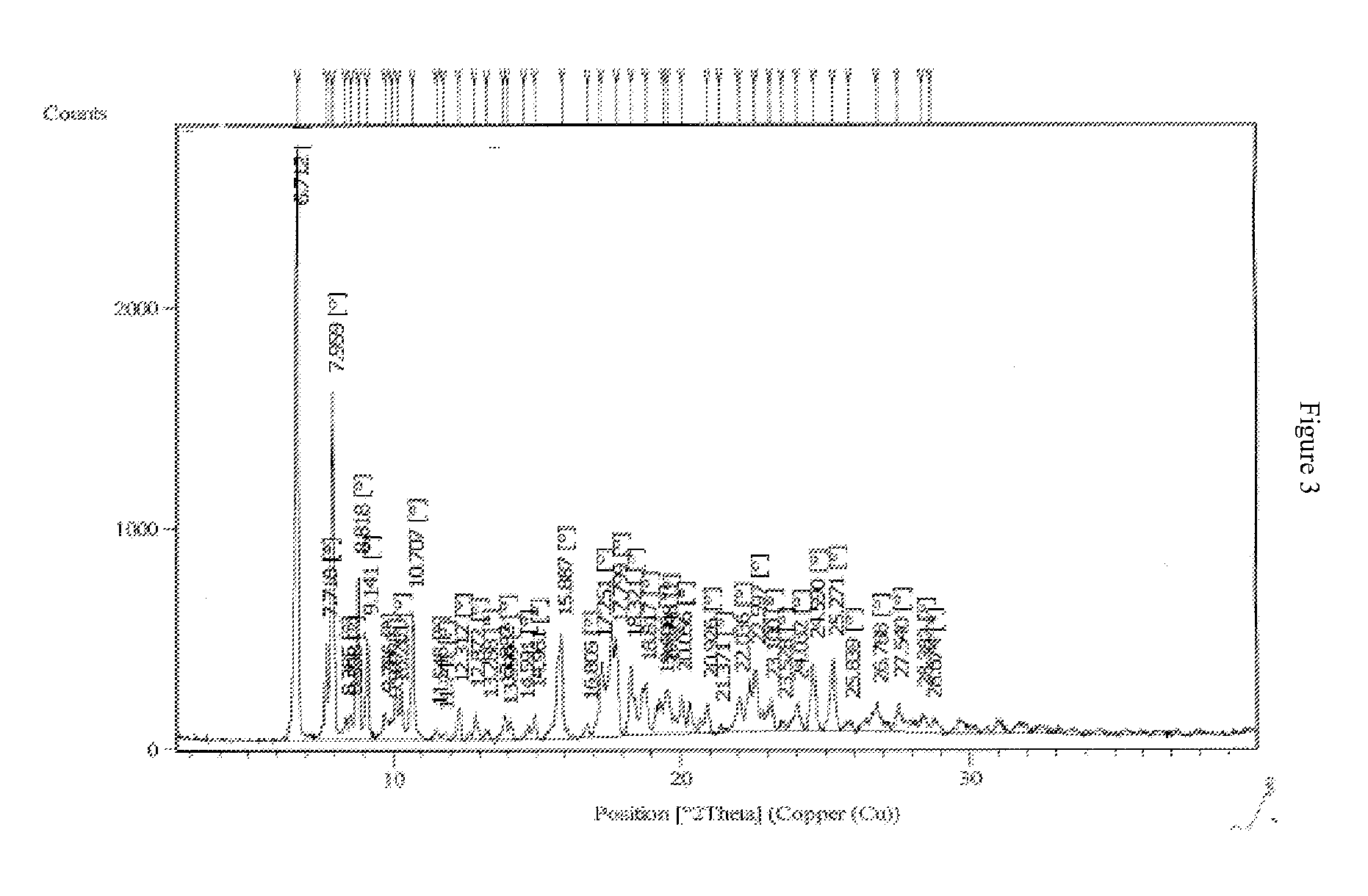
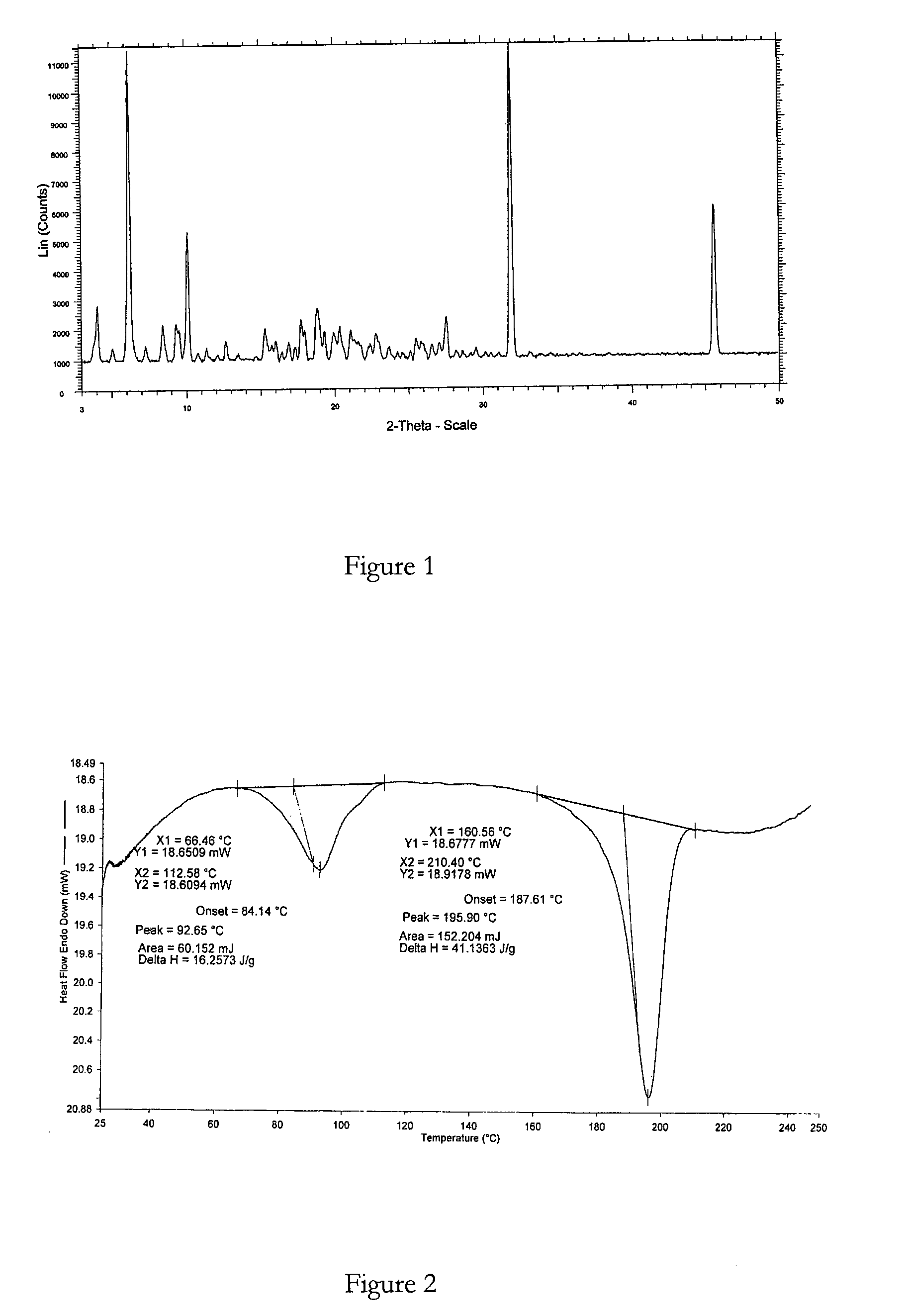
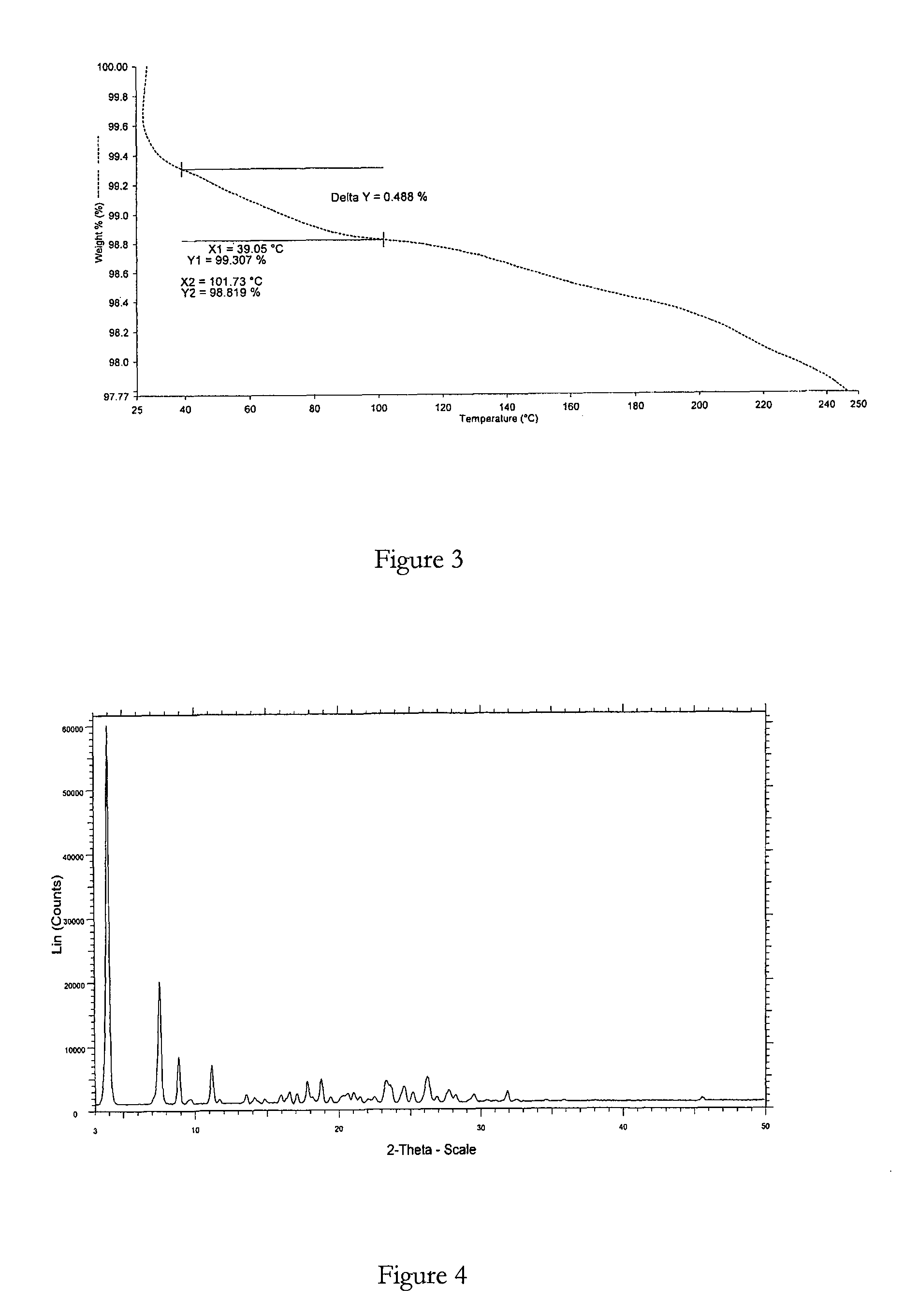
Polymorphic forms
ActiveUS8288401B2Improve solubilityImprove bioavailabilityOrganic active ingredientsBiocideAnginaBosentan
Novel polymorphic forms of bosentan and processes for their preparation are disclosed. Further, pharmaceutical compositions comprising said polymorphic forms and the use of said compositions in the treatment of patients suffering from endothelin receptor mediated disorders, for example, cardiovascular disorders such as hypertension, pulmonary hypertension, ischemia, vasospasm and angina pectoris are disclosed.
Owner:GENERICS UK LTD
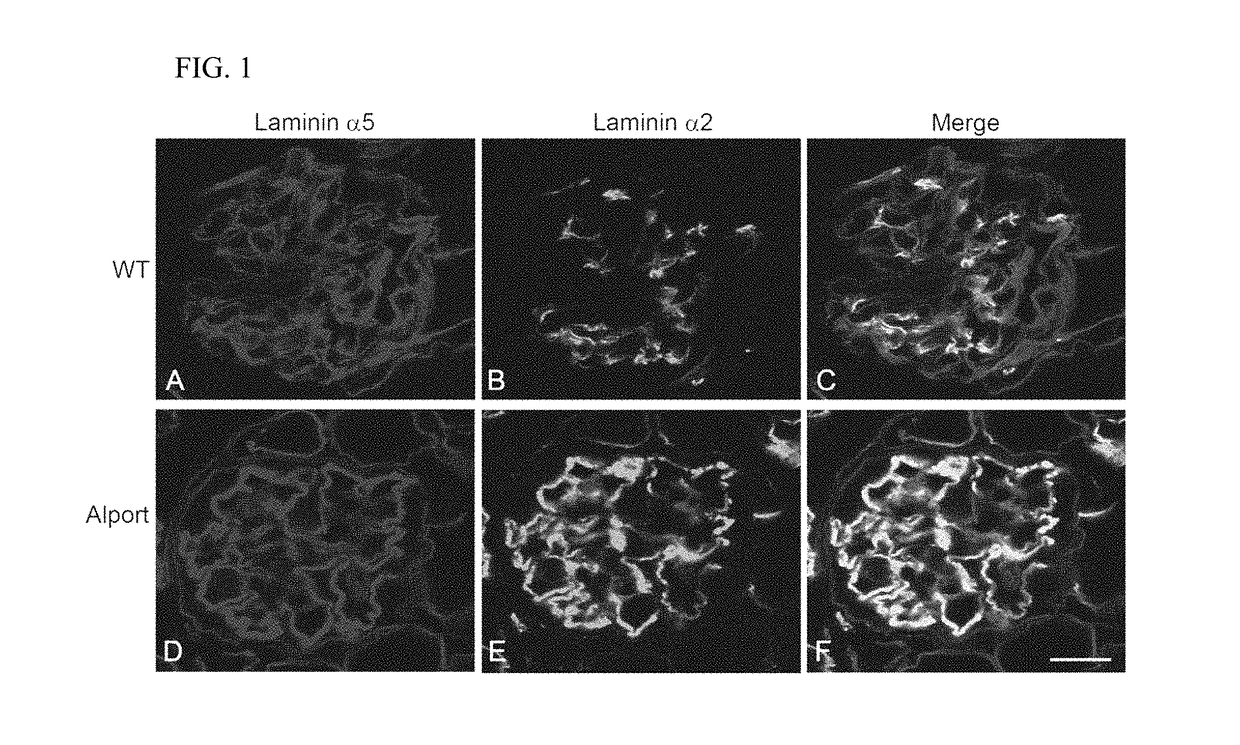
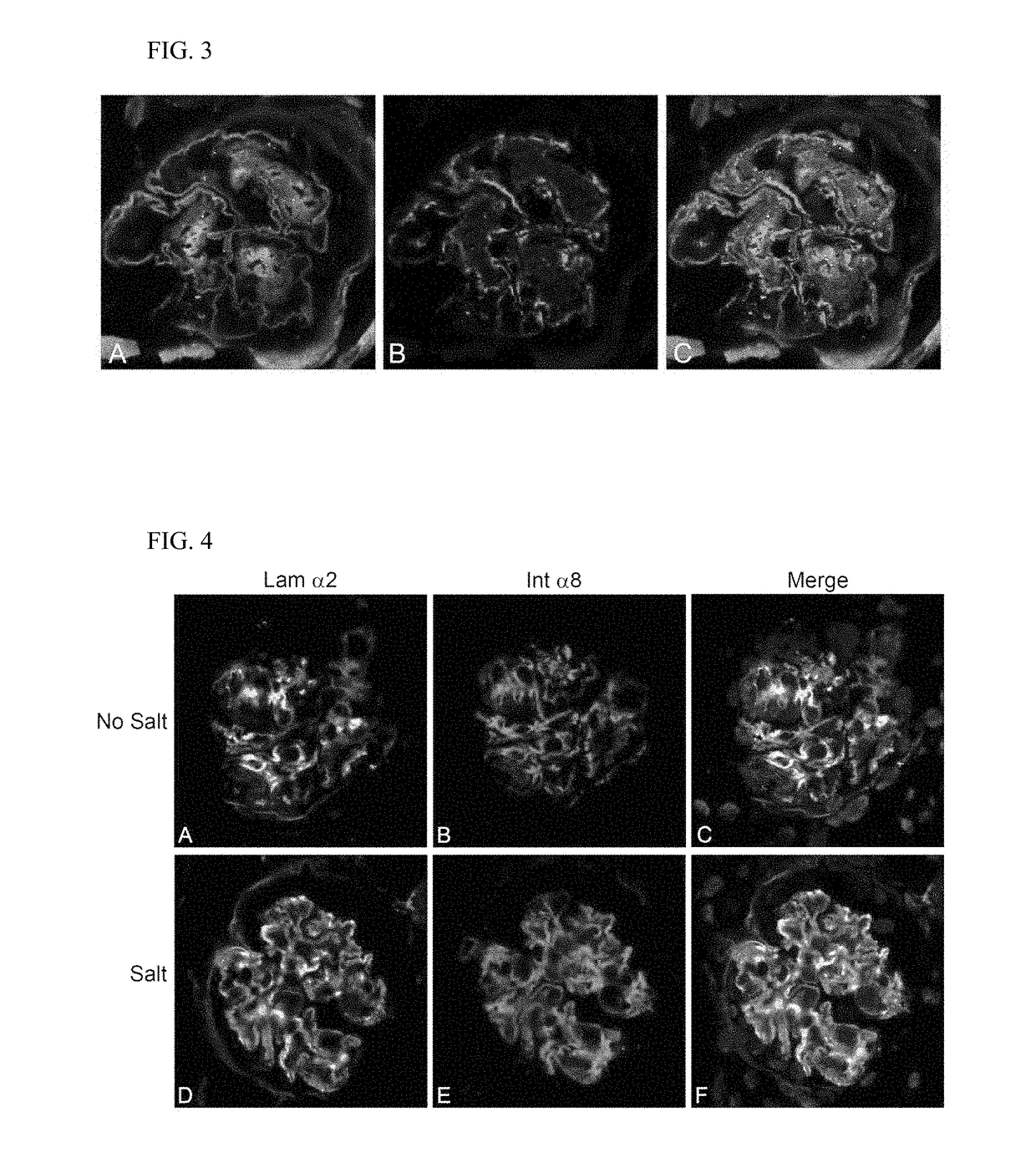
Rac1 inhibitors for the treatment of alport glomerular disease
The present invention provides methods of treating Alport syndrome in a subject by the administration of an agent that can blocks the activation of RAC1 / CDC42 members of the rho family of small GTPases. Such agents include, but are not limited to, the endothelin receptor antagonists such as bosentan and letairis and neutralizing antibodies to endothelin-1. Such administration prevents invasion of the glomerular capillary tufts by mesangial lamellipodial / filopodial processes, blocks mesangial process invasion abrogates the deposition of laminin 211 in the GBM, and prevents the activation of maladaptive expression of proteins known to contribute to glomerular disease progression.
Owner:FATHER FLANAGANS BOYS HOME DOING BUSINESS AS BOYS TOWN NAT RES HOSPITAL
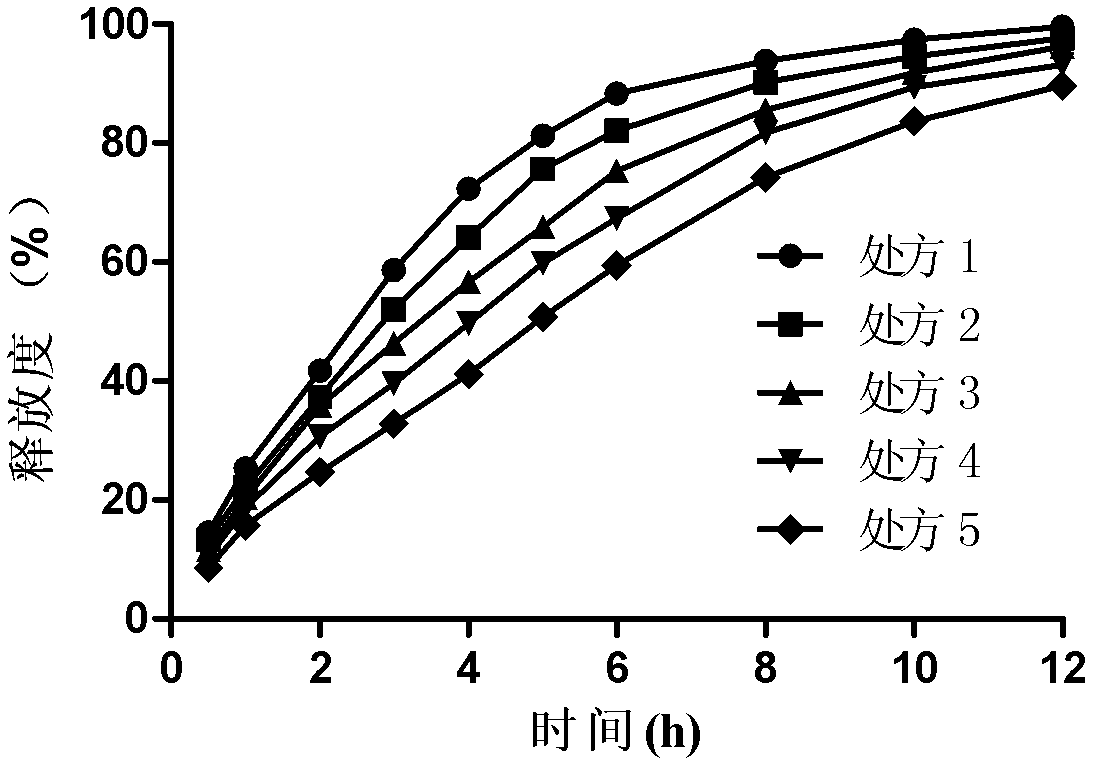
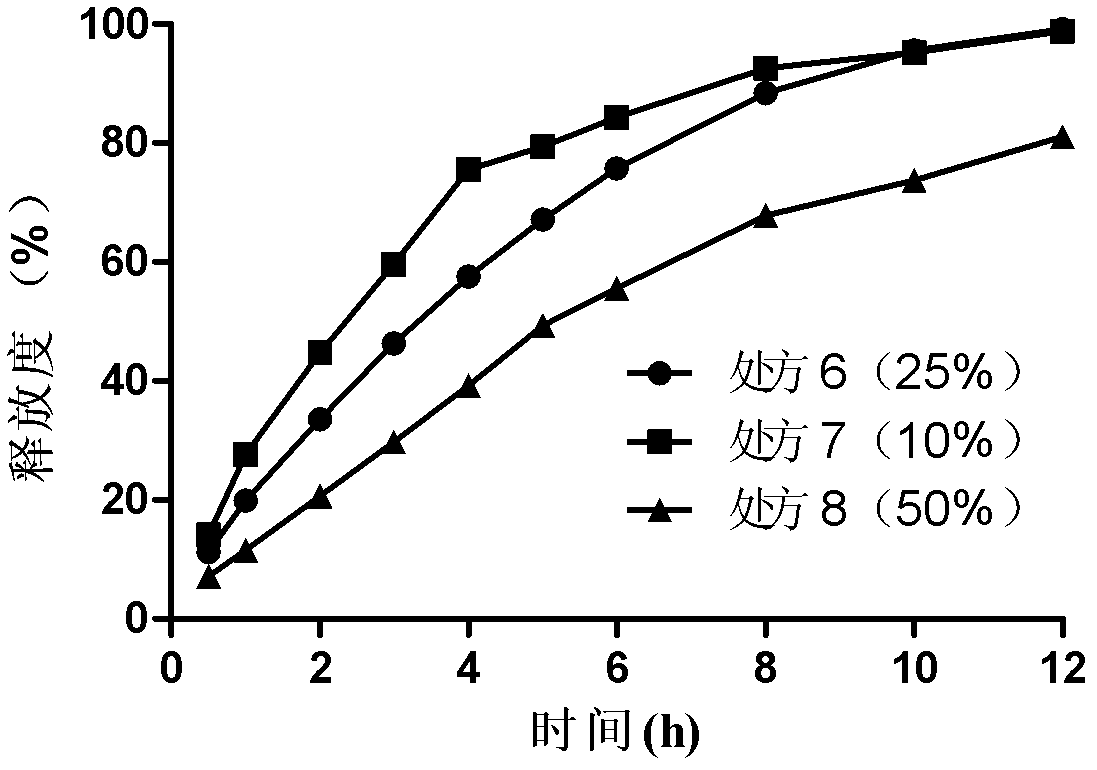
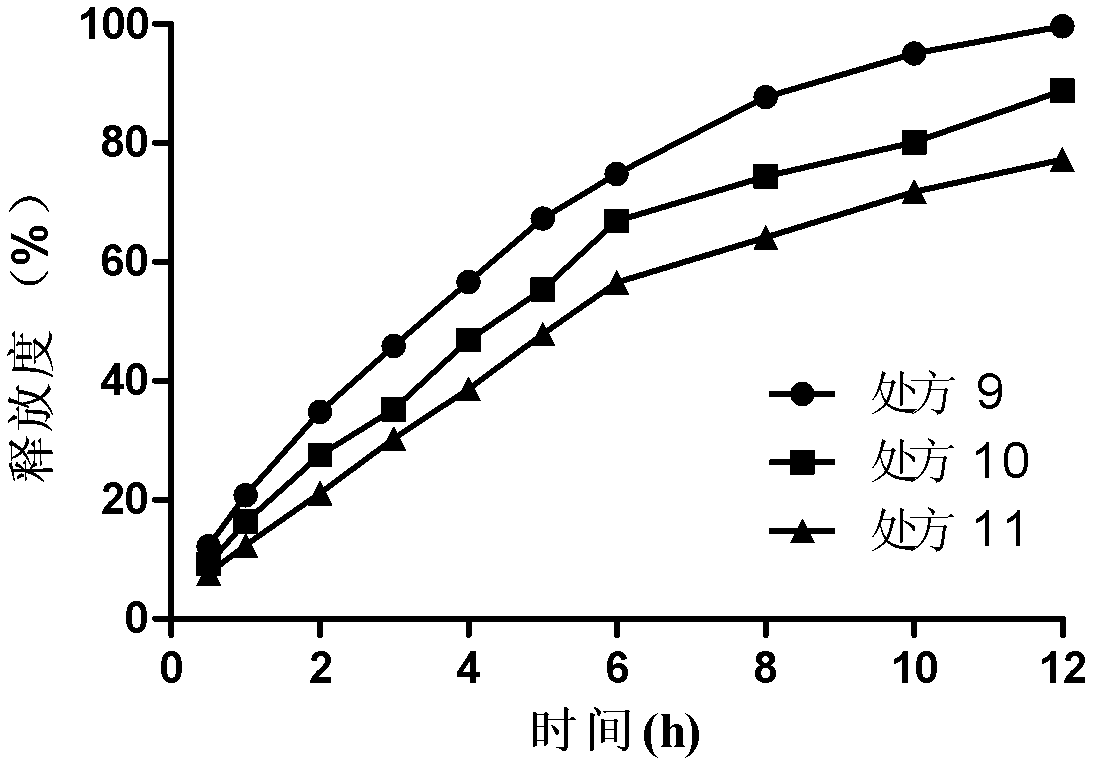
Bosentan sustained-release matrix tablet and preparation method thereof
InactiveCN102600094ALasting effectSmall toxicityOrganic active ingredientsPharmaceutical delivery mechanismProlonged-release tabletBosentan
The invention discloses a bosentan sustained-release matrix tablet and a preparation method thereof, which are characterized in that bosentan is adopted as an active ingredient, auxiliary materials such as sustained-release matrix materials, fillers, lubricants, pH adjusting agents, moistening agents and the like are added, and a wet pelleting technology or a dry direct tabletting technology is adopted, so that a gel sustained-release matrix tablet is prepared. Experimental results show that the prepared sustained-release tablet is slowly and durably released both inside and outside a human body, and a good biological availability inside the human body can be realized.
Owner:XIAN LIBANG ZHAOXIN BIOTECH CO LTD
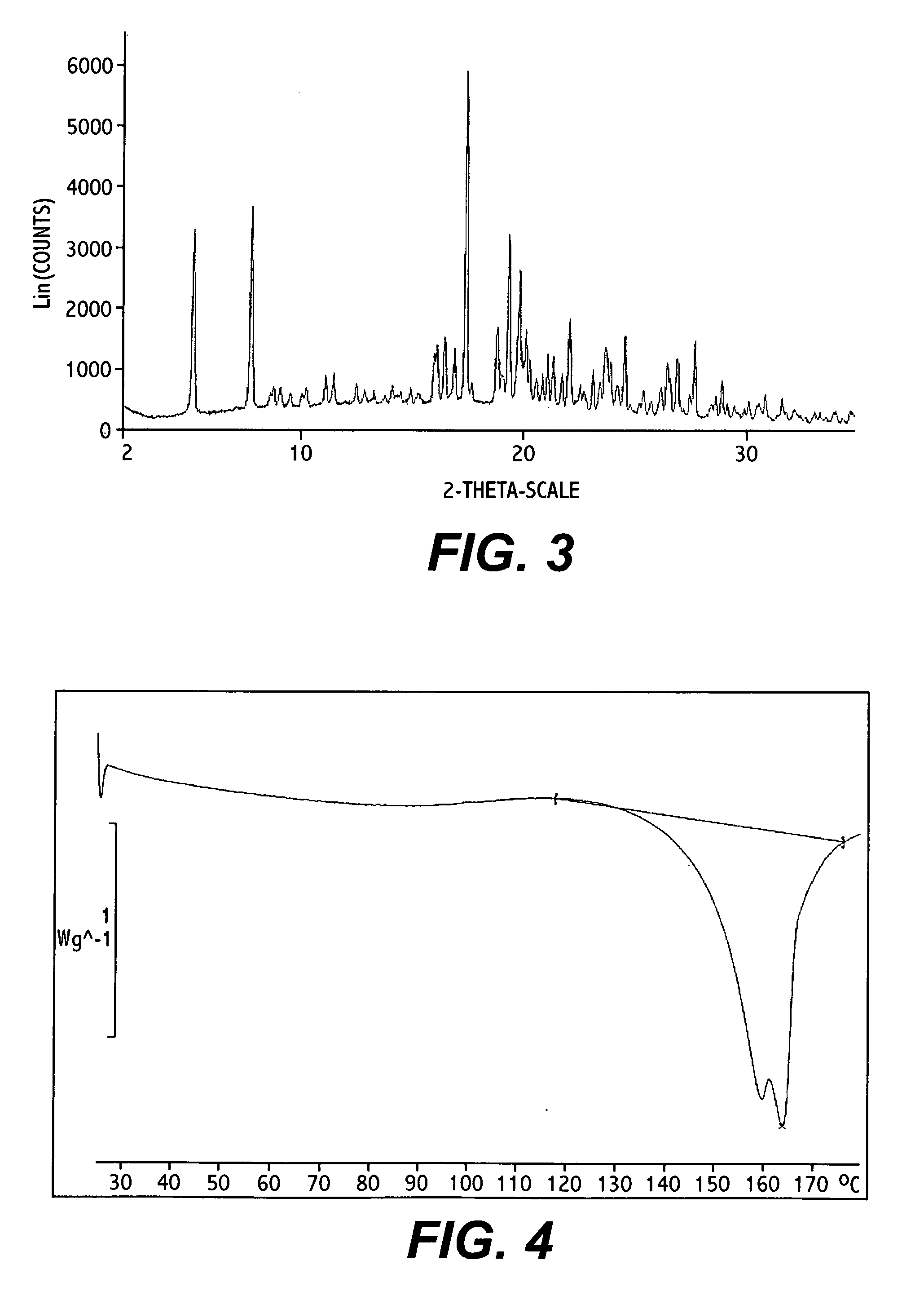
Novel polymorphic forms
ActiveUS20100261742A1Maintain good propertiesImprove solubilityBiocideOrganic active ingredientsAnginaBosentan
Novel polymorphic forms of bosentan and processes for their preparation are disclosed. Further, pharmaceutical compositions comprising said polymorphic forms and the use of said compositions in the treatment of patients suffering from endothelin receptor mediated disorders, for example, cardiovascular disorders such as hypertension, pulmonary hypertension, ischemia, vasospasm and angina pectoris are disclosed.
Owner:GENERICS UK LTD
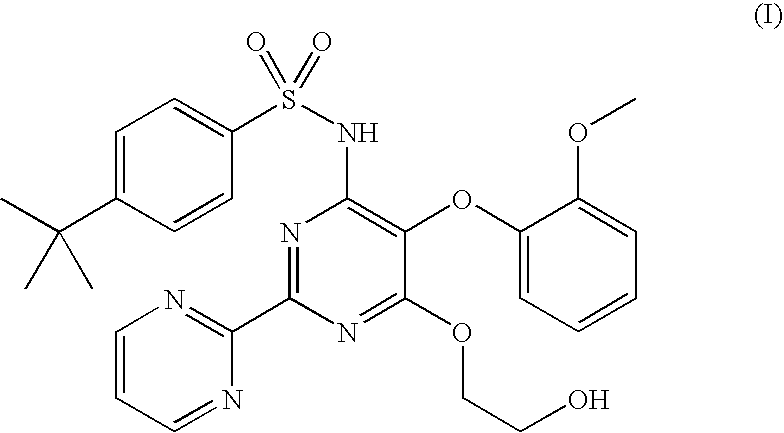
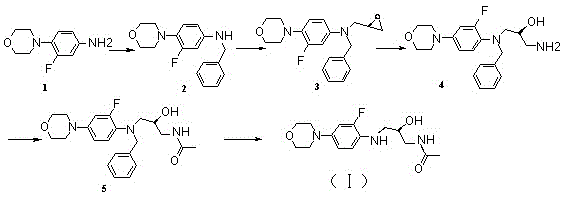
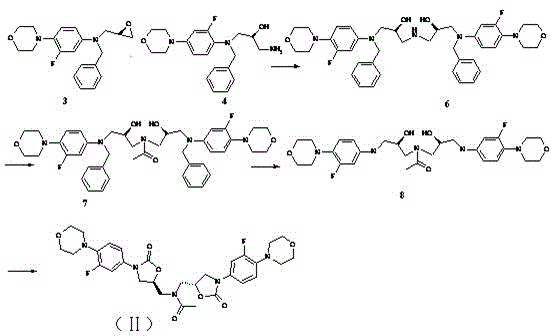
Process for preparation of endothelial receptor antagonist (bosentan)
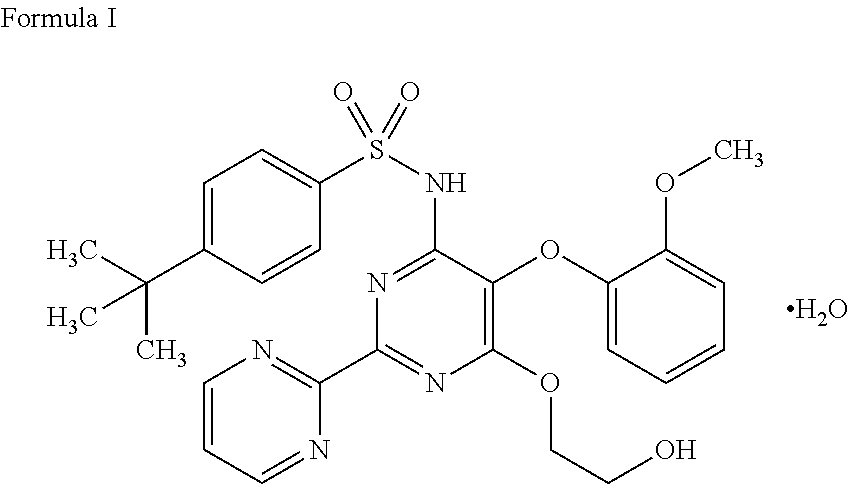
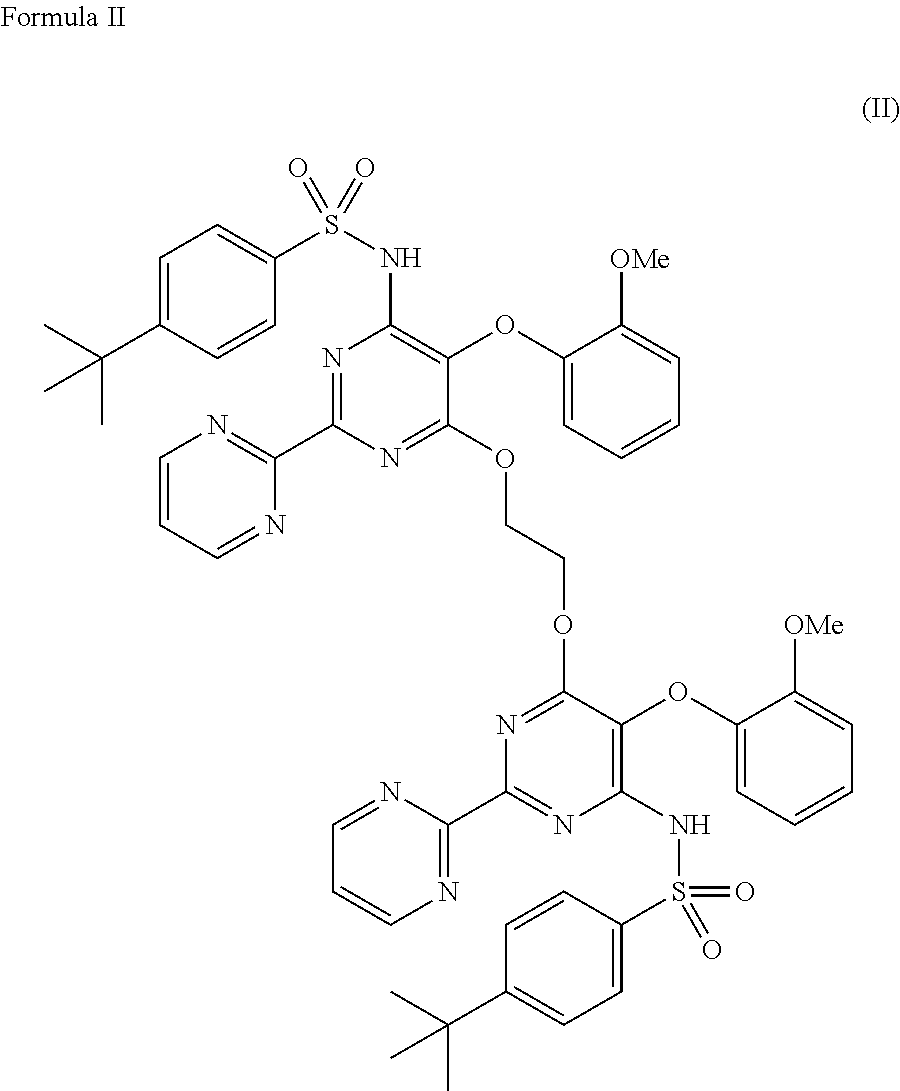
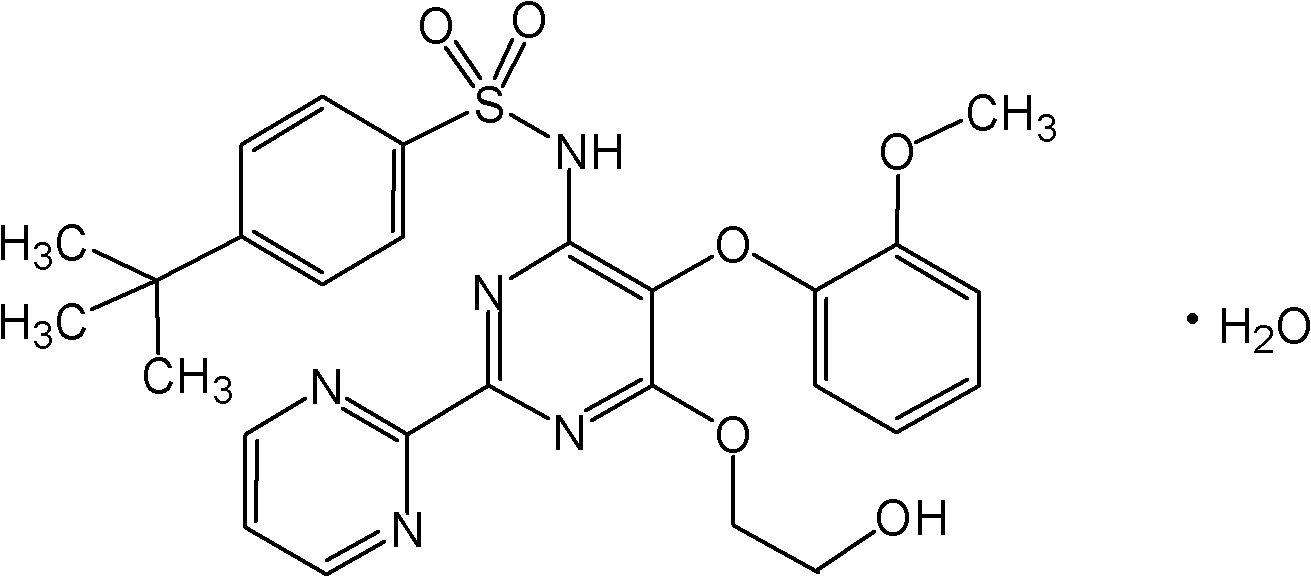
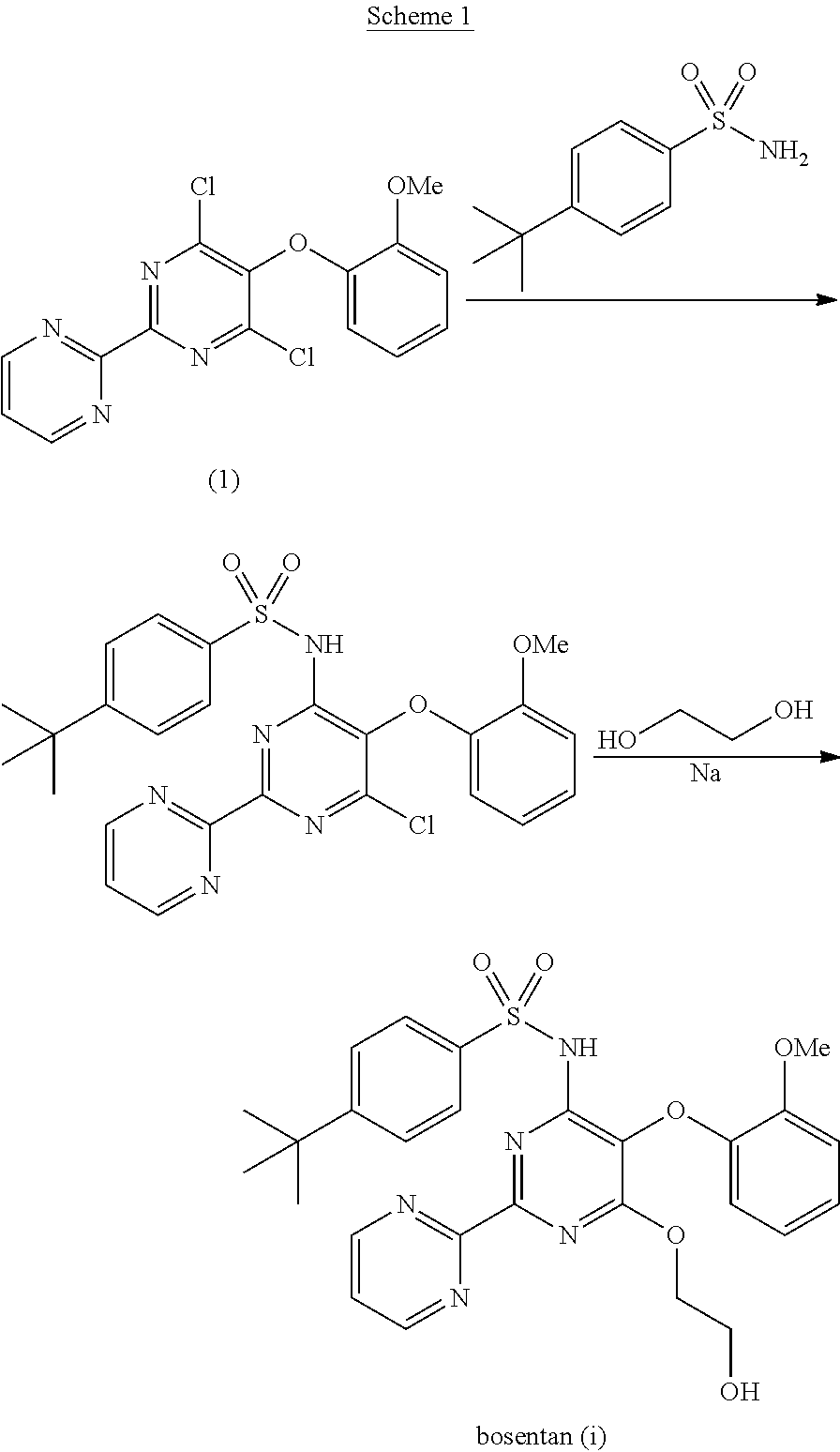
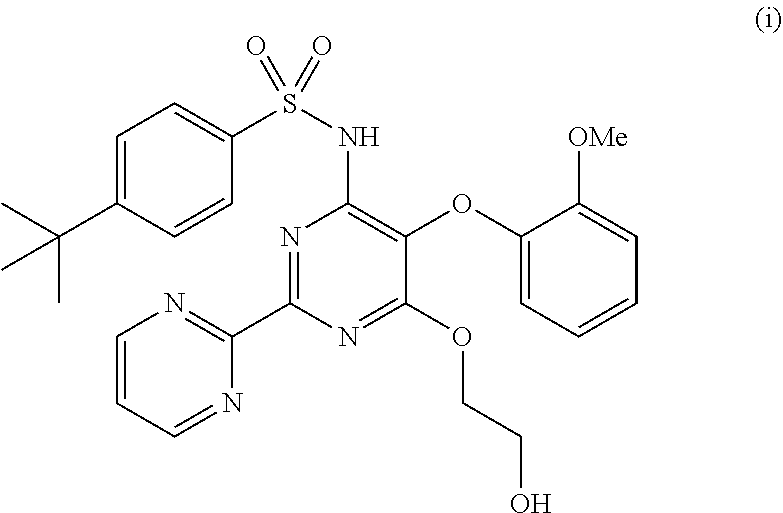
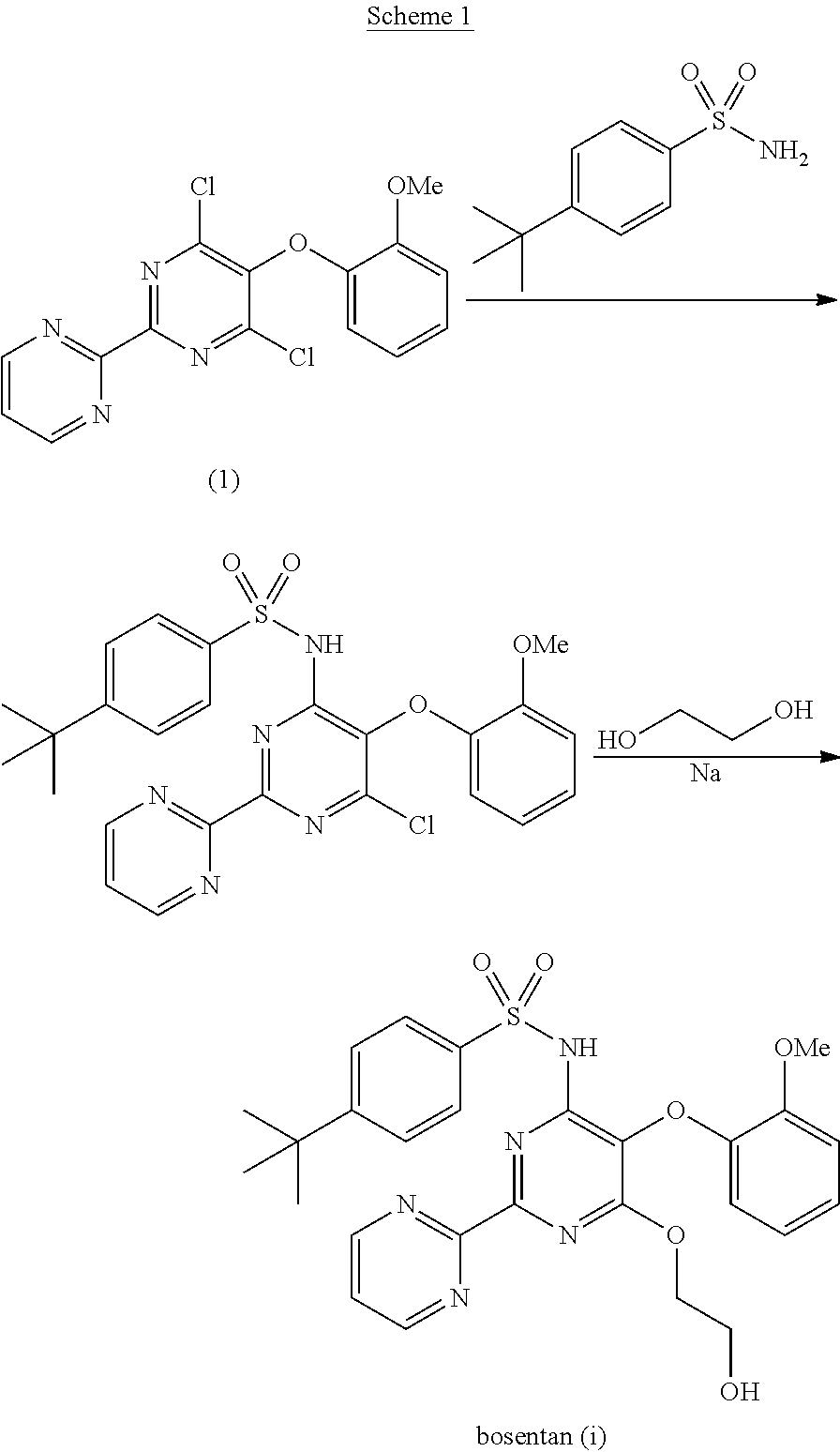
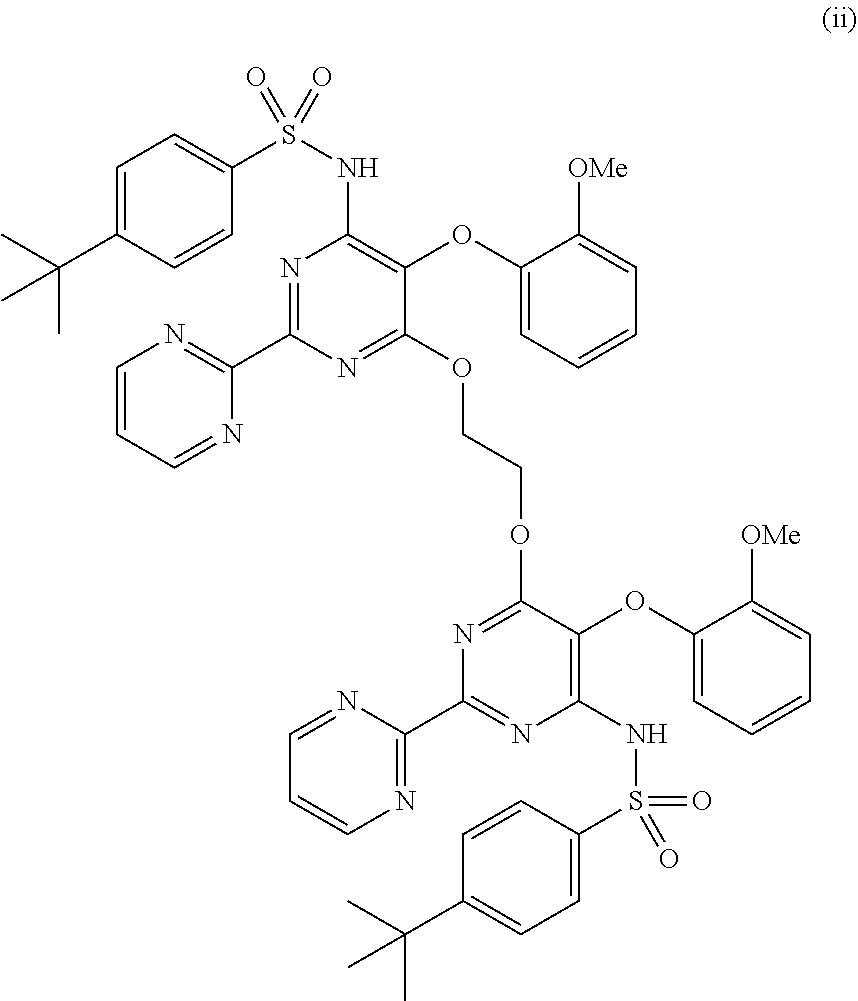
The present invention relates to processes for the preparation of an endothelial receptor antagonist. The present invention particularly relates to synthesis of 4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-(2-pyrimidinyl)-4-pyrimidinyl]benzene sulfonamide (bosentan).
Owner:SANDOZ AG
Preparation method of linezolid derivative
The invention discloses a preparation method of a linezolid derivative. Abundant screening experiments are performed to determine the optimal reactant consumption, reaction temperature, reaction time, reaction solvent and the like in the preparation technique; and the whole preparation technique has the advantages of high operability, high preparation efficiency and low production cost, and can implement industrialized mass production. The bosentan metabolite (hydroxy bosentan) prepared by the method has the advantages of higher bioavailability and lower untoward effect, and directly can have the antihypertensive effect.
Owner:TLC NANJING PHARMA RANDD CO LTD
Process for preparation of endothelial receptor antagonist (bosentan)
The present invention relates to processes for the preparation of an endothelial receptor antagonist. The present invention particularly relates to synthesis of 4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-(2-pyrimidinyl)-4-pyrimidinyl] benzene sulfonamide (bosentan).
Owner:SANDOZ LTD
RAC1 inhibitors for the treatment of alport glomerular disease
The present invention provides methods of treating Alport syndrome in a subject by the administration of an agent that can block the activation of RAC1 / CDC42 members of the rho family of small GTPases. Such agents include, but are not limited to, the endothelin receptor antagonists such as bosentan and letairis and neutralizing antibodies to endothelin-1. Such administration prevents invasion of the glomerular capillary tufts by mesangial lamellipodial / filopodial processes, blocks mesangial process invasion abrogates the deposition of laminin 211 in the GBM, and prevents the activation of maladaptive expression of proteins known to contribute to glomerular disease progression.
Owner:FATHER FLANAGANS BOYS HOME DOING BUSINESS AS BOYS TOWN NAT RES HOSPITAL
Process for preparing bosentan
Owner:ALEMBIC PHARMA
Bosentan controlled release oral preparation
InactiveUS20140377346A1Ease of administrationEase of patient complianceBiocideOrganic active ingredientsControl releaseOral medication
Provided are an extended release preparation for oral administration of bosentan including bosentan, a pharmaceutically acceptable salt thereof or a solvate thereof as an active ingredient and a hydrophilic polymer as a sustained release excipient, and a method of manufacturing the same. Accordingly, the number of administrations of the bosentan, a pharmaceutically acceptable salt thereof or a solvate thereof may be reduced, thereby enhancing ease of administration and patient compliance. In addition, the extended release preparation for oral administration of bosentan may be effectively manufactured.
Owner:HANALL PHARMA CO LTD
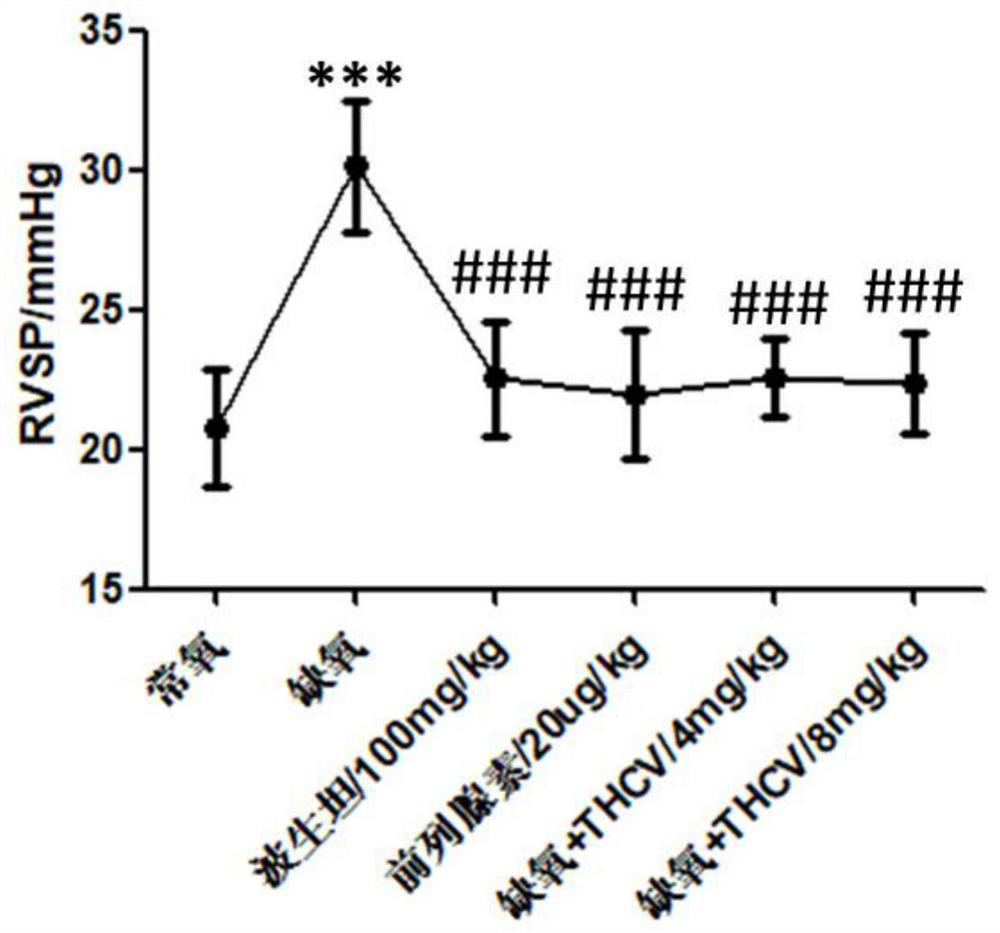
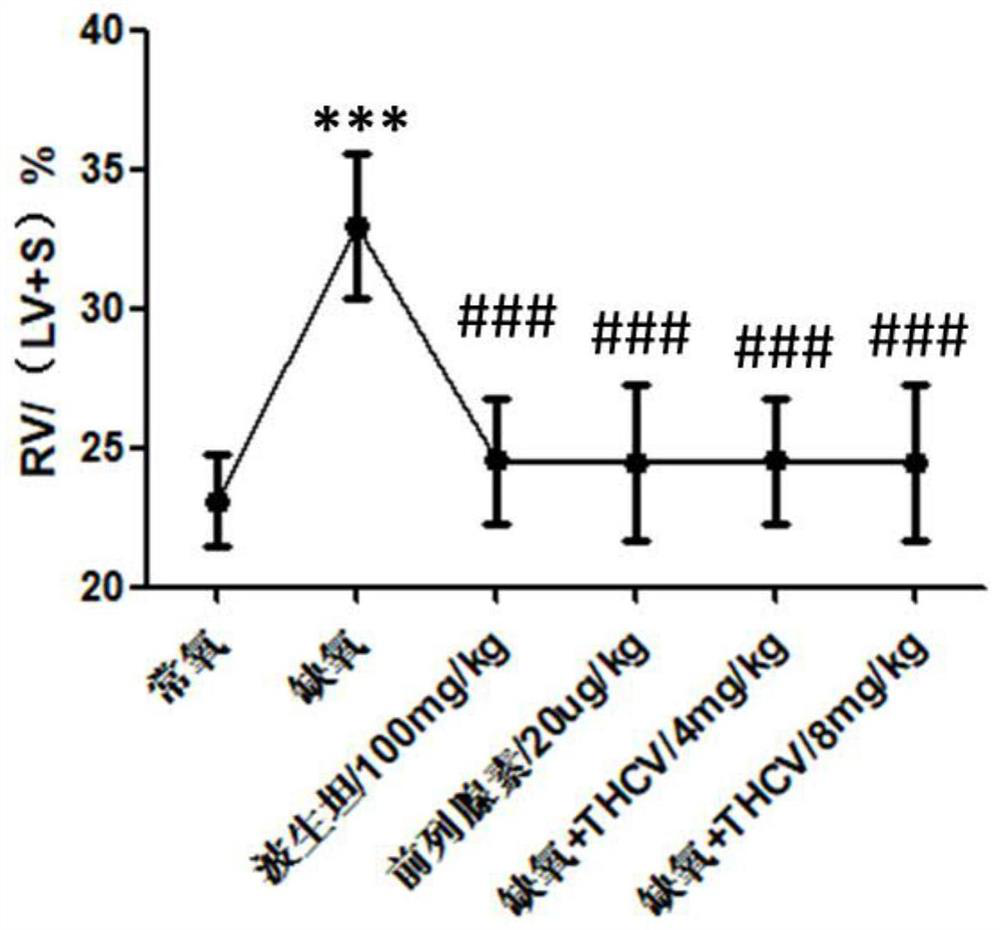
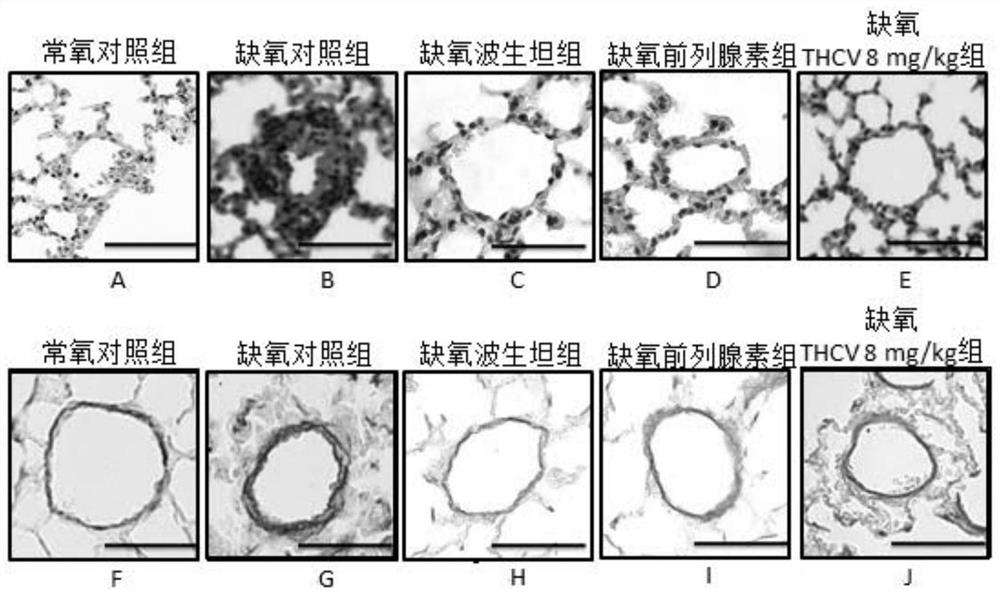
Application of tetrahydrocannabidivarin in preparation of medicine for treating pulmonary arterial hypertension and medicine composition containing tetrahydrocannabidivarin
ActiveCN112569220ALittle side effectsOrganic active ingredientsRespiratory disorderSide effectBosentan
The invention discloses a novel application of tetrahydrocannabidivarin (THCV). The application comprises preparation of a medicine for treating pulmonary arterial hypertension, and application to combined use with bosentan for reducing the side effect of bosentan in treating pulmonary arterial hypertension. The invention also provides a pharmaceutical composition for treating pulmonary arterial hypertension. The pharmaceutical composition comprises tetrahydrocannabidivarin and a medicinal carrier suitable for treating pulmonary arterial hypertension. The invention also provides a pharmaceutical composition for treating pulmonary arterial hypertension in combination with bosentan, wherein the pharmaceutical composition comprises tetrahydrocannabidivarin, bosentan and a medicinal carrier suitable for treating pulmonary arterial hypertension, and is used for treating pulmonary arterial hypertension and reducing liver toxicity caused by the use of bosentan.
Owner:YUNNAN HEMPMON PHARMA CO LTD
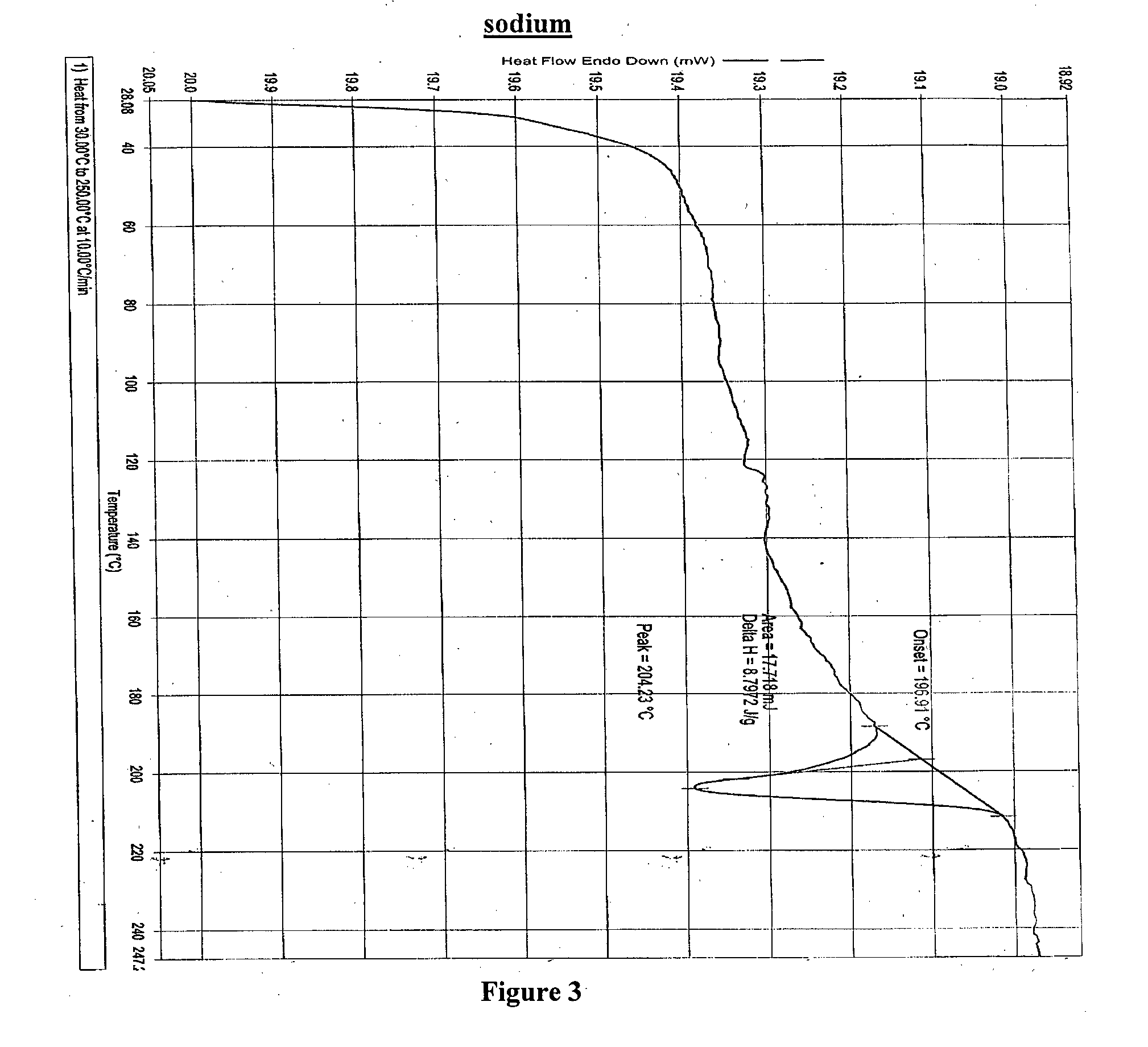
4-tert-butyl-n-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2(2-pyrimidinyl)-pyrimidine-4-yl)-benzen esulfonamide sodium
Owner:RAO DAVULURI RAMAMOHAN
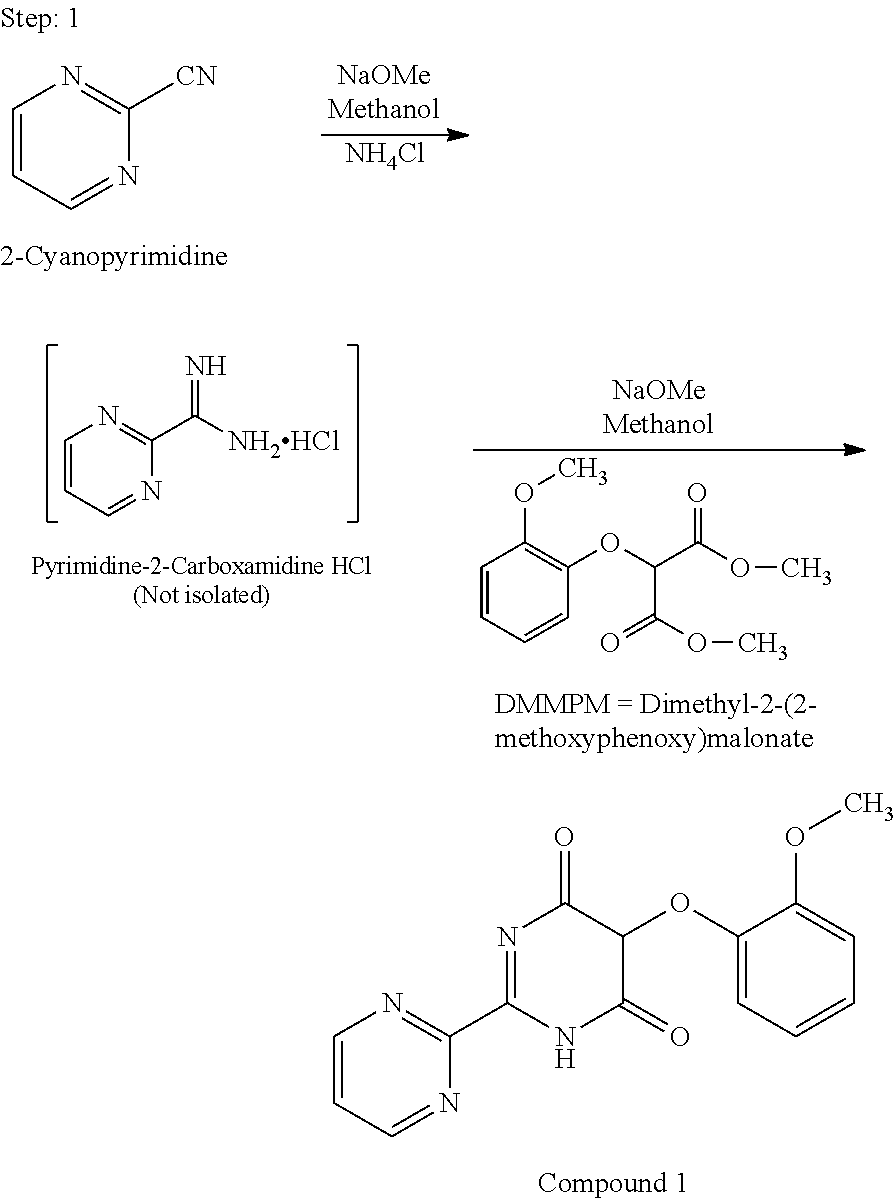
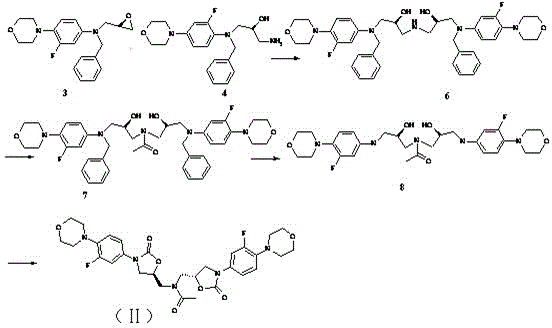
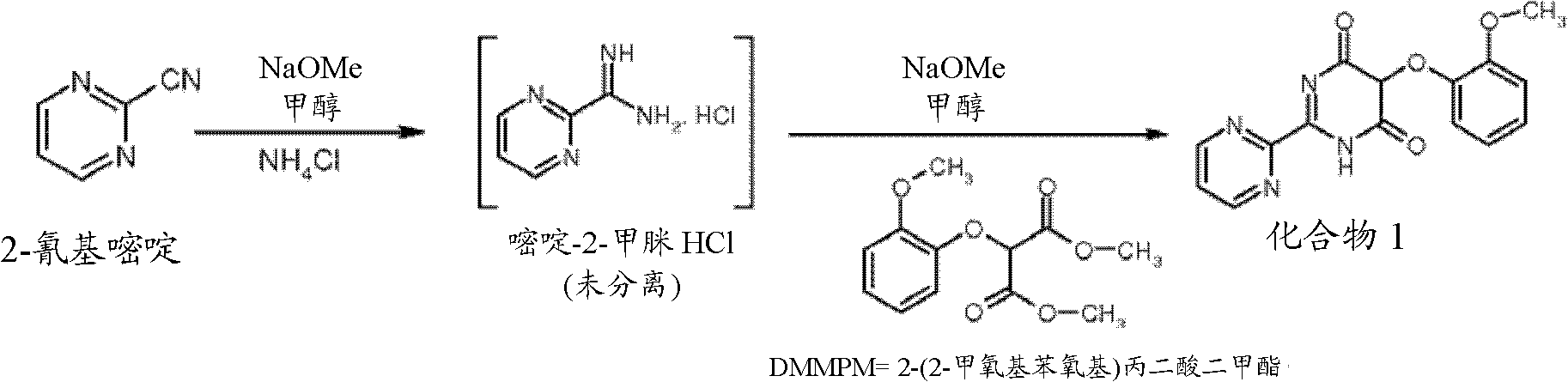
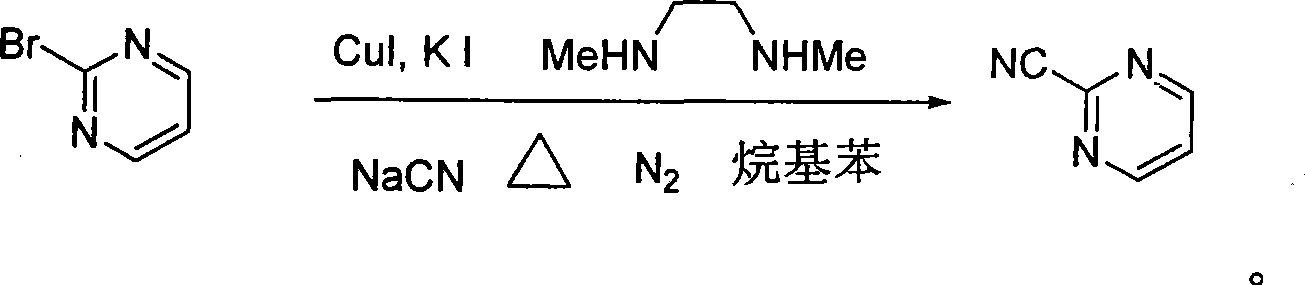
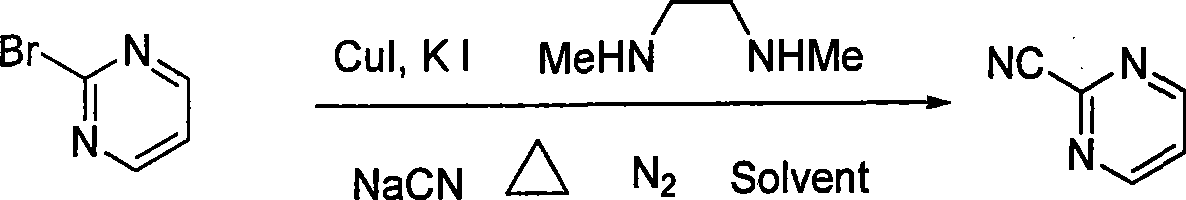
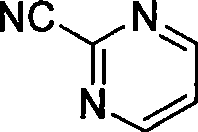
Preparation process of key intermediate 2-cyano pyrimidine of bosentan
InactiveCN100494183CMild reaction conditionsThe reaction process is shortOrganic chemistryIodideBosentan
The invention discloses a preparing method of key Boston drug intermediate 2-cyano pyrimidine, which comprises the following steps: adopting alkyl benzene as reacting solvent; making 5-30% cuprous iodide, 1.5-3% potassium iodide and 1-1.5% N, N'-dimethyl ethanediamine as composite catalyst; reacting 2-bromine pyrimidine and sodium cyanide with molar rate at 1: 1.0-2.0 protected by nitrogen at 100-150 Deg C for 20-48h; filtering; washing the filtrate; drying; condensing; recrystallizing to separate to form the product through ligroine.
Owner:ZHEJIANG UNIV
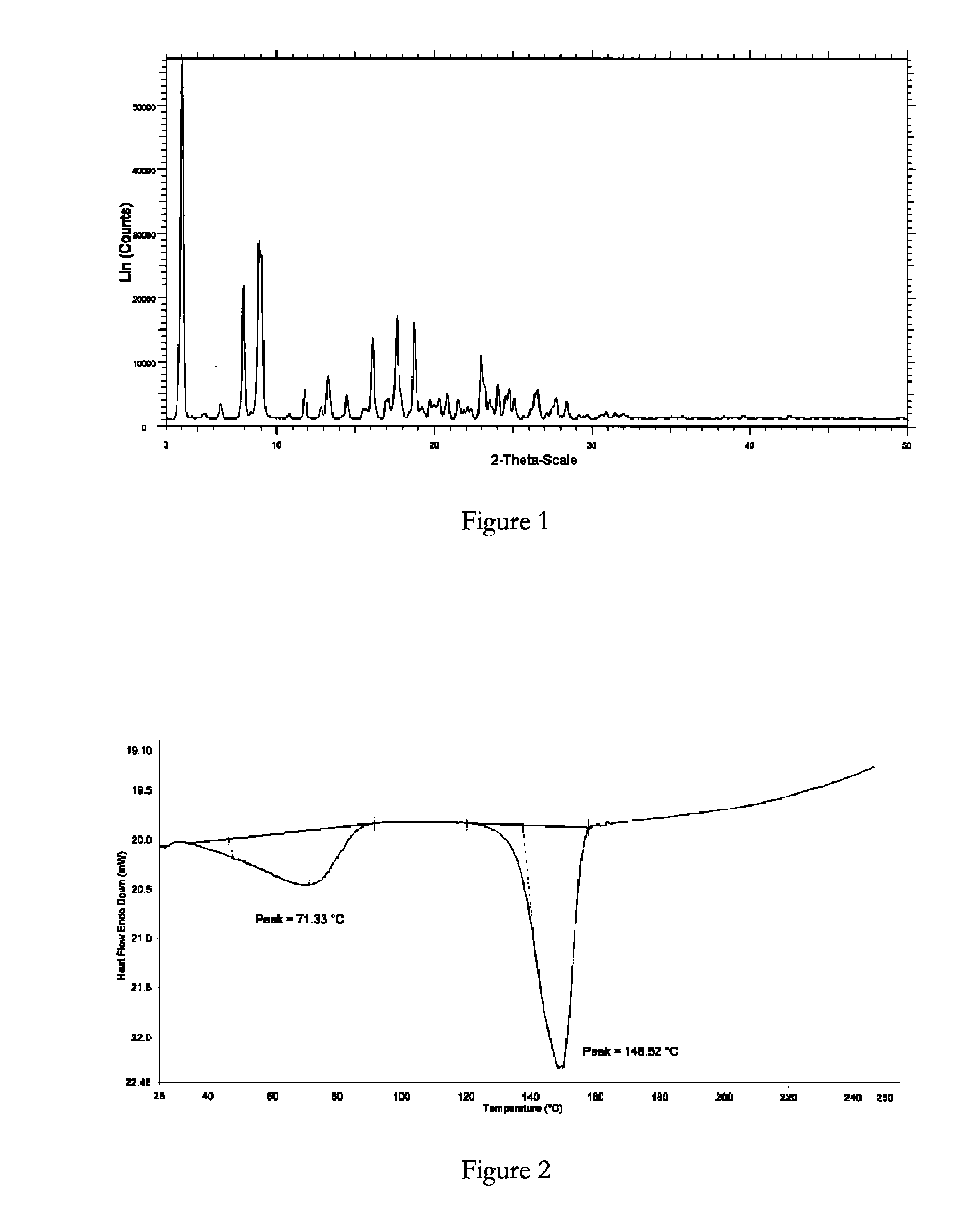
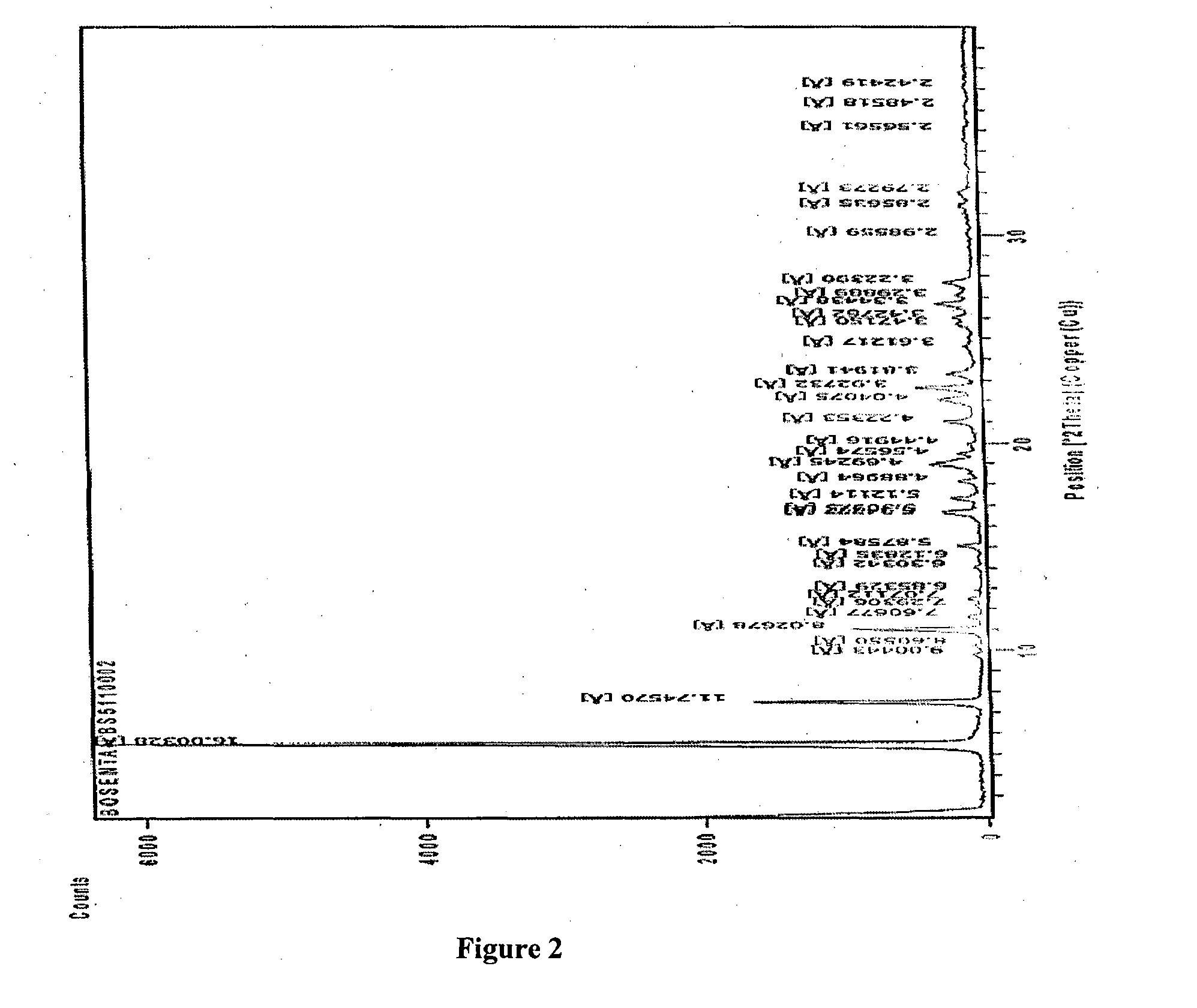
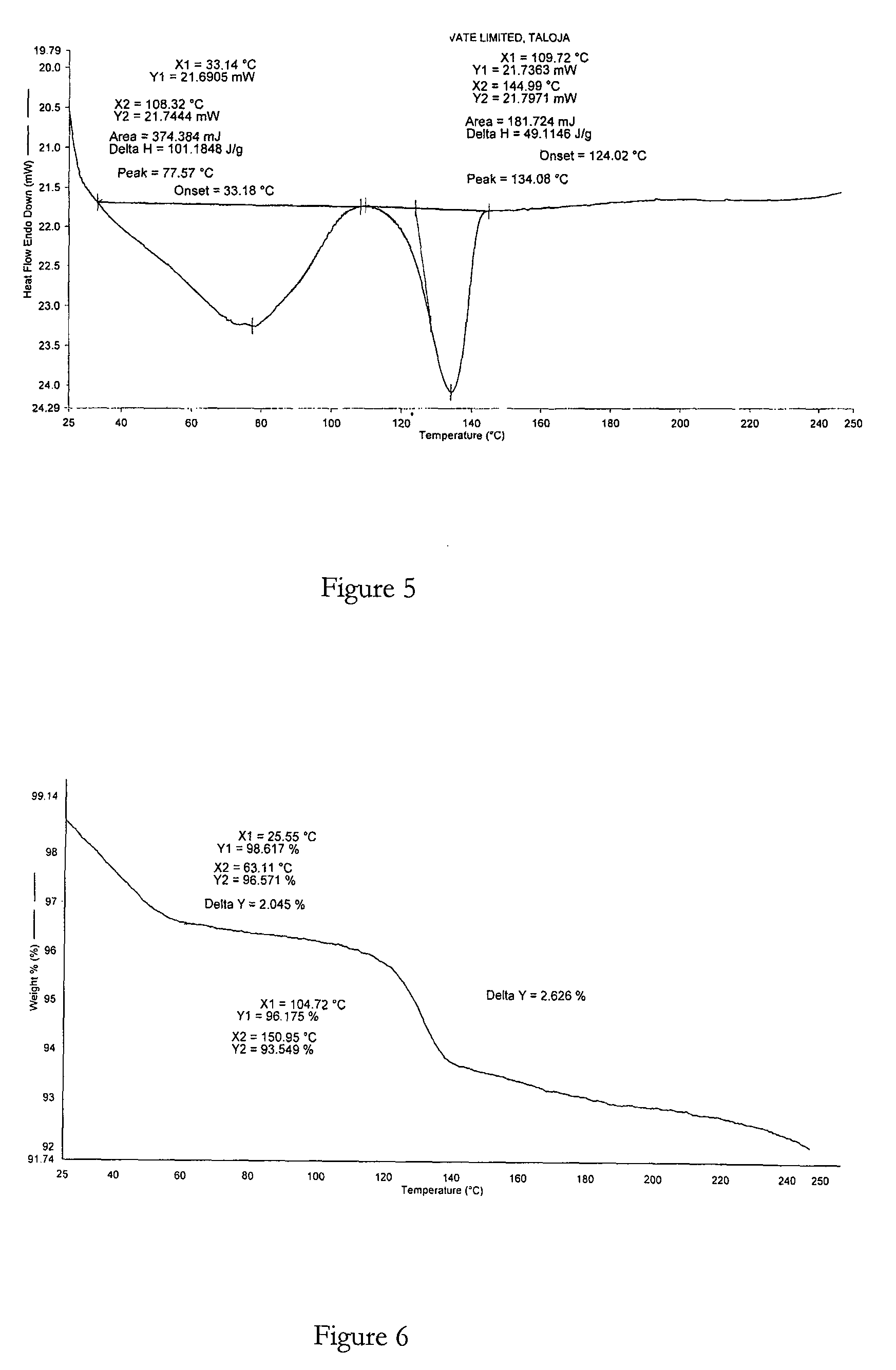
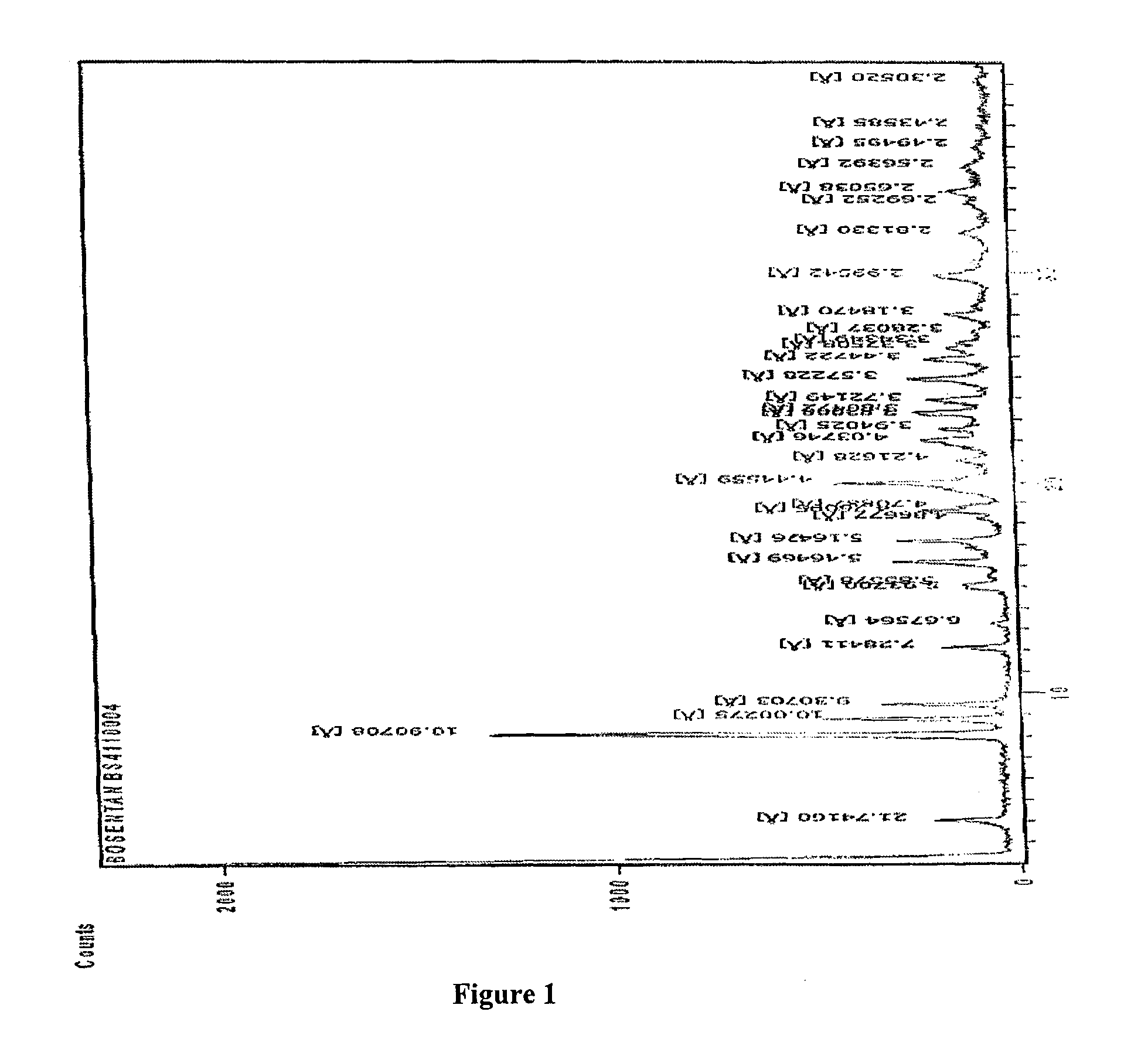
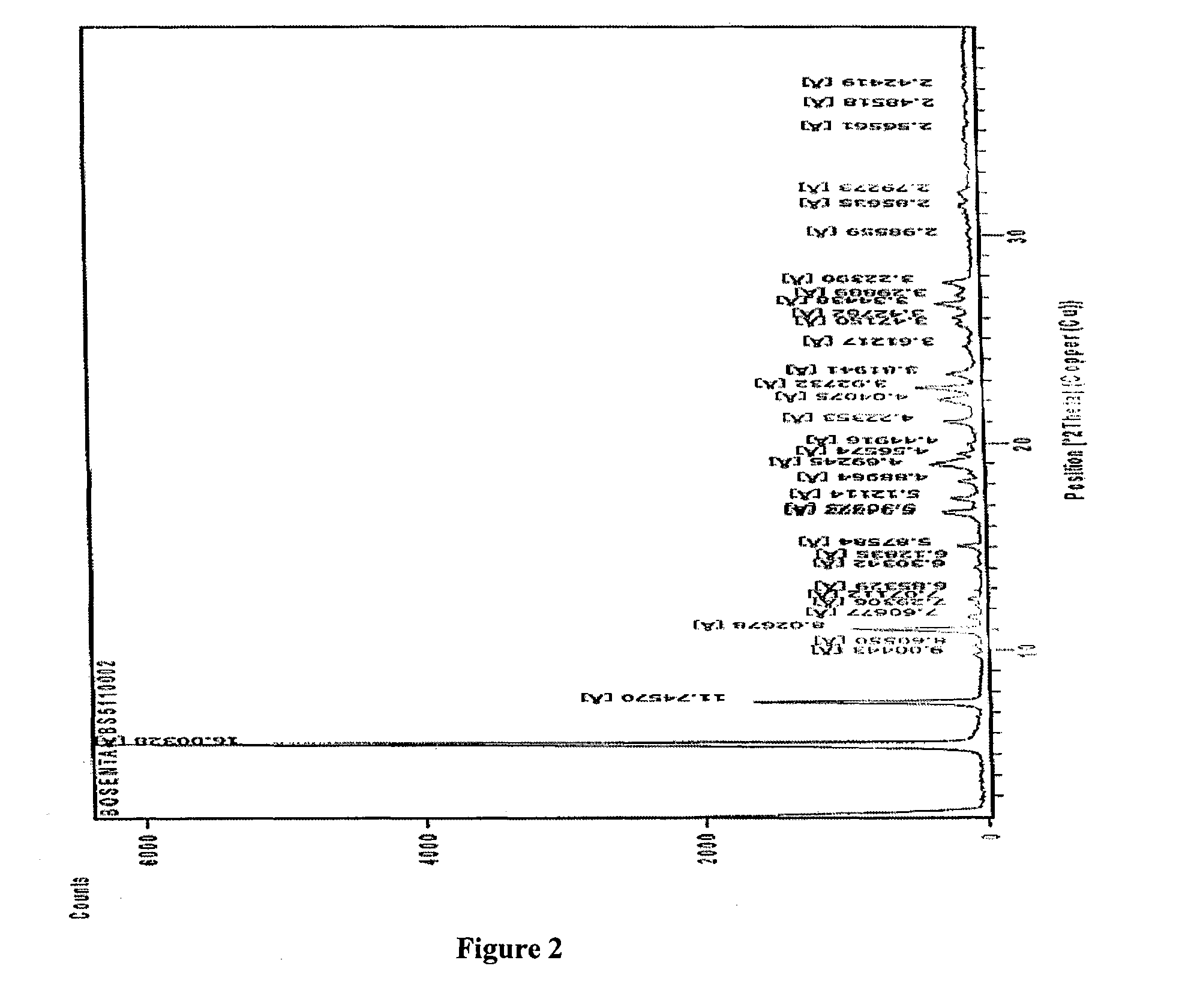
Crystalline forms of bosentan
InactiveUS8530488B2Improve solubilityImprove bioavailabilityBiocideOrganic active ingredientsMedicineAngina
The present invention relates to novel crystalline forms of bosentan and processes for their preparation. Further, the invention relates to pharmaceutical compositions comprising said crystalline forms and use of said compositions in the treatment of patients suffering from endothelin receptor mediated disorders, for example, cardiovascular disorders such as hypertension, pulmonary hypertension, ischemia, vasospasm and angina pectoris.
Owner:GENERICS UK LTD
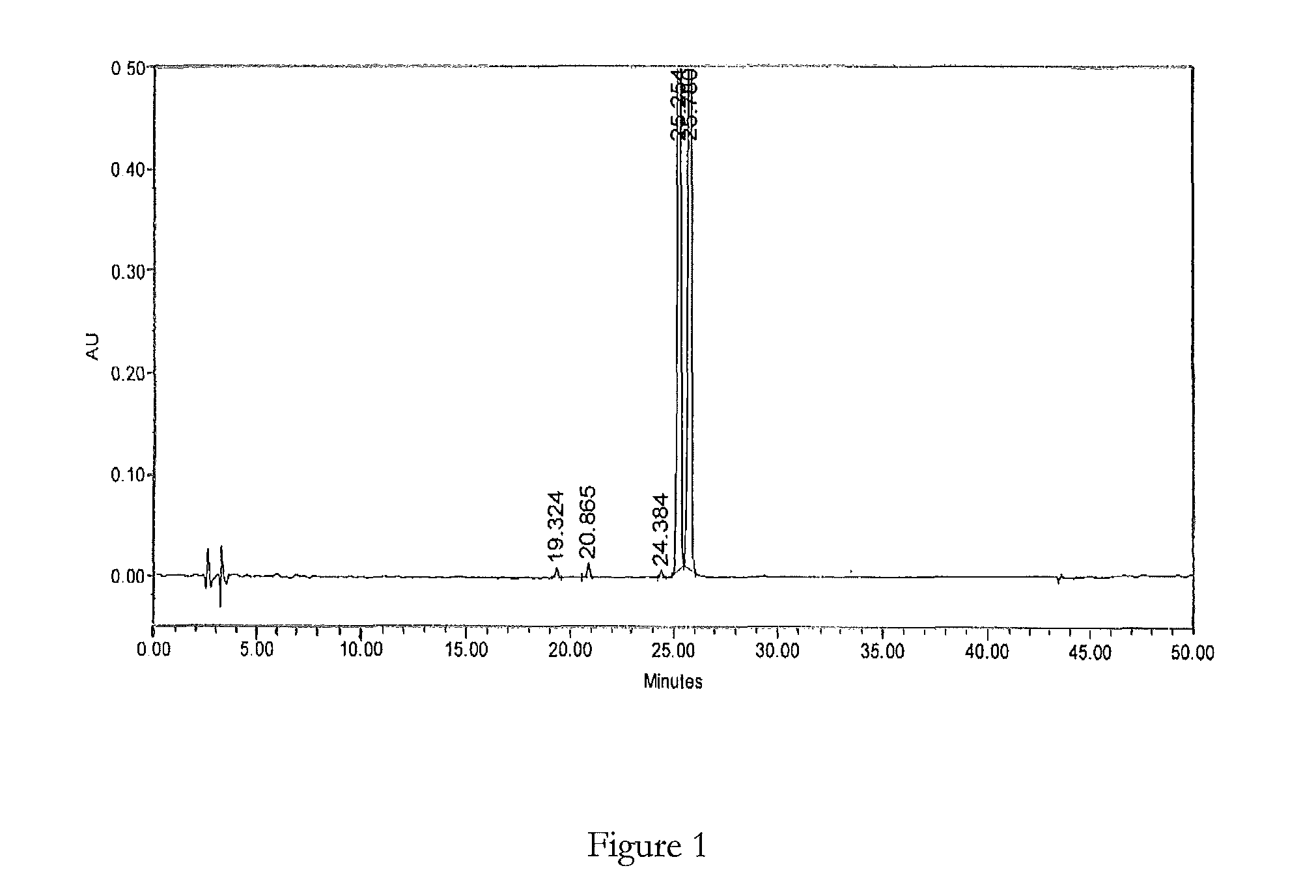
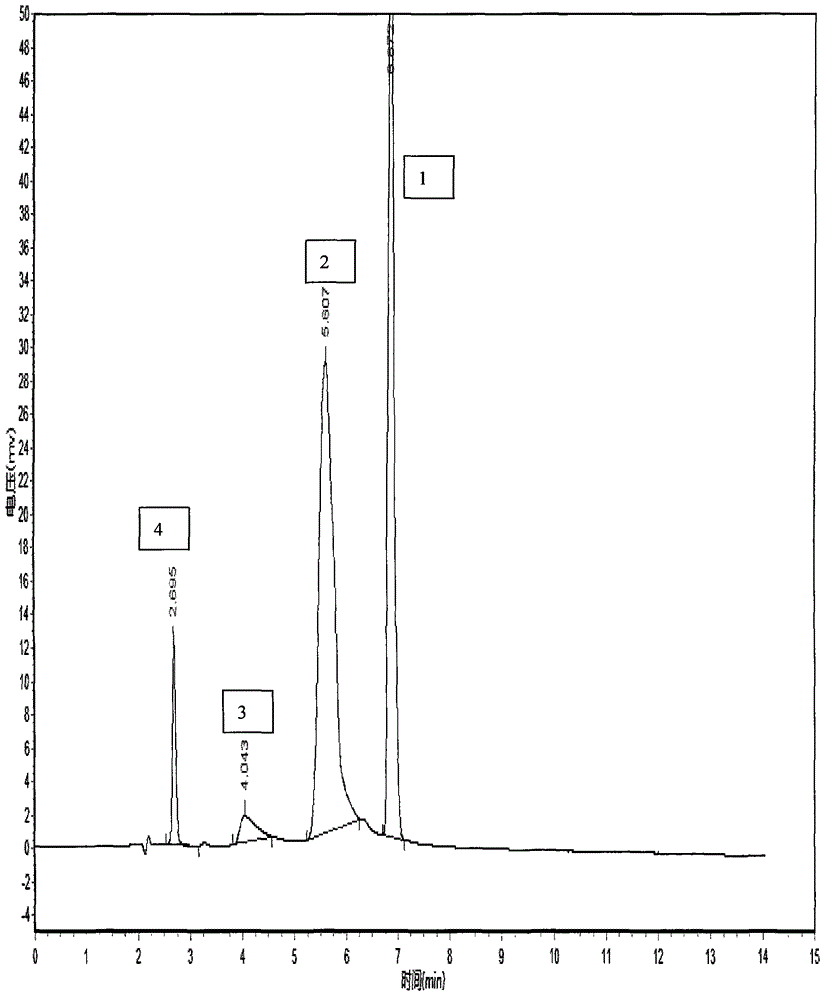
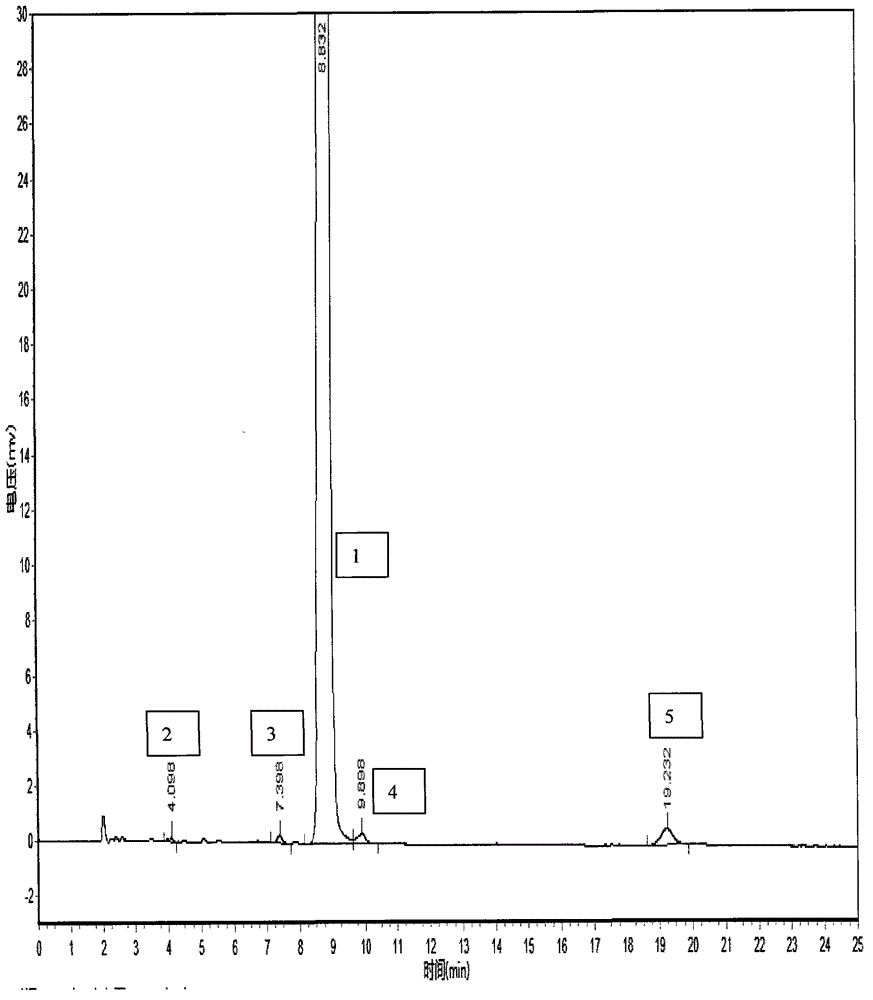
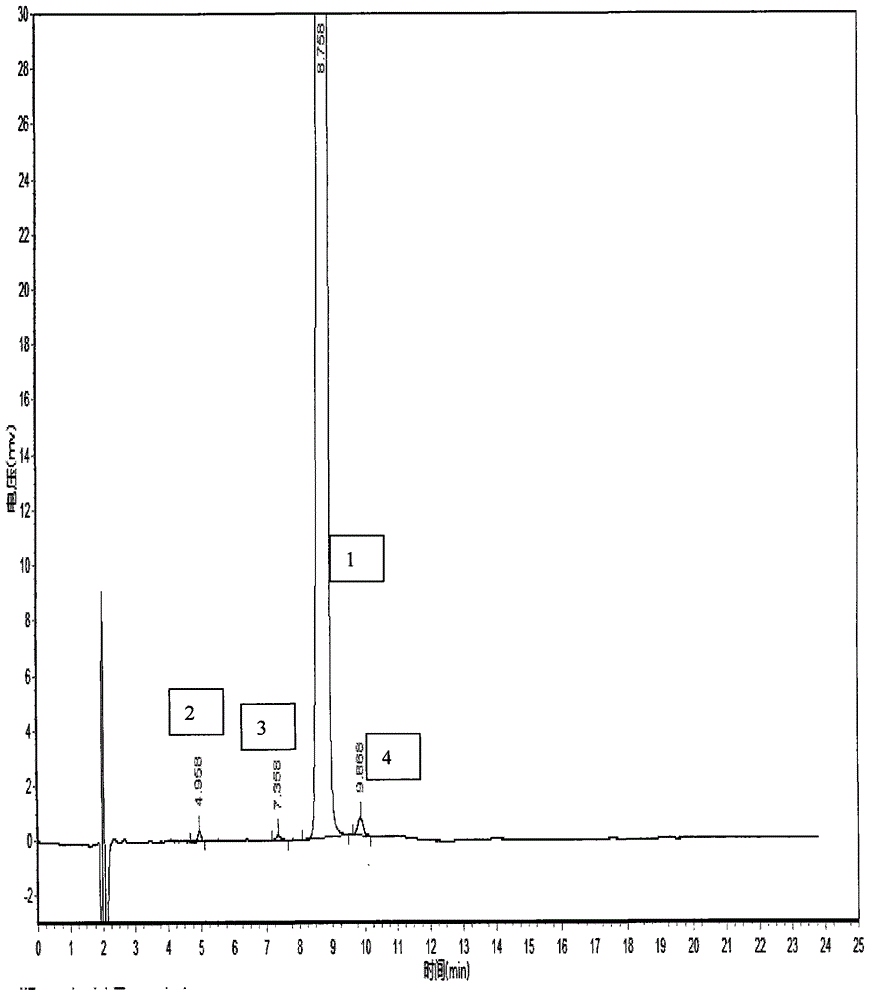
HPLC method for the analysis of bosetan and related substances and use of these substances as reference standards and markers
InactiveUS8975402B2Selective, sensitive, linear, precise, accurate and robustSensitive highOrganic chemistryComponent separationHplc methodAssociated substance
The present invention relates to a new HPLC method for the analysis of the drug substance bosentan and related substances and to the use of said substances as reference standards and markers.
Owner:GENERICS UK LTD
Preparation method of small-particle-size bosentan
InactiveCN109320466AReduce pollutionImprove working environmentOrganic chemistryPurification methodsMicrometer
The invention relates to a preparation method of small-particle-size bosentan, and belongs to the technical field of raw material medicine preparation. According to a purifying method of the small-particle-size bosentan, firstly, an bosentan crude product is dissolved in dichloromethane, and filtering is conducted; filter liquid is cooled to 0 to 5 DEG C, the temperature is maintained, and 50-60%ethanol water with volume 1.8-3 times of that of the solvent in the first step is added in a flowing mode; stirring speed is 220-250 rpm, the speed of adding the ethanol water in a flowing mode is 10-15 ml / min, and after flowing adding is completed, stirring is conducted continuously for 4-5 hours; filtering is conducted, 50-60% ethanol water is used for washing filter cake, and drying is conducted. The invention provides the preparation method of the small-particle-size bosentan. The prepared bosentan D90 can be controlled to be between 16.89 micrometers and 32.56 micrometers, and a raw material medicine which can be used directly is provided for an bosentan preparation.
Owner:WEIHAI YUNRUI INFORMATION TECH CO LTD
Preparation method of linezolid derivative
InactiveCN103554058BHigh purityReduce manufacturing costOrganic chemistryMetaboliteReaction temperature
The invention discloses a preparation method of a linezolid derivative. Abundant screening experiments are performed to determine the optimal reactant consumption, reaction temperature, reaction time, reaction solvent and the like in the preparation technique; and the whole preparation technique has the advantages of high operability, high preparation efficiency and low production cost, and can implement industrialized mass production. The bosentan metabolite (hydroxy bosentan) prepared by the method has the advantages of higher bioavailability and lower untoward effect, and directly can have the antihypertensive effect.
Owner:TLC NANJING PHARMA RANDD CO LTD
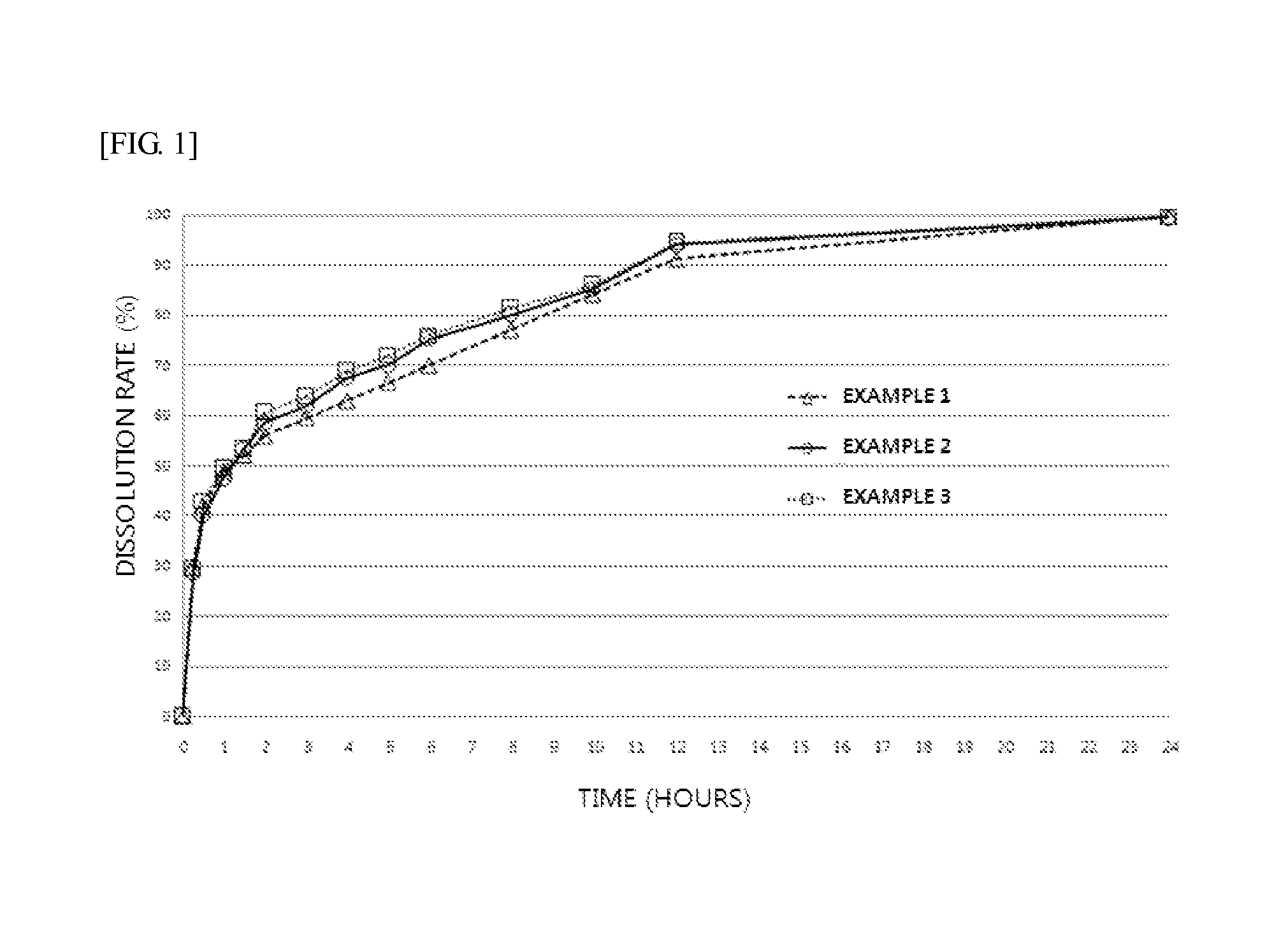
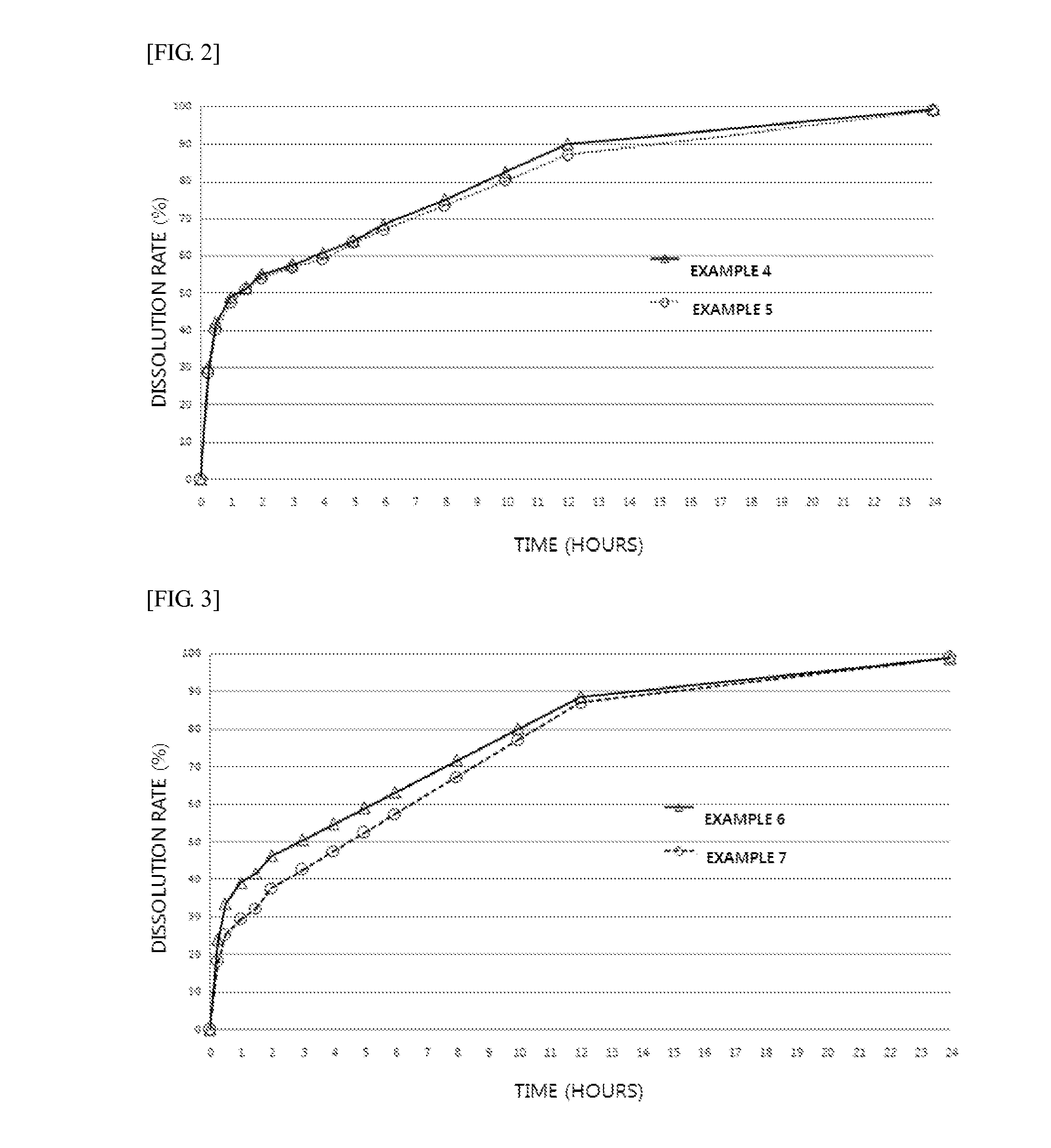
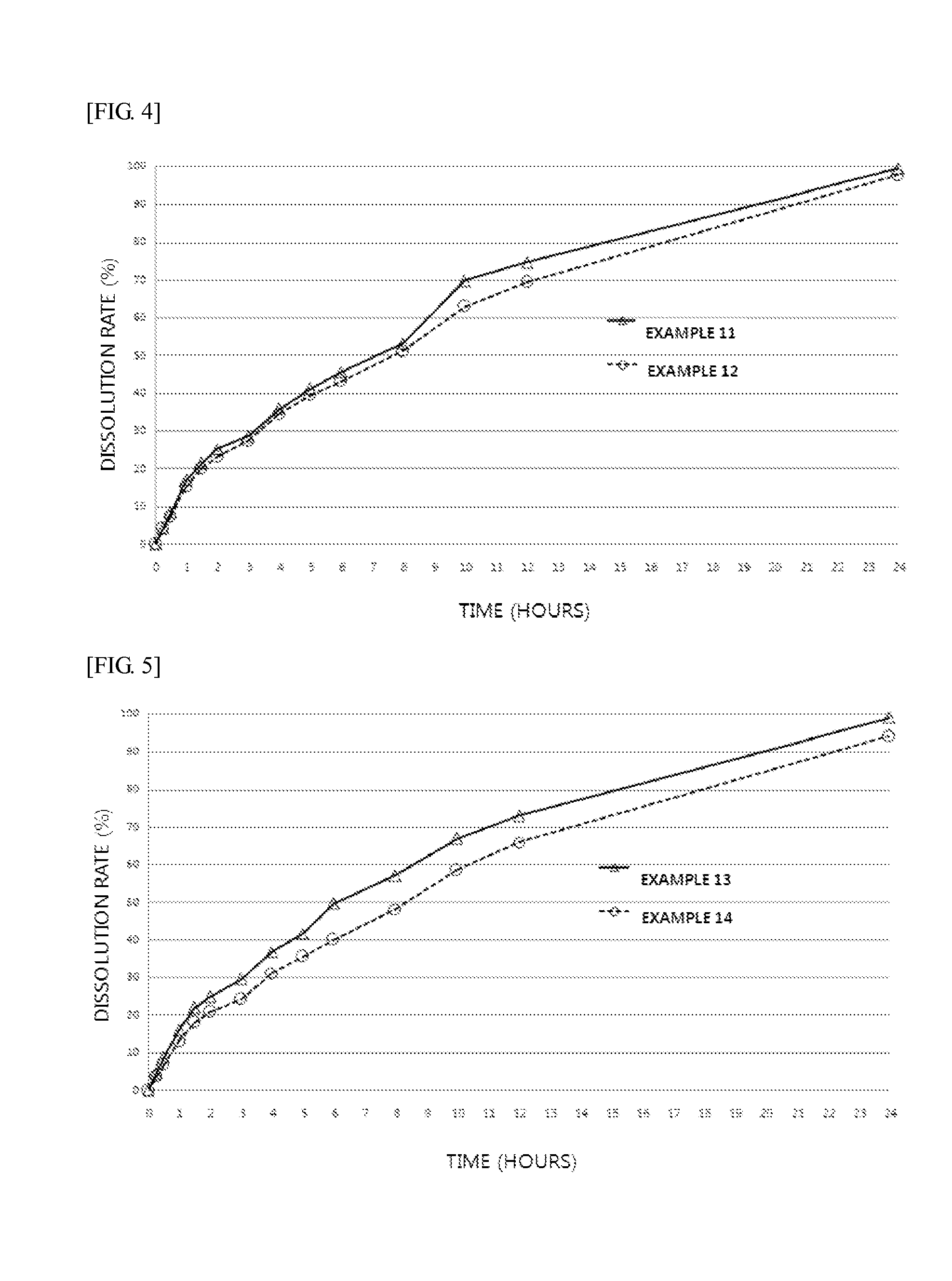
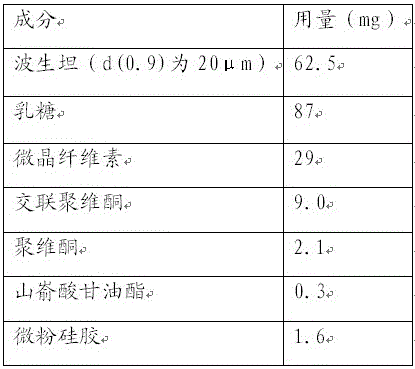
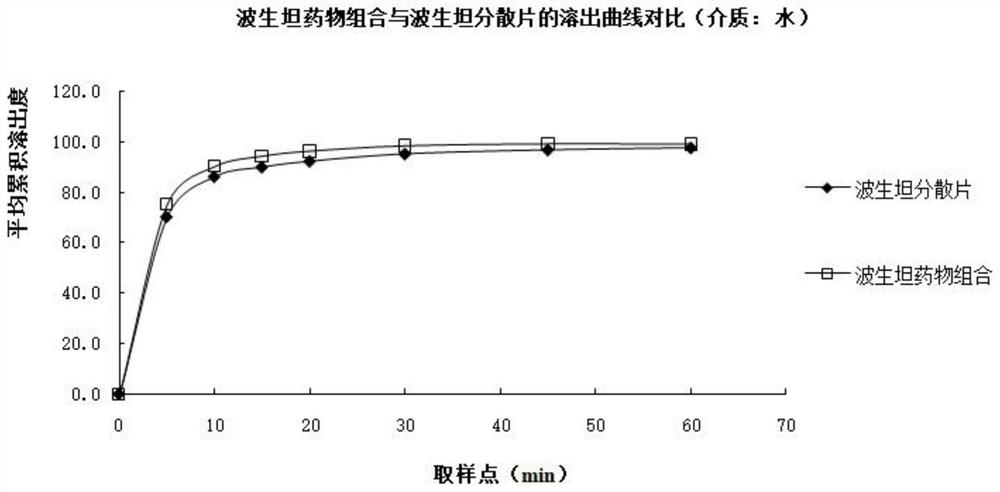
A kind of bosentan pharmaceutical composition
ActiveCN103768068BHigh dissolution rateLow impurity contentOrganic active ingredientsRespiratory disorderMedicineBosentan
The invention relates to a pharmaceutical composition of Bosentan, and dissolution rate of the composition is increased, thereby improving stability of the preparation; the invention also relates to the preparation method of the Bosentan pharmaceutical composition, and the preparation method is simple and easy to be operated, and is suitable for industrial production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Bosentan tablet composition and preparation method thereof
InactiveCN110151723AImprove bioavailabilityReduce adverse reactionsOrganic active ingredientsInorganic non-active ingredientsPharmacySodium bicarbonate
The invention relates to the technical field of pharmacy, in particular to a bosentan tablet composition and a preparation method thereof. The bosentan tablet composition is prepared from the following components in parts by mass: 65 to 130 parts of bosentan main medicine, 65 to 130 parts of sodium bicarbonate, 55 to 100 parts of skeleton material, 12 to 24 parts of disintegrating agent, 2 to 5 parts of flow aid, 2 to 5 parts of lubricant and 0.1 to 1.2 parts of enteric coating material. The lubricant, the enteric coating material, the disintegrating agent and the sodium bicarbonate are addedin the bosentan tablet composition, so that the water dissolution rate is effectively increased, the bioavailability of the medicine is improved and the untoward effect on the stomach after use is reduced. Granules prepared by the preparation method are uniform in granularity and high in flowability, loose tablets, sticking and astringent flushing phenomena of the tablets are avoided, the hardnessof the tablet can reach to above 5 kg and the preparation is suitable for coating operation; and the sodium bicarbonate is added, so that the pH value is increased, dissolution of the bosentan main medicine is benefited and the bioavailability is improved.
Owner:JIANGSU YABANG QIANGSHENG PHARMA
Bosentan solid pharmaceutical composition and preparation method thereof
PendingCN114533738AGood solubilization effectOvercoming the status quo of poor compliancePowder deliveryOrganic active ingredientsPharmacyBosentan
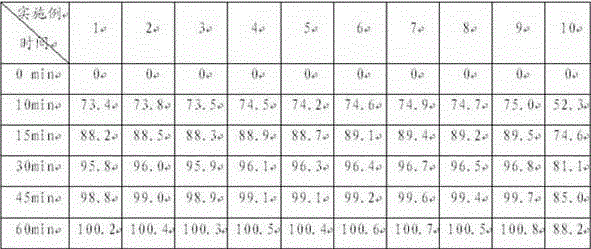
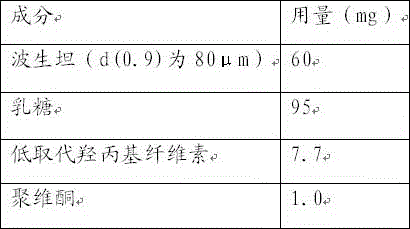
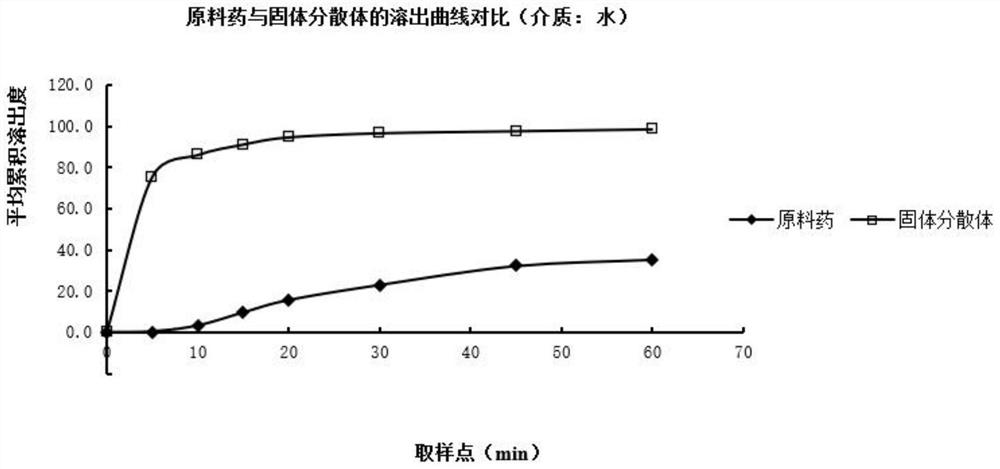
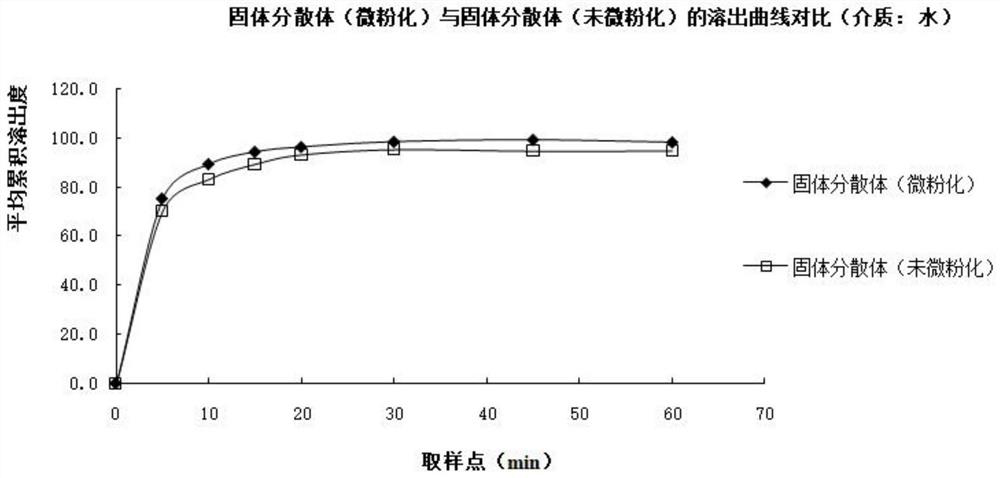
The invention relates to a bosentan solid pharmaceutical composition and a preparation method thereof, and belongs to the technical field of pharmacy. According to the technical scheme, the bosentan solid pharmaceutical composition comprises a bosentan solid dispersion, a filling agent, a suspending aid, a sweetening agent, buffer salt, a flavoring agent and a lubricating agent, and the bosentan solid dispersion is prepared through a hot melt extrusion method. The invention overcomes the defect of poor use compliance of children in the prior art, and has a good clinical application prospect. The preparation method is simple and convenient, is suitable for industrial production, and has great practical value.
Owner:苏州海景医药科技有限公司
Medicine for treating atrophic gastritis
InactiveCN106511376AReasonable combinationSignificant effectHeavy metal active ingredientsOrganic active ingredientsSodium bicarbonateSide effect
The invention discloses a medicine for treating atrophic gastritis. The medicine for treating atrophic gastritis mainly comprises, by weight, 8-14 parts of lamivudine, 5-10 parts of artemisinin, 5-7 parts of triazolam, 6-10 parts of polyvinylpyrrolidone, 0.04-0.15 part of scopoletin , 2-7 parts of bosentan, 4-8 parts of metronidazole, 1-3 parts of biotin, 4-6 parts of bismuth subnitrate and 10-20 parts of sodium bicarbonate. The medicine for treating atrophic gastritis has the advantages that the medicine, which is a western medicine compound preparation, is reasonable in formula, remarkably effective through clinical experiment results and capable of treating symptoms of the atrophic gastritis effectively; the medicine is small in dose, convenient to take, quick in action, exact in curative effect, safe, reliable, free of side effect and low in cost.
Owner:HENAN BALING ELECTRONICS TECH CO LTD
Process for preparing bosentan
ActiveUS8785461B2Less sulfonamideHigh purityOrganic active ingredientsBiocideBosentanCombinatorial chemistry
Owner:GENERICS UK LTD
Method for measuring content of related substances in bosentan or preparation of bosentan
The invention relates to a method for measuring content of related substances in bosentan or preparation of bosentan. The method is a high performance liquid chromatography, wherein the chromatographic column: octadecyl silane serves as the filler; the moving phase: acetonitrile-0.025mol / L sodium heptanesulfonate solution (regulating the PH to be 3.0 through phosphoric acid); the volume ratio of the acetonitrile and the sodium heptanesulfonate solution is (60:40)-(70-30); and the detection wavelength is 212-222nm. By means of the high performance liquid chromatography, content of samples, intermediates and impurities can be accurately measured simultaneously. The samples are thoroughly separated in the analyzing process, the analyzing results are good in reproducibility, and the method simply and reliably ensures quality control and sample quality in the production process.
Owner:WUHAN WUYAO SCI & TECH
Process for preparing bosentan
ActiveUS20110039871A1High purityLess sulfonamideOrganic active ingredientsBiocideBosentanCombinatorial chemistry
The present invention relates to a novel intermediate useful in the preparation of bosentan and to processes for the preparation of said intermediate and bosentan. The invention further relates to compositions comprising bosentan prepared according to the processes of the invention and their use in the treatment of endothelin-receptor mediated disorders.
Owner:GENERICS UK LTD
4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2(2-pyrimidinyl)-pyrimidine-4-yl)-benzen esulfonamide sodium
ActiveUS9296705B2Organic active ingredientsOrganic chemistry methodsCrystallographyPhenylsulfonamide
Owner:RAO DAVULURI RAMAMOHAN
Pharmaceutical for treating chronic gastritis
InactiveCN106491639AReasonable combinationSignificant effectHeavy metal active ingredientsDigestive systemSodium bicarbonateSide effect
The invention discloses a pharmaceutical for treating chronic gastritis, prepared mainly from, by weight, 8-14 parts of glucurolactone, 5-10 parts of artemisinin, 5-7 parts of Gamma-hydroxybutyric acid, 6-10 parts of polyvinylpyrrolidone, 0.04-0.15 part of scopoletin, 2-7 parts of bosentan, 4-8 parts of allicin, 1-3 parts of biotin, 4-6 parts of bismuth subnitrate, and 10-20 parts of sodium bicarbonate. The western medicine compound preparation provided herein has reasonable composition, clinical test results show that the preparation has significant effect and is effective in treating chronic gastritis symptoms; the preparation has the advantages of low dosage, good convenience of administration, high acting speed, clear therapeutic effect, good safety and reliability, zero side effects and low cost.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
Pharmaceutical composition for treating chronic gastritis and preparation method of pharmaceutical composition
InactiveCN106822178AReasonable combinationSignificant effectHeavy metal active ingredientsDigestive systemSide effectClinical trial
The invention discloses a pharmaceutical composition for treating chronic gastritis. The pharmaceutical composition for treating the chronic gastritis is prepared from the following main raw materials in parts by weight: 8 to 14 parts of sodium rabeprazole, 5 to 10 parts of artemisinin, 5 to 7 parts of gamma-hydroxybutyric acid, 6 to 10 parts of polyvinylpyrrolidone, 0.04 to 0.15 part of scopoletin, 2 to 7 parts of bosentan, 4 to 8 parts of garlicin, 1 to 3 parts of biotin, 4 to 6 parts of bismuth subnitrate and 10 to 20 parts of sodium hydrogen carbonate. A Western medicine compound preparation provided by the invention has a reasonable prescription and a clinical test result shows that the curative effect is remarkable; the pharmaceutical composition can be used for effectively treating chronic gastritis symptoms; the pharmaceutical composition has the advantages of small dosage, convenience for orally taking, rapid effect, accurate curative effect, safety and reliability, no side effect and low cost.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
Chemical synthesis method of bosentan metabolite
ActiveCN113072504AImprove bioavailabilityEasy to operateOrganic chemistryChemical synthesisMetabolite
The invention discloses a chemical synthesis method of bosentan metabolite. According to the method disclosed by the invention, the bosentan metabolite is obtained from 2, 2-dimethyl methyl phenylacetate through six-step reaction. The whole preparation method is high in operability, high in preparation efficiency and low in production cost, and industrial mass production can be achieved. The bosentan metabolite prepared by the invention is higher in bioavailability, can directly play a role in resisting hypertension, and is lower in adverse reaction.
Owner:四川摩尔生物制药有限公司
Bosentan tablet composition
InactiveCN109481407ASolve looseFix stability issuesOrganic active ingredientsInorganic non-active ingredientsCalcium bicarbonateBosentan
The invention relates to bosentan tablet composition and belongs to the technical field of pharmacy. The bosentan tablet composition is composed of, by weight per 1000 tablets, 62.5 g of bosentan, 48-78 g of lactose anhydrous, 8-16 g of calcium bicarbonate, 10-20 g of mannitol, 0.1-0.8 g of lauryl sodium sulfate, 8-12 g of superfine silica powder, 20-32 g of microcrystalline cellulose, 1.5-4 g ofcrossarmellose sodium and 0.8-1.6 g of magnesium stearate. The bosentan tablet composition can meet the requirements on dissolution rate and meanwhile solve the problems of tablet loosening and related substance content increasing during storage.
Owner:WEIHAI YUNRUI INFORMATION TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com