Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32results about How to "Ease of administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
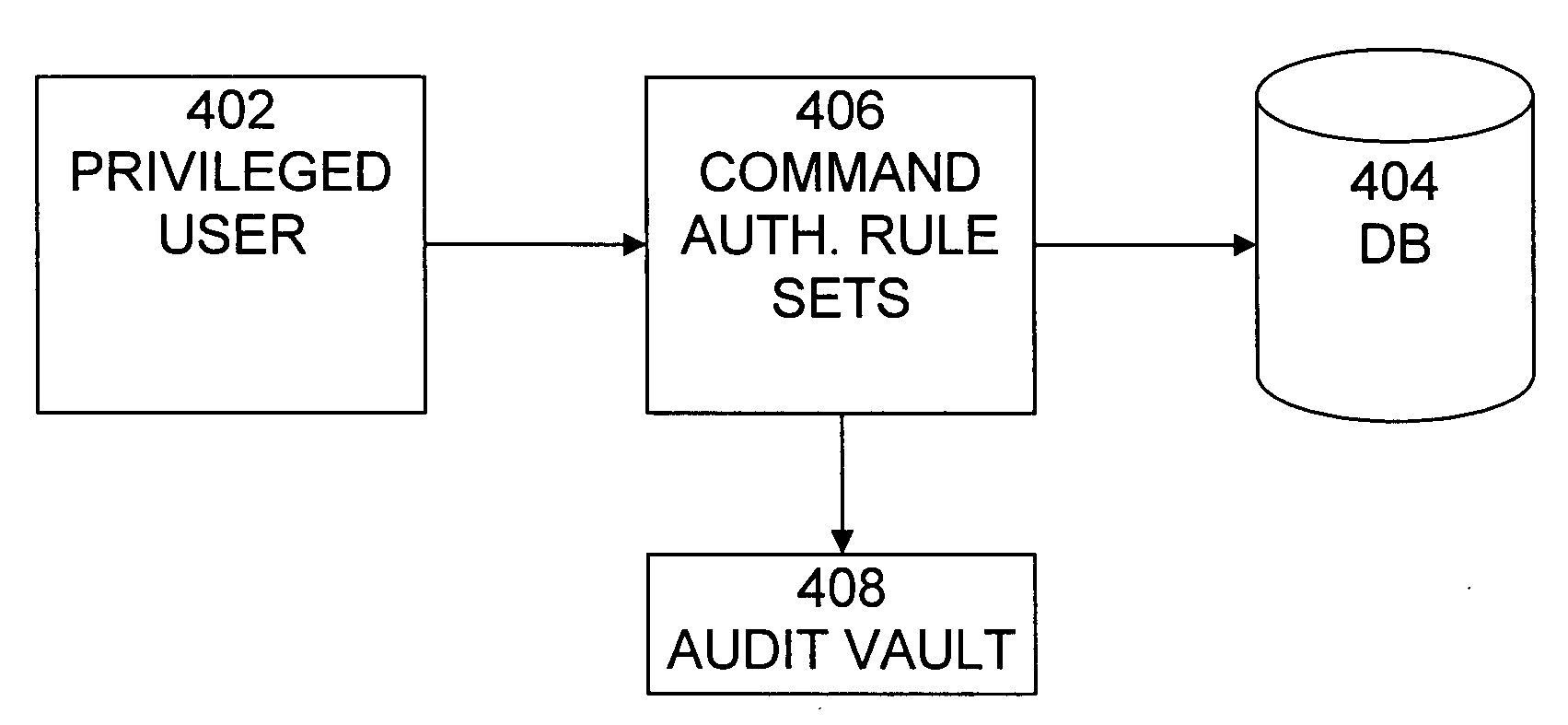
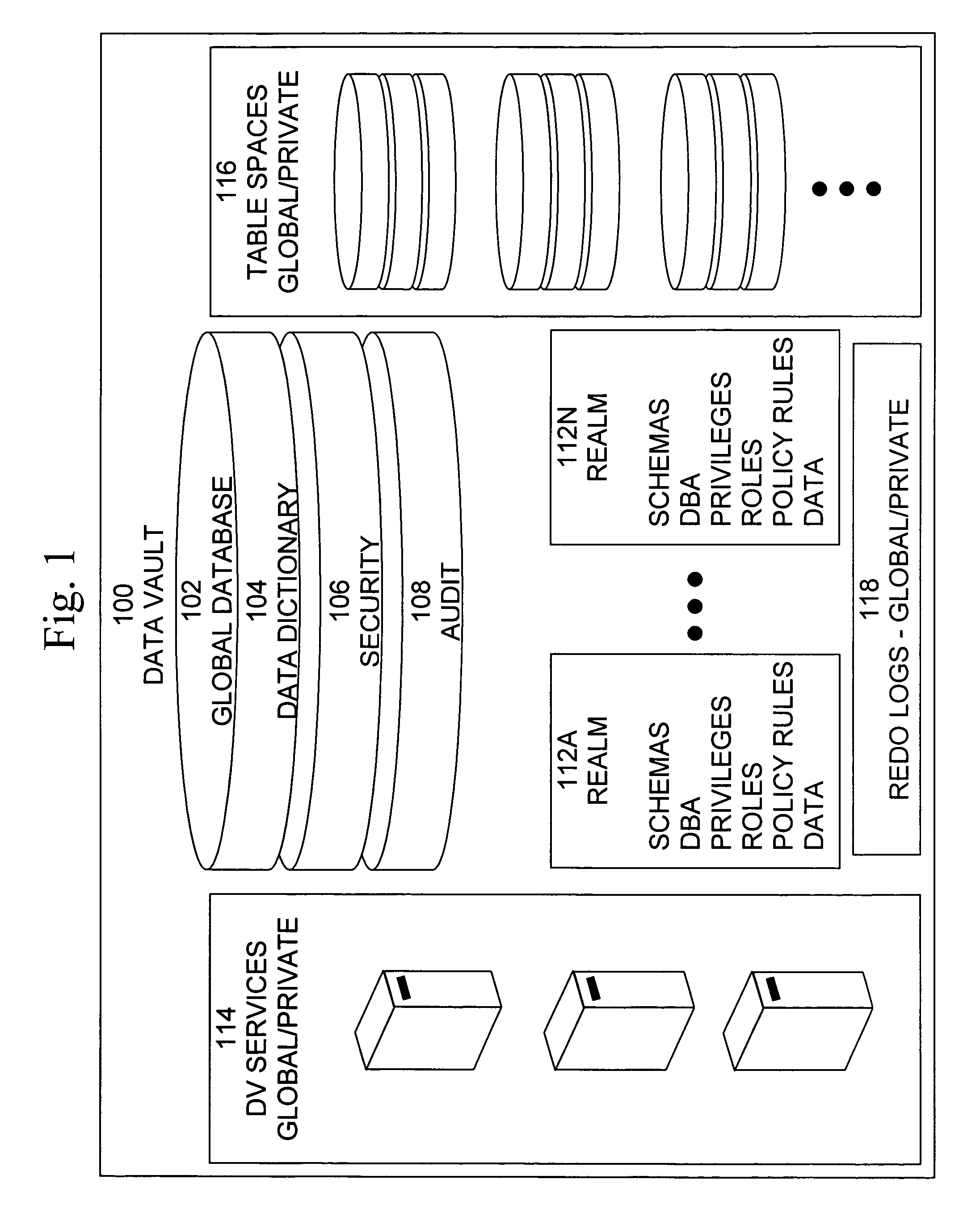
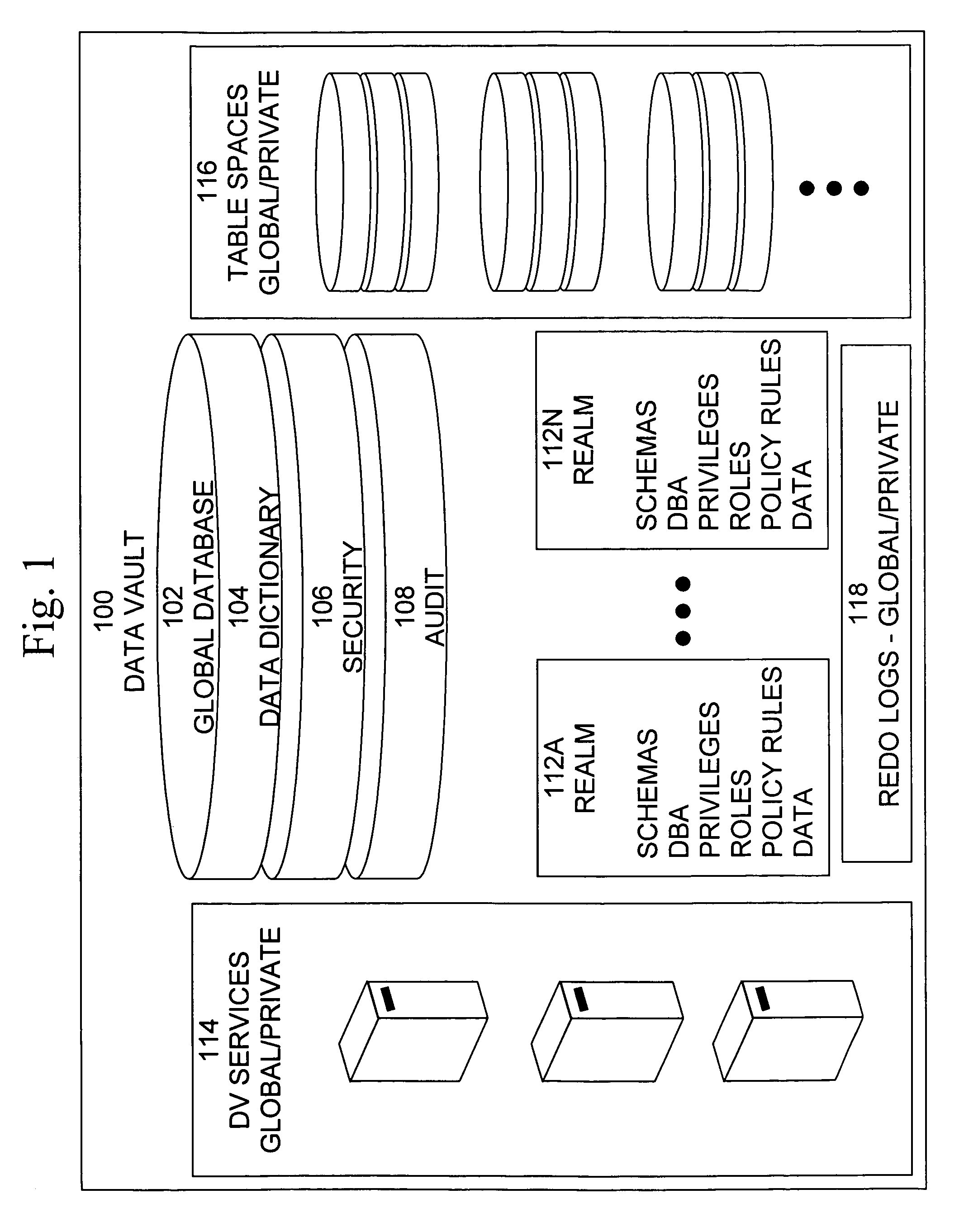
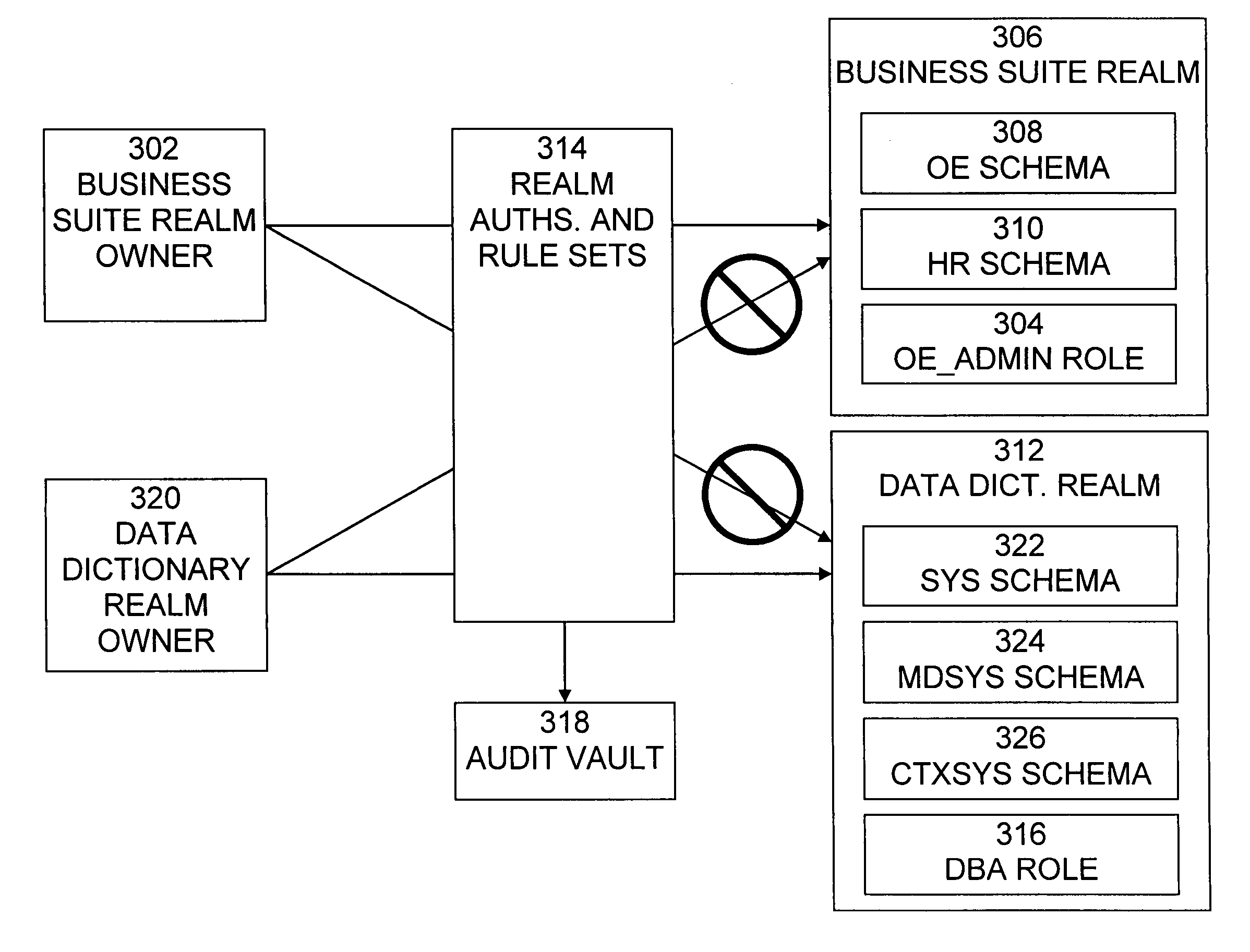
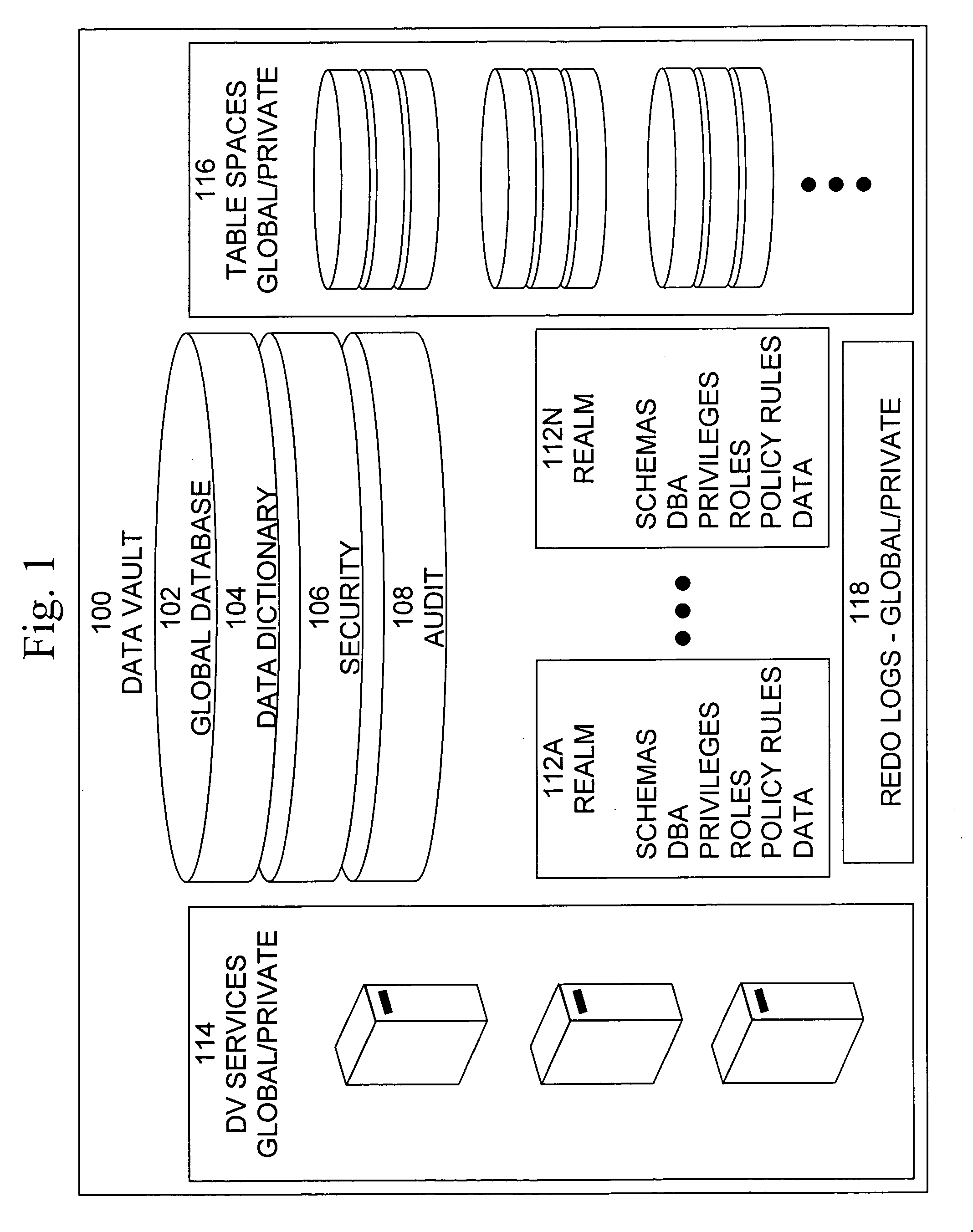
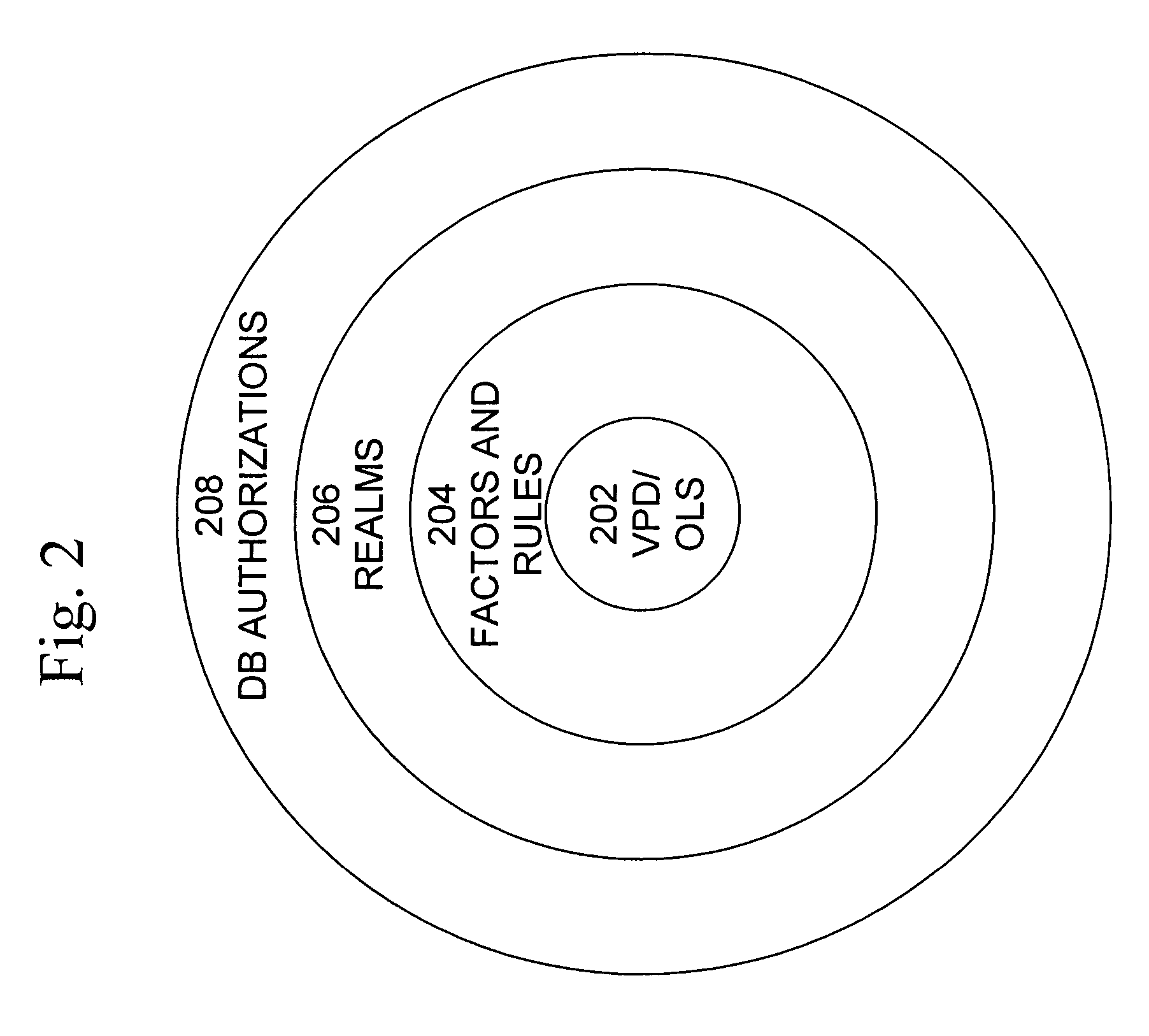
Cross-domain security for data vault
ActiveUS20060248599A1Ease of administrationEnsure effective implementationDigital data processing detailsUser identity/authority verificationDatabase securitySecurity level
A secure database appliance leverages database security in a consistent framework provides consistent, flexible, and adaptable security using mandatory access controls in addition to user and role based security for access control and accountability. A database system communicatively connected to a plurality of network domains, each network domain having a level of security, the database system comprises at least one database accessible from all of the plurality of network domains, the database comprising data, each unit of data having a level of security and access control security operable to provide access to a unit of data in the database to a network domain based on the level of security of the network domain and based on the level of security of the unit of data.
Owner:ORACLE INT CORP
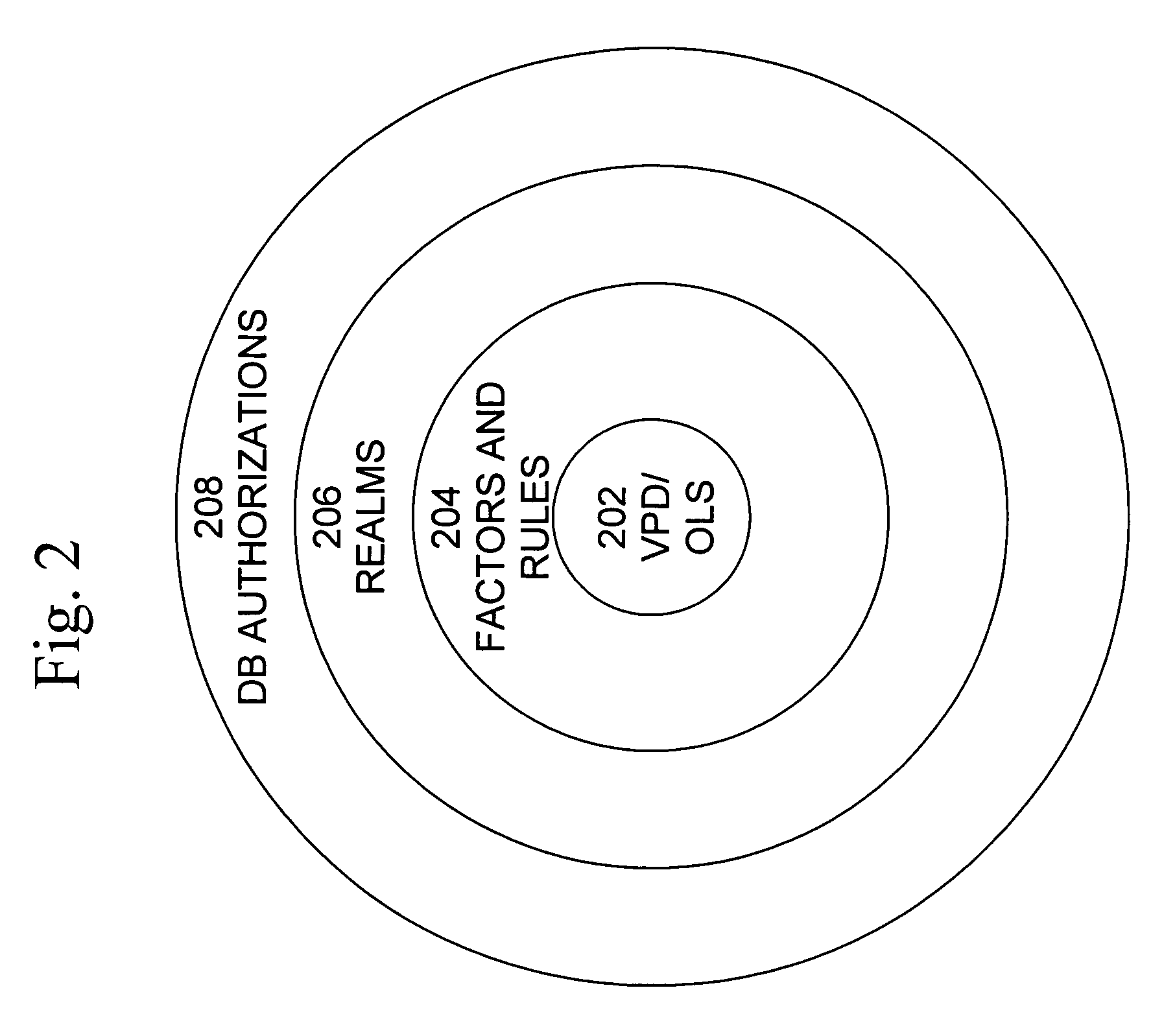
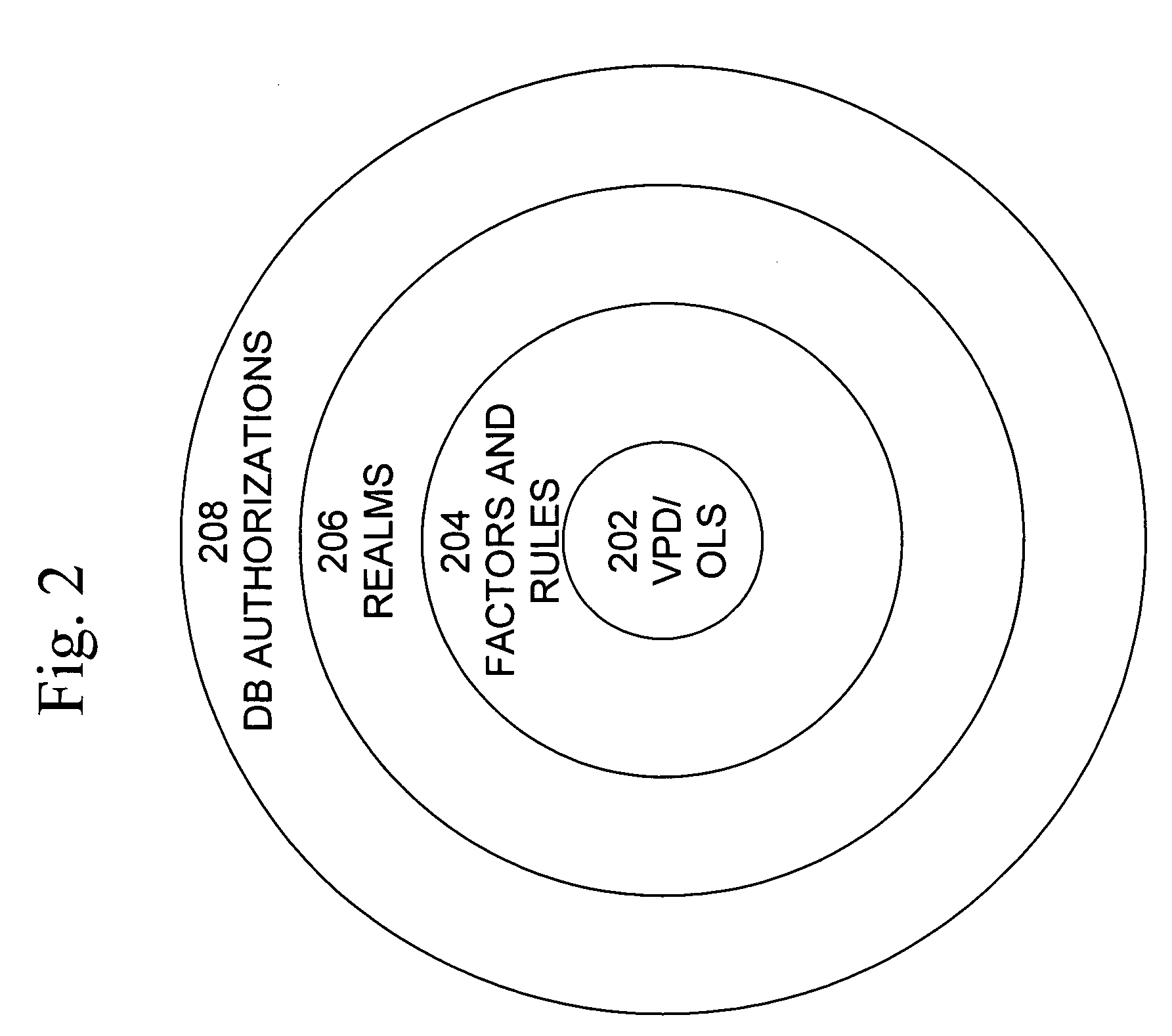
Mandatory access control label security
ActiveUS7831570B2Ease of administrationEnsure effective implementationDigital data processing detailsComputer security arrangementsData setData access
A secure database appliance leverages database security in a consistent framework provides consistent, flexible, and adaptable security using mandatory access controls in addition to user and role based security for access control and accountability. A database system comprises a plurality of datasets, each dataset including a plurality of data, and a plurality of database objects, each object having a security label comprising a security classification of the object, at least one database session, the database session having a security label indicating a security classification of the database session, wherein, the database system is operable to allow or deny access to data to a database session based on a security label of a database object and on a security label of the database session.
Owner:ORACLE INT CORP
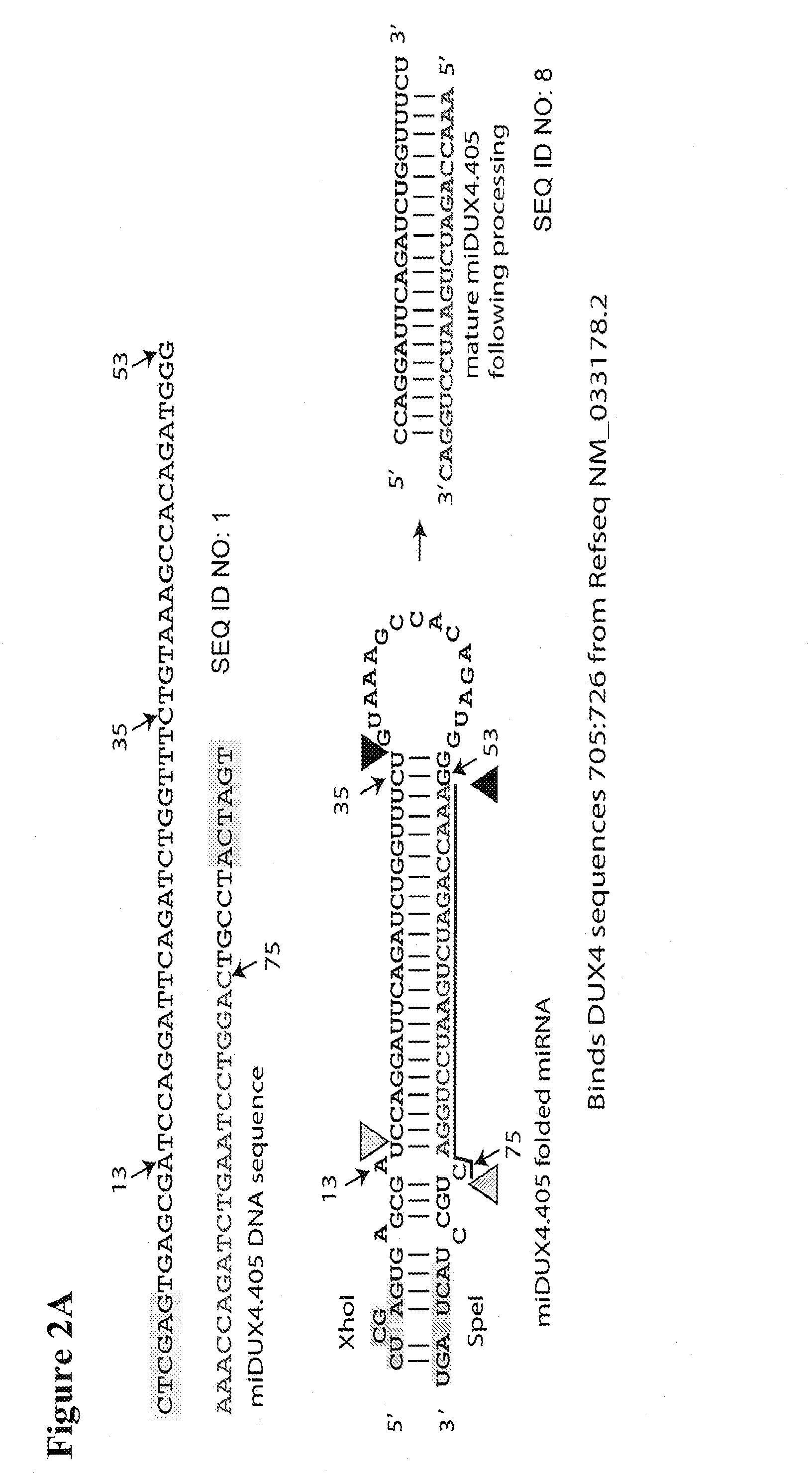
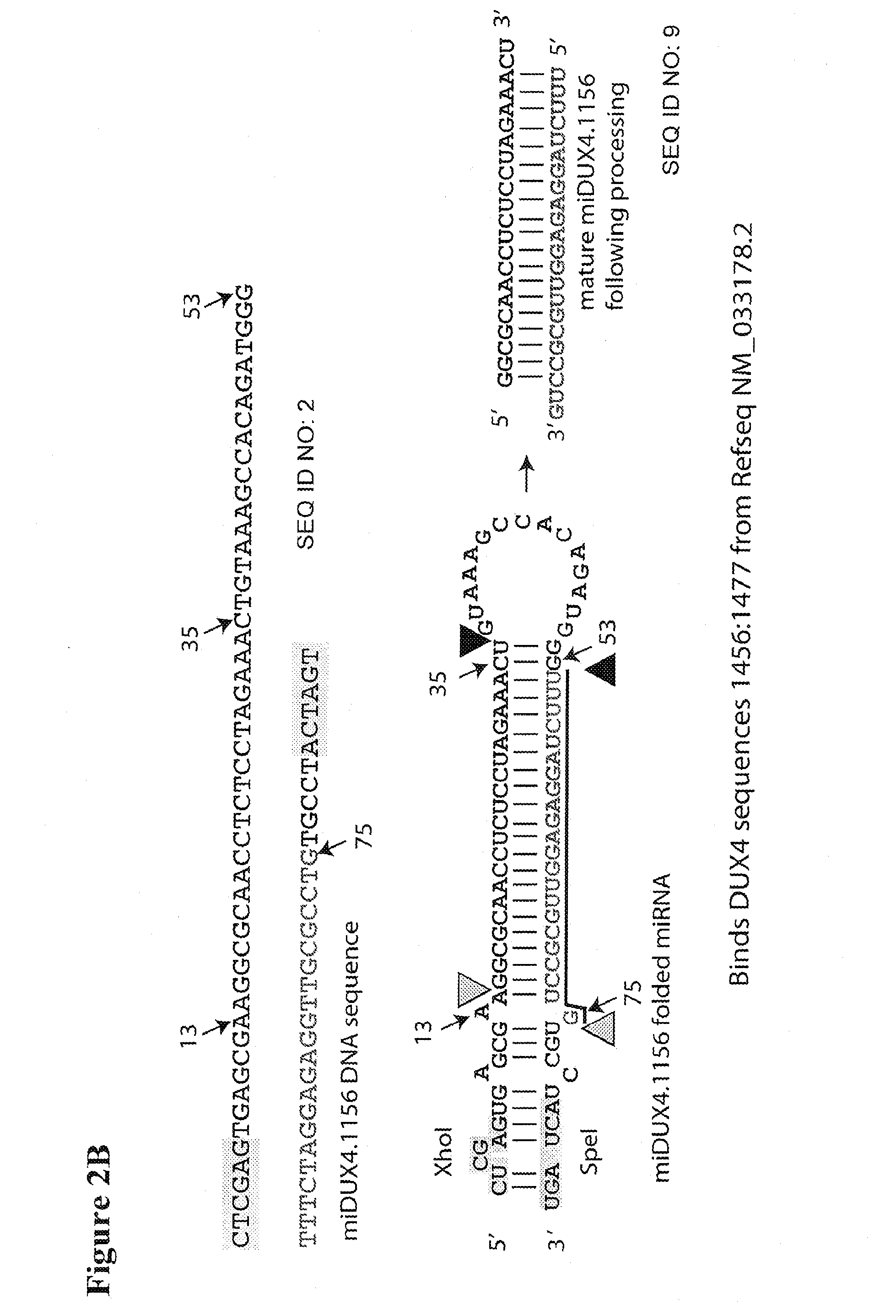
Recombinant Virus Products and Methods for Inhibition of Expression of DUX4
ActiveUS20140322169A1Ease of administrationEase of handlingBiocideSugar derivativesAdeno associate virusDna encoding
The present invention relates to RNA interference-based methods for inhibiting the expression of the DUX4 gene, a double homeobox gene on human chromosome 4q35. Recombinant adeno-associated viruses of the invention deliver DNAs encoding microRNAs that knock down the expression of DUX4. The methods have application in the treatment of muscular dystrophies such as facioscapulohumeral muscular dystrophy.
Owner:RES INST AT NATIONWIDE CHILDRENS HOSPITAL
Mandatory access control base
ActiveUS7593942B2Ease of administrationEnsure effective implementationComputer security arrangementsSpecial data processing applicationsData accessDatabase security
Owner:ORACLE INT CORP
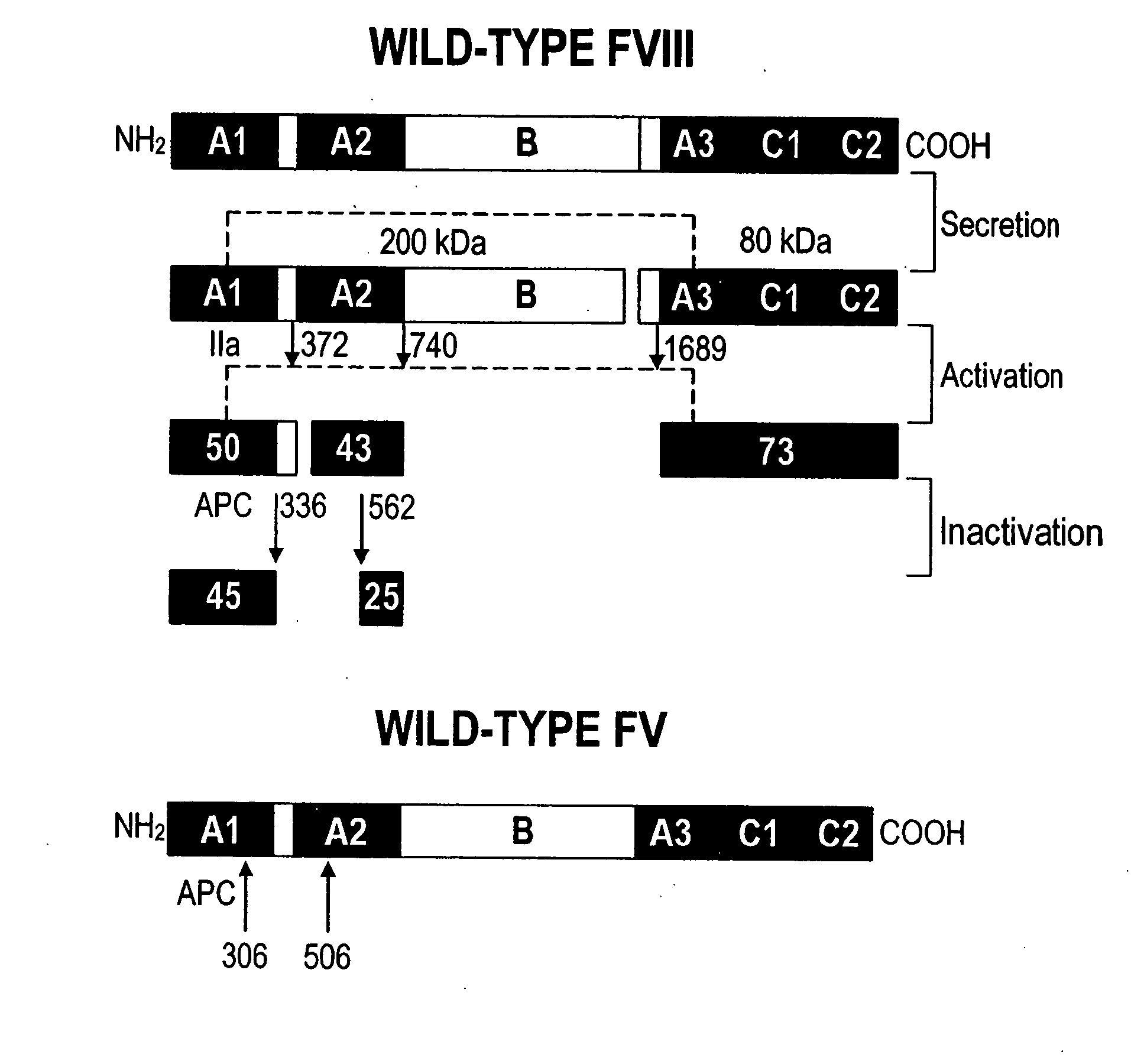
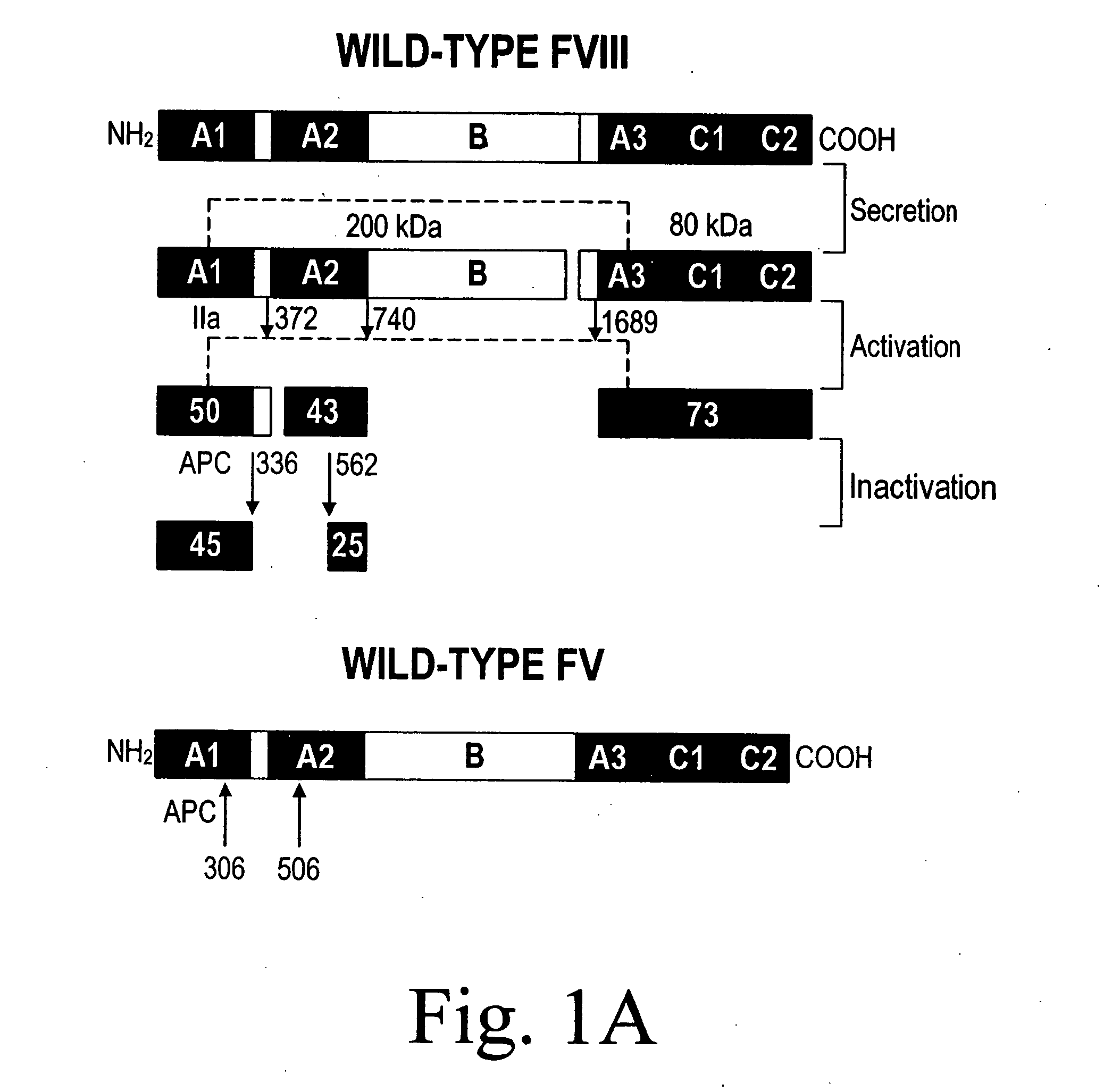
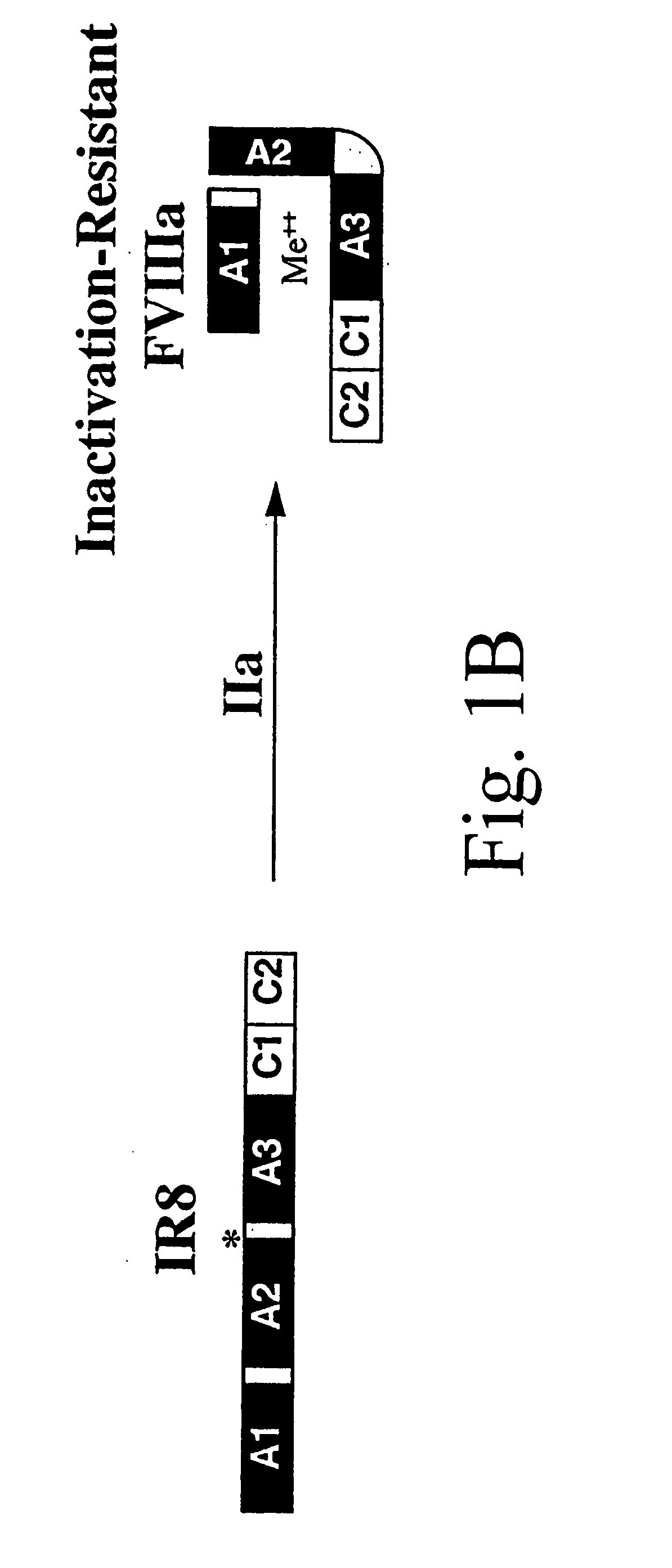
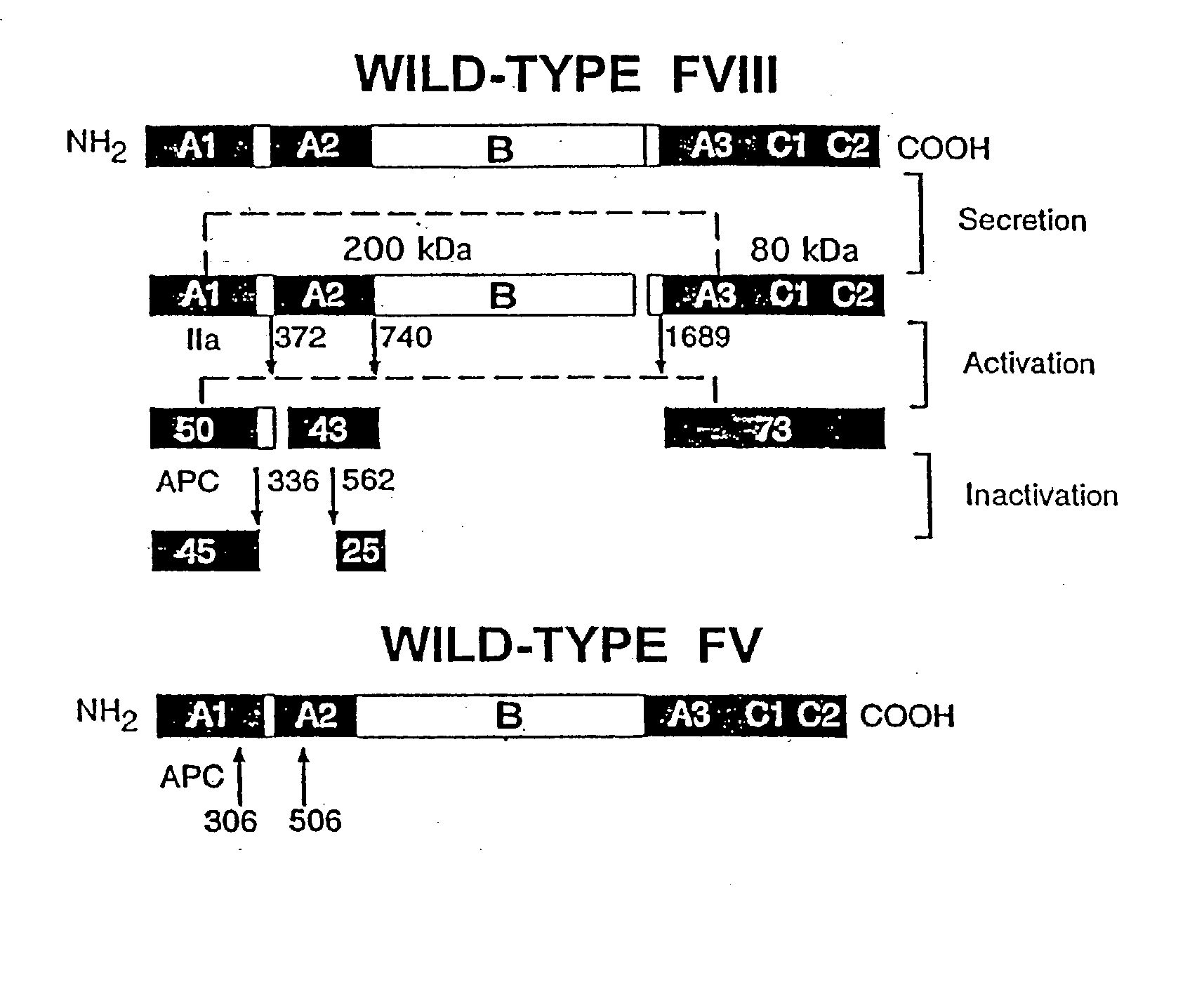
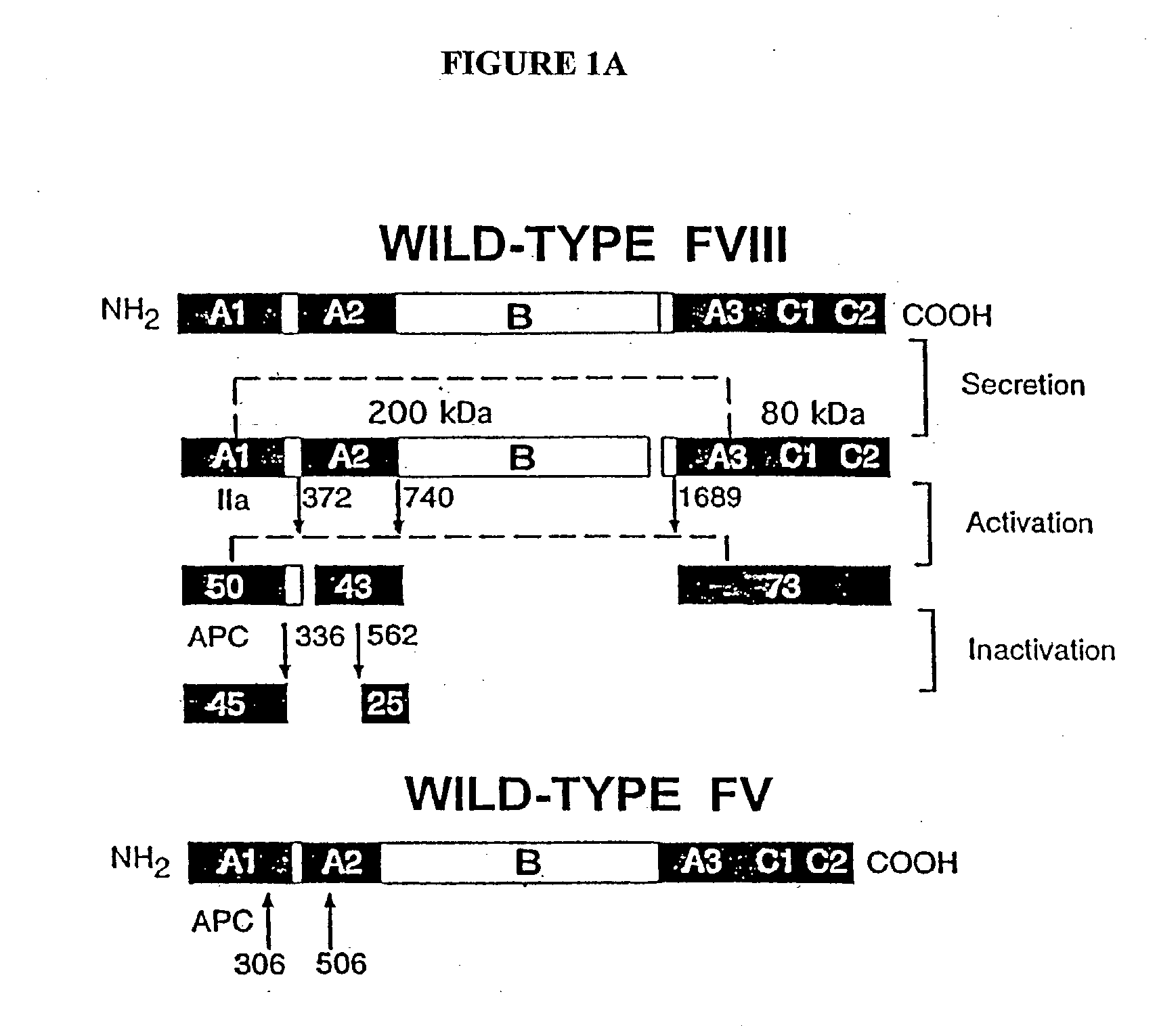
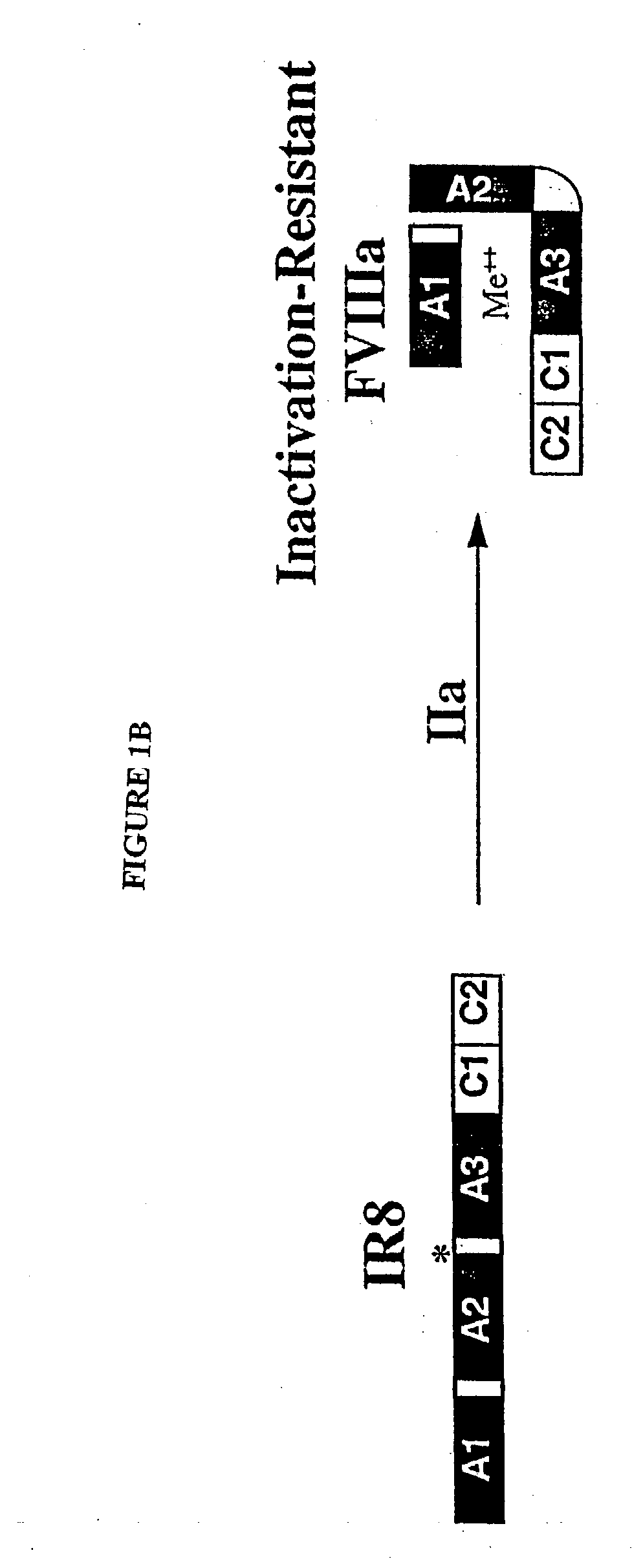
Inactivation resistant factor VIII
InactiveUS20040092442A1Increase secretionIncrease FVIII expressionFactor VIIBacteriaNucleotideBinding site
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe3O9 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated, an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:UNIV OF MICHIGAN THE
Cross-domain security for data vault
ActiveUS8732856B2Ease of administrationEnsure effective implementationDigital data processing detailsAnalogue secracy/subscription systemsDatabase securitySecurity level
A secure database appliance leverages database security in a consistent framework provides consistent, flexible, and adaptable security using mandatory access controls in addition to user and role based security for access control and accountability. A database system communicatively connected to a plurality of network domains, each network domain having a level of security, the database system comprises at least one database accessible from all of the plurality of network domains, the database comprising data, each unit of data having a level of security and access control security operable to provide access to a unit of data in the database to a network domain based on the level of security of the network domain and based on the level of security of the unit of data.
Owner:ORACLE INT CORP
Method of improving antioxidant status of an infant
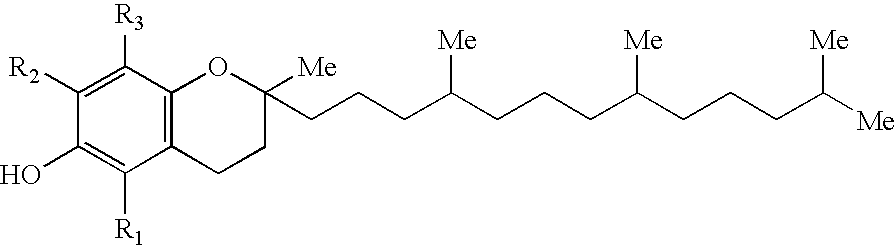
InactiveUS20060171993A1Ease of administrationImproving the antioxidant status of an infantBiocideFood ingredient as antioxidantTocopherolAntioxidative status
The present invention relates generally to a method of improving the antioxidant status of an infant. More particularly, the present invention relates to a method of improving the antioxidant status of an infant by administering a mixture of natural tocopherols. The natural tocopherol mixture is an effective blend of α- and γ-tocopherol. For ease of administration and improved taste, the mixture of natural tocopherols are typically delivered in vehicle which may be in the form, for example, of a tablet, capsule, liquid, and nutritional formula. The present invention also relates to a method of improving the antioxidant status of an infant by supplementing the lactating woman wherein the supplemented breast milk is fed to the infant. Additionally, the present invention relates to a method of improving the antioxidant status of a newborn infant by supplementing the pregnant woman.
Owner:ABBOTT LAB INC
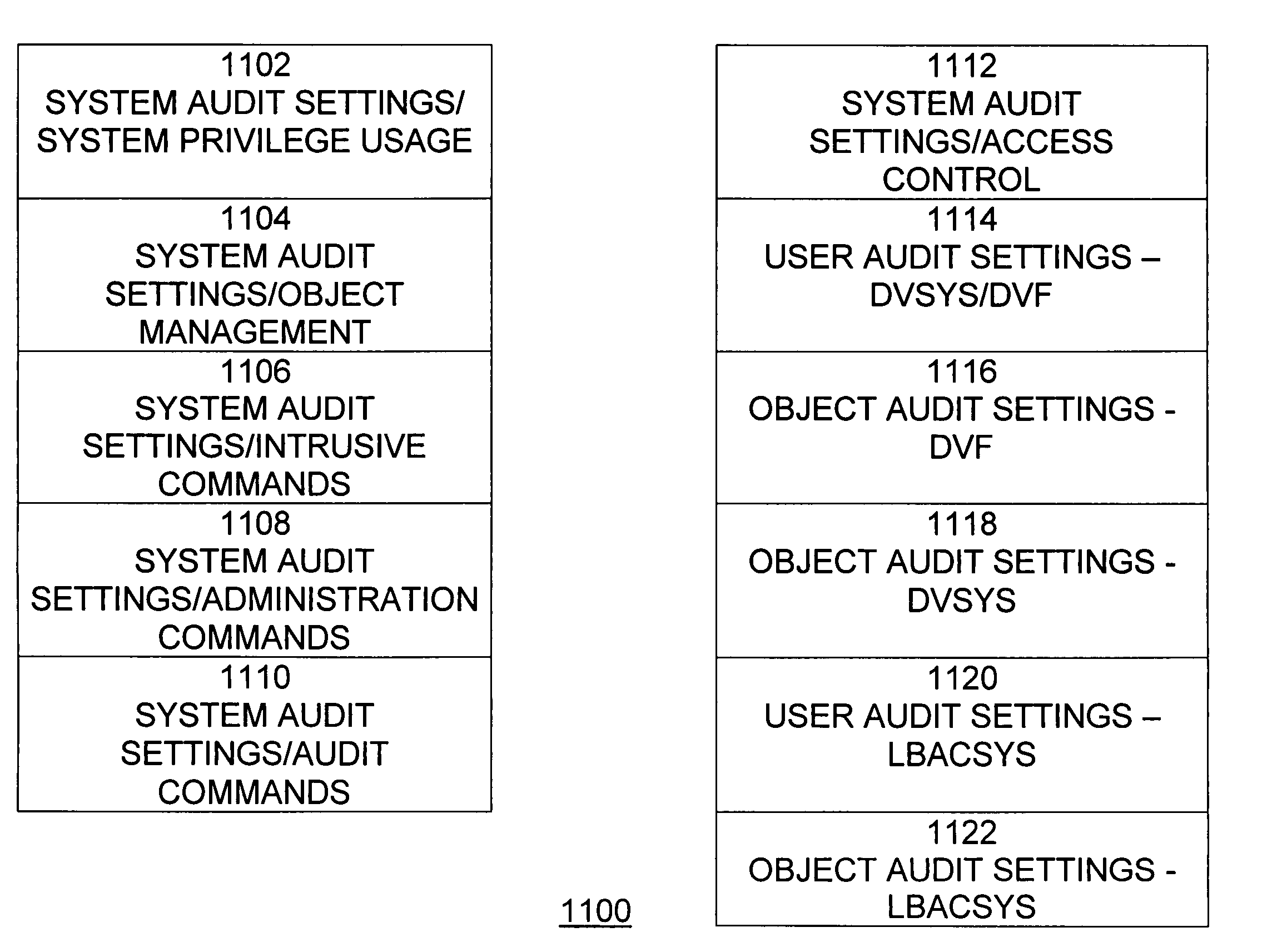
Dynamic auditing
ActiveUS7814075B2Ease of administrationEnsure effective implementationDigital data processing detailsComputer security arrangementsDatabase securitySecurity level
A secure database appliance leverages database security in a consistent framework provides consistent, flexible, and adaptable security using mandatory access controls in addition to user and role based security for access control and accountability. A database system comprises a plurality of database objects, each database object having a level of security, a plurality of factors, each factor representing a characteristic of a user of the database system, at least one database session of the user in the database, the database session having a level of security, the user connected to the database with a network domain, each network domain having a level of security, wherein the database system is operable to grant or deny access to the data to a user based on the factors associated with the user, based on the level of security of the data, based on the level of security of the database session, and based on the level of security of the network domain.
Owner:ORACLE INT CORP
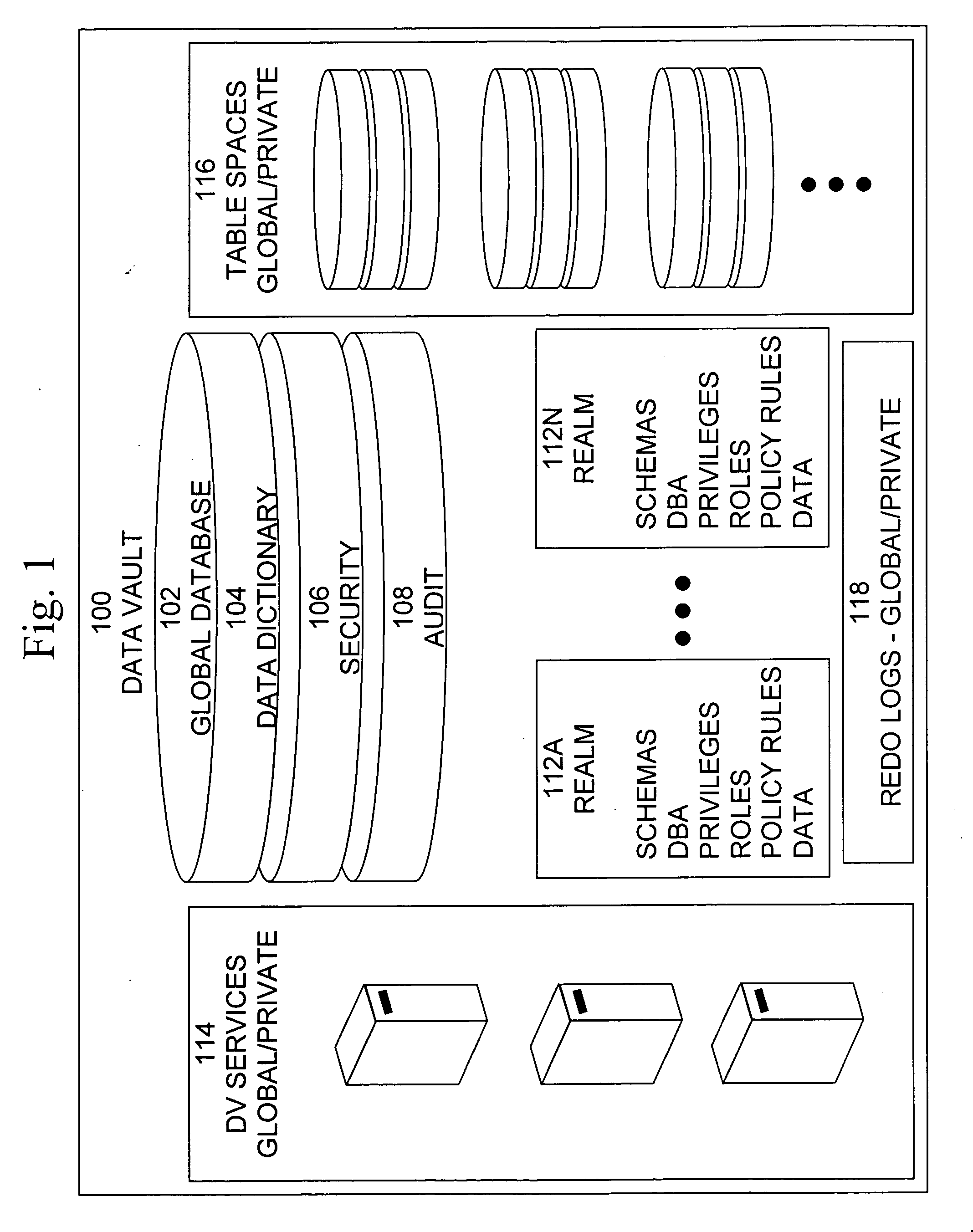
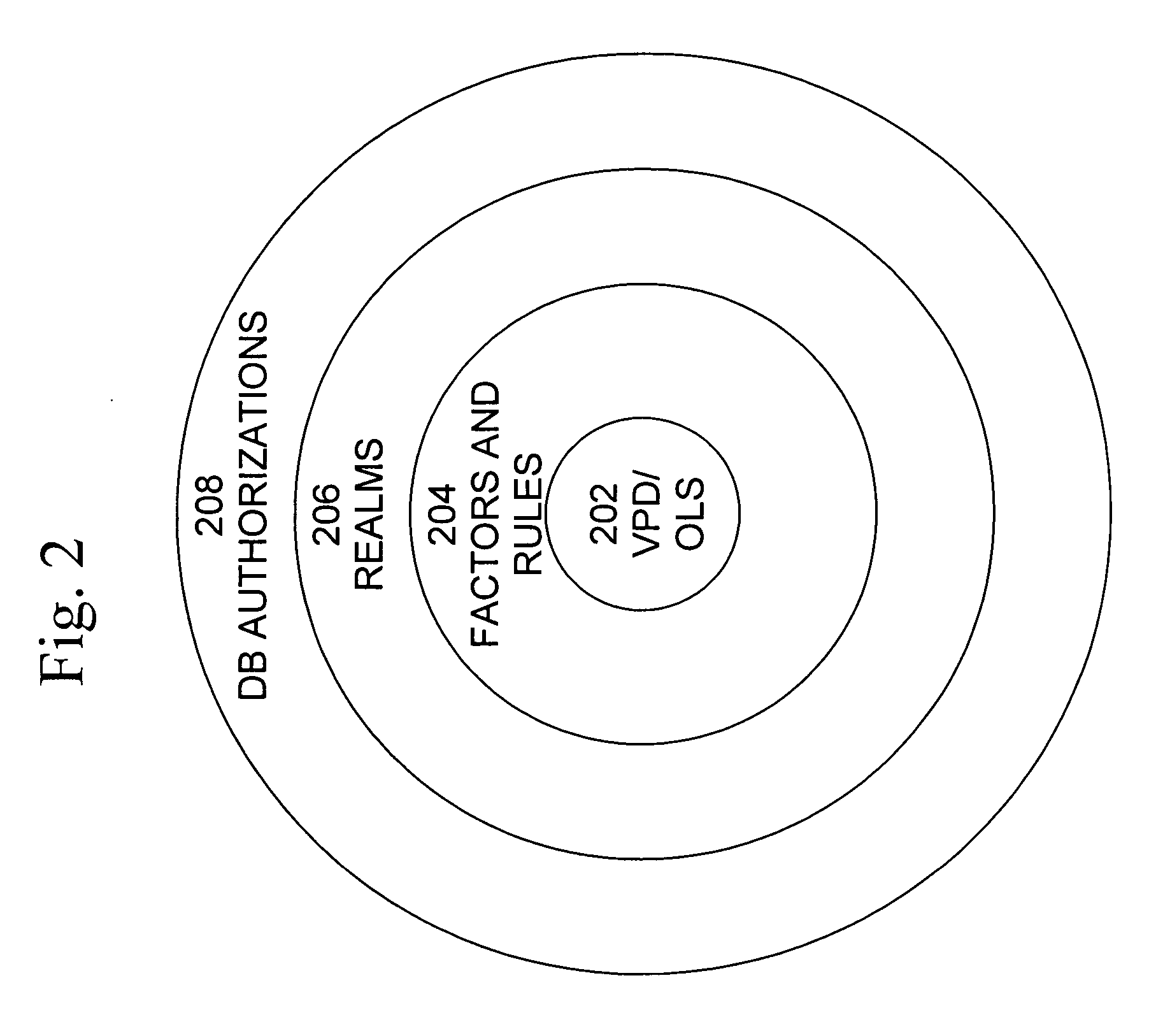
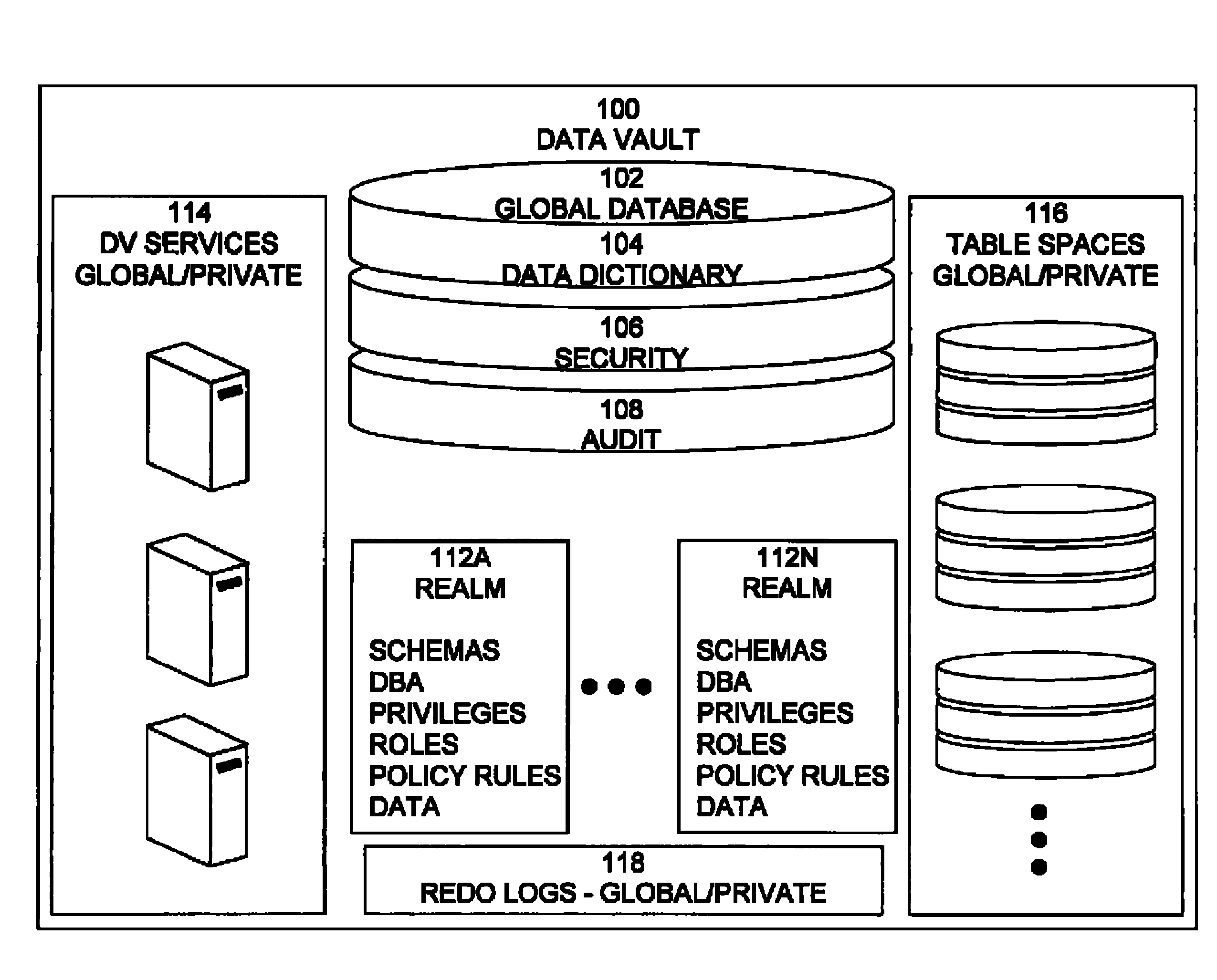
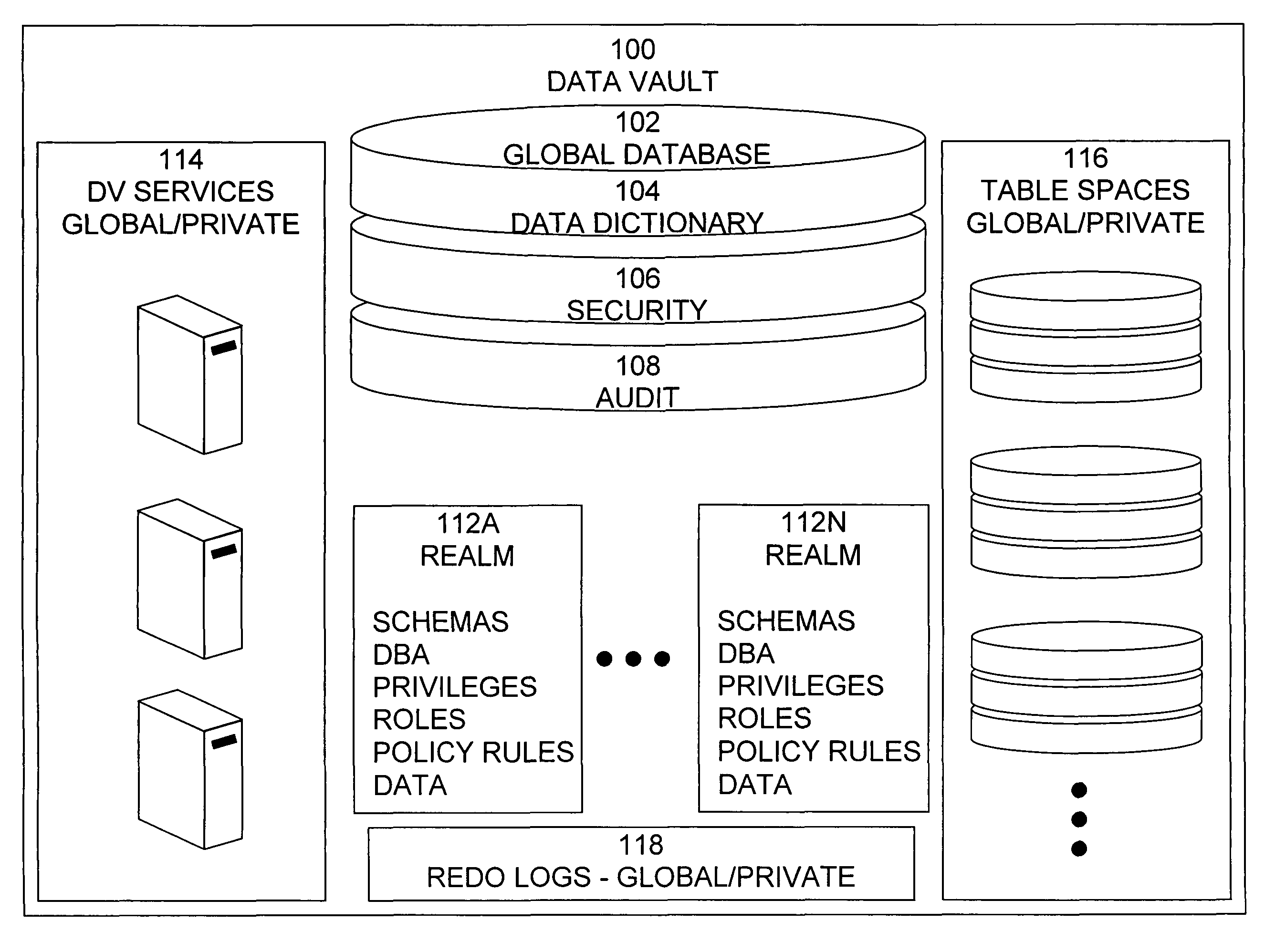
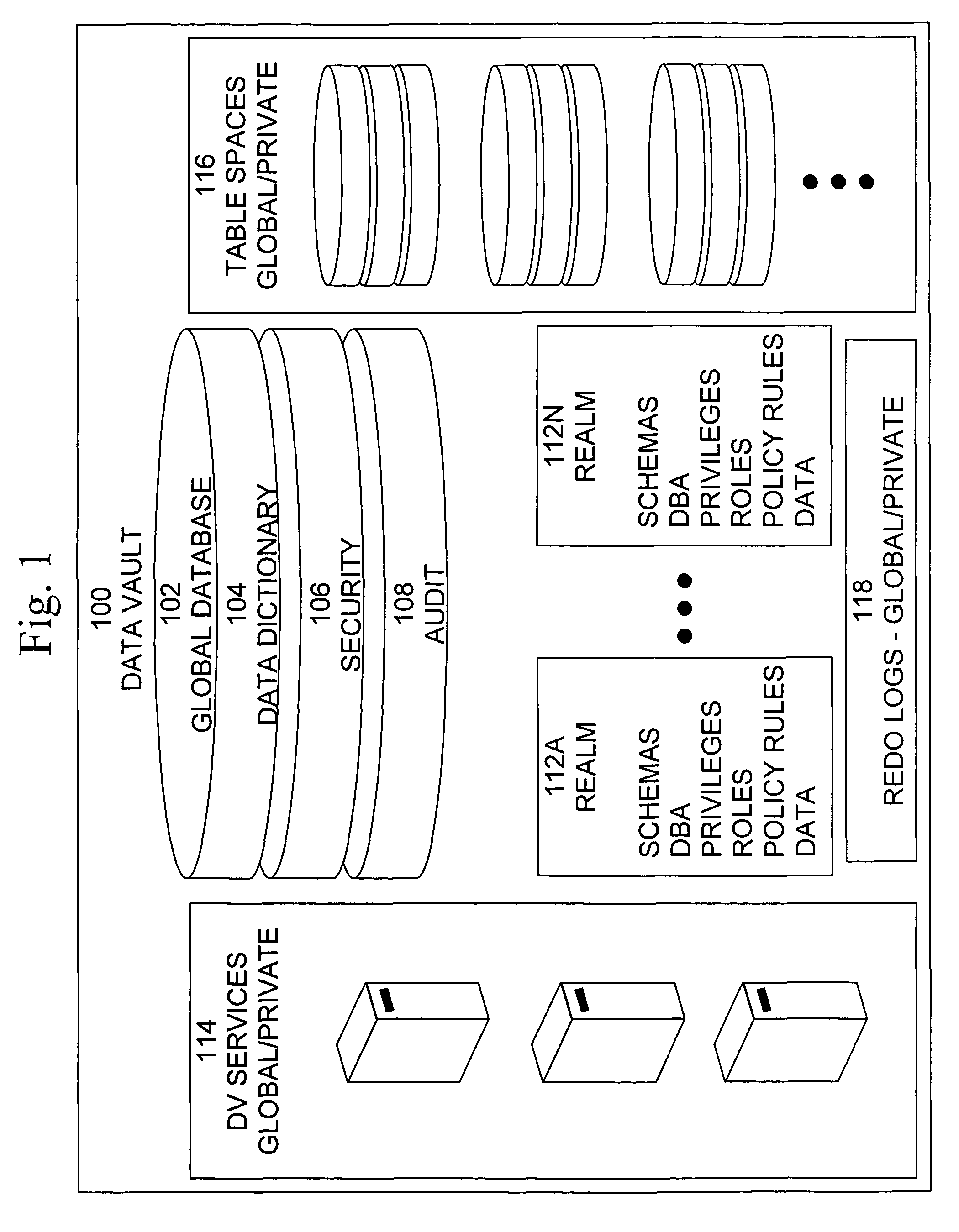
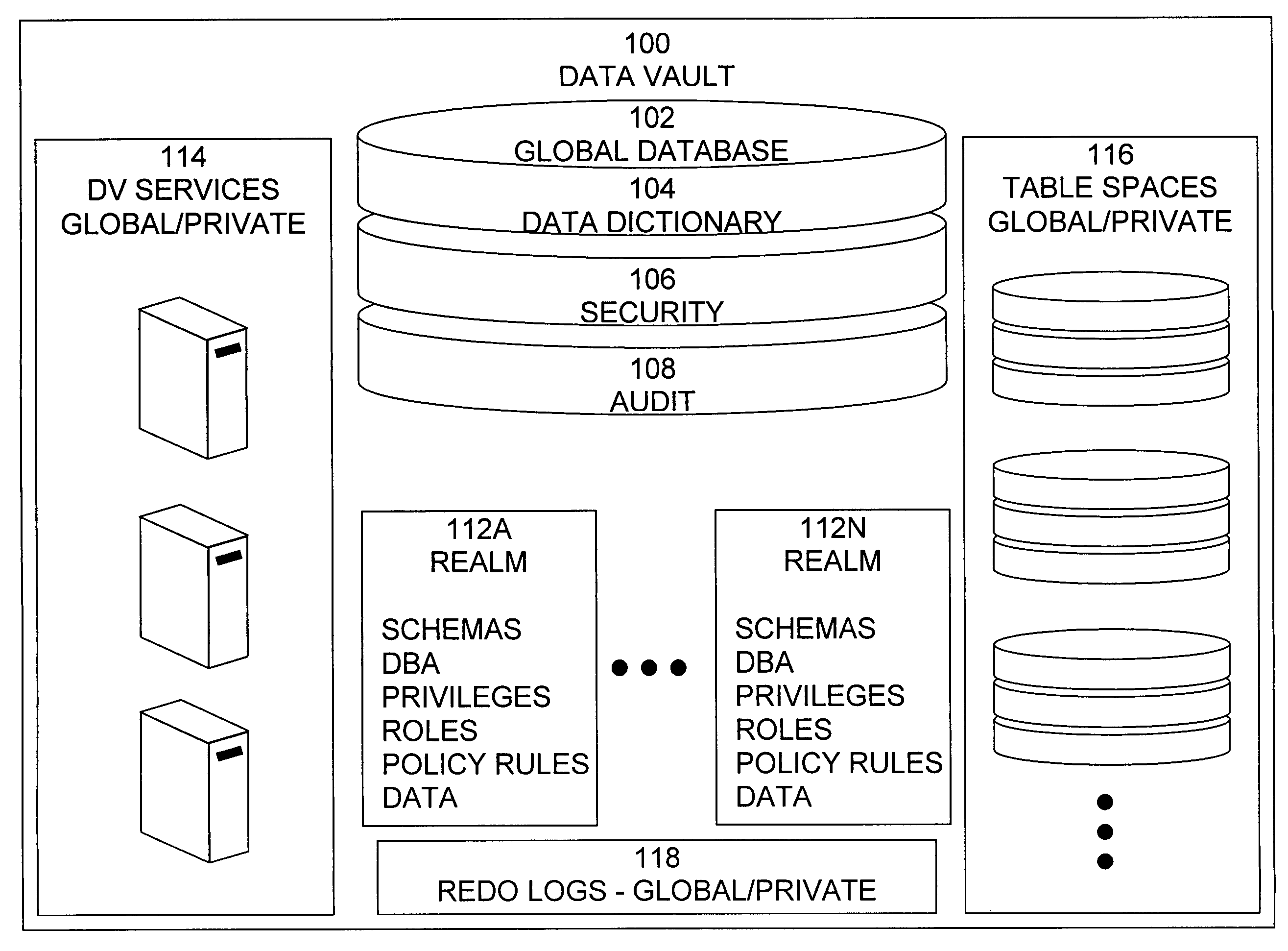
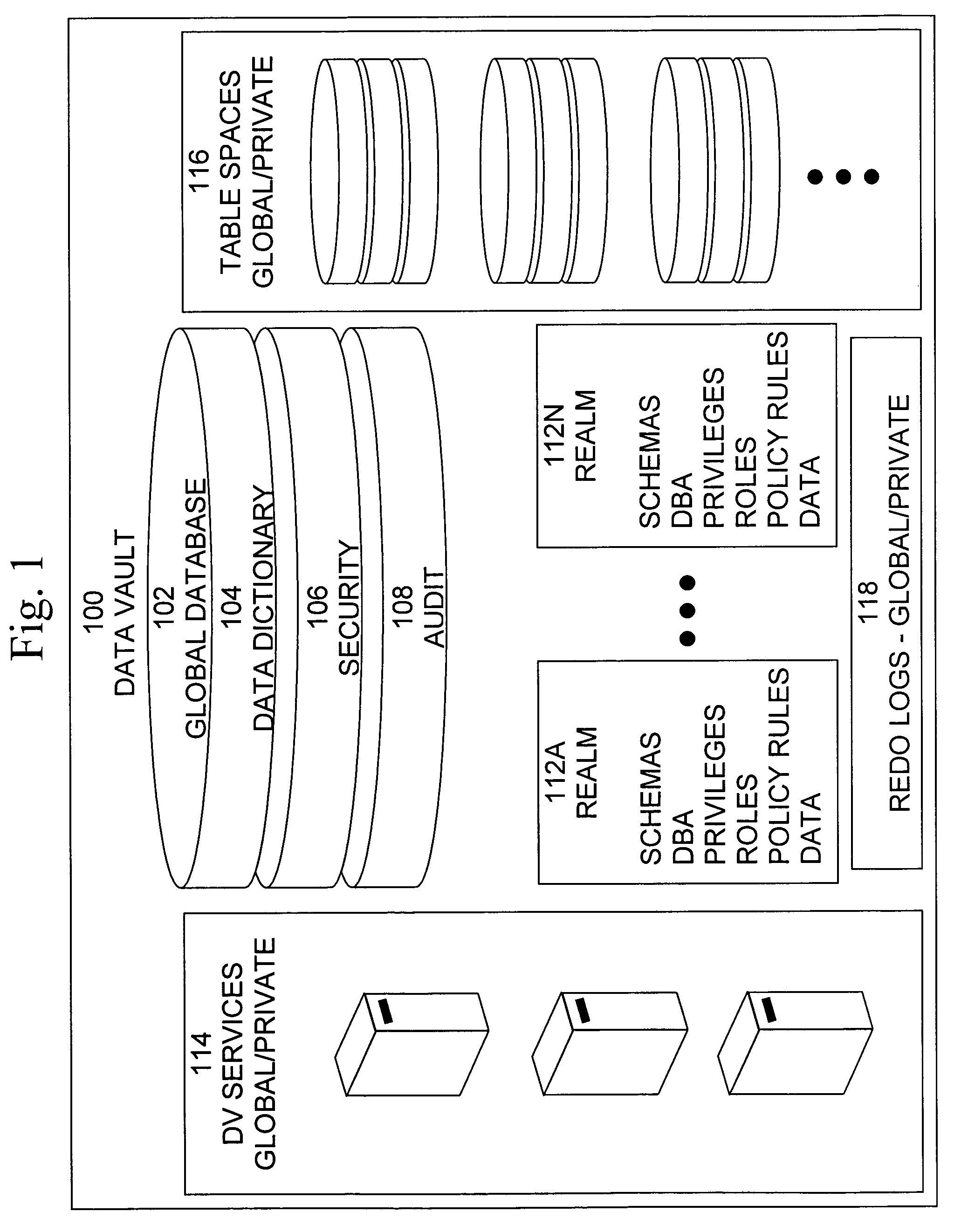
Data vault
ActiveUS7814076B2Ease of administrationEnsure effective implementationDigital data processing detailsComputer security arrangementsDatabase securitySecurity level
A secure database appliance leverages database security in a consistent framework providing consistent, flexible, and adaptable security using mandatory access controls in addition to user and role based security for access control and accountability. A database system comprises a plurality of database objects, each database object having a level of security, a plurality of factors, each factor representing a characteristic of a user of the database system, at least one database session of the user in the database, the database session having a level of security, the user connected to the database with a network domain, each network domain having a level of security, wherein the database system is operable to grant or deny access to the data to a user based on the factors associated with the user, based on the level of security of the data, based on the level of security of the database session, and based on the level of security of the network domain.
Owner:ORACLE INT CORP
Methods and kits for co-administration of nutritional supplements
ActiveUS20080152725A1Supplement nutritional deficiencyEase of administrationBiocideHeavy metal active ingredientsCo administrationVitamin C
The present invention relates to methods of co-administration of various vitamin and mineral compositions, and in a specific embodiment, said methods comprise co-administering one composition comprising vitamin A, vitamin D, vitamin C, vitamin E, folic acid, vitamin B1, vitamin B2, vitamin B6, vitamin B12, niacin, calcium, iron, magnesium, zinc, and / or copper, and a second composition comprising omega-3 fatty acids such as DHA, to supplement the nutritional needs of individuals within physiologically stressful states; and kits provided for co-administration of various vitamin and mineral compositions, and in a specific embodiment, said kits comprise one composition comprising vitamin A, vitamin D, vitamin C, vitamin E, folic acid, vitamin B1, vitamin B2, vitamin B6, vitamin B12, niacin, calcium, iron, magnesium, zinc, and / or copper, and a second composition comprising omega-3 fatty acids such as DHA, to supplement the nutritional needs of individuals within physiologically stressful states.
Owner:EVERETT LAB
Compositions and methods for nutrition supplementation
InactiveUS20060034916A1Ease of administrationEase of complianceHeavy metal active ingredientsBiocideDiseaseVitamin C
The present invention relates to compositions that may be swallowable, chewable or dissolvable, comprising various vitamins and minerals, and in a specific embodiment comprising vitamin A, beta carotene, B-complex vitamins, vitamin C, vitamin D3, vitamin E, iron, magnesium and zinc, and methods for using these compositions for nutritional supplementation in subjects undergoing physiologically stressful events, such as, for example and without limitation, pregnancy, lactation or any disease state.
Owner:EVERETT LAB
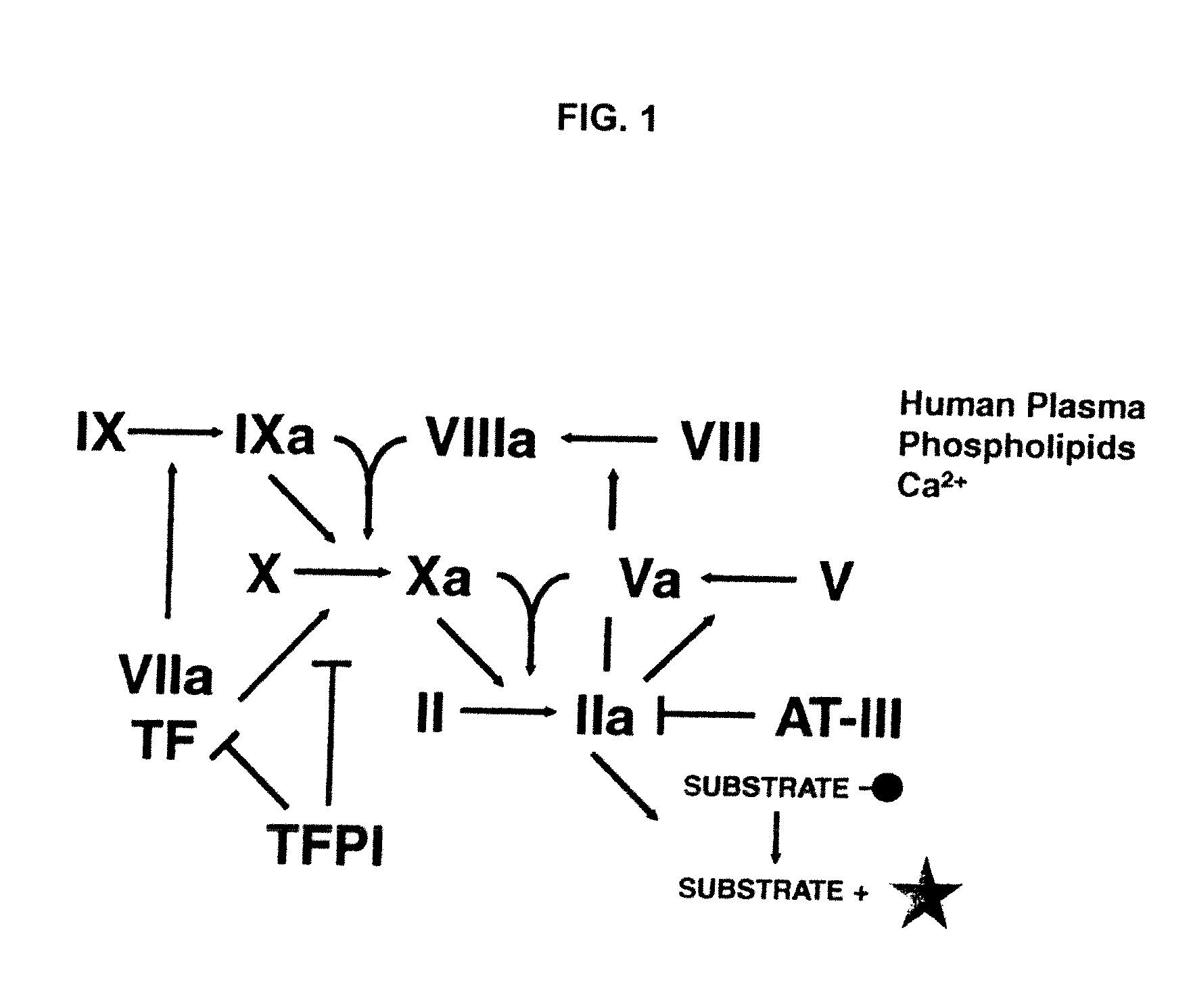
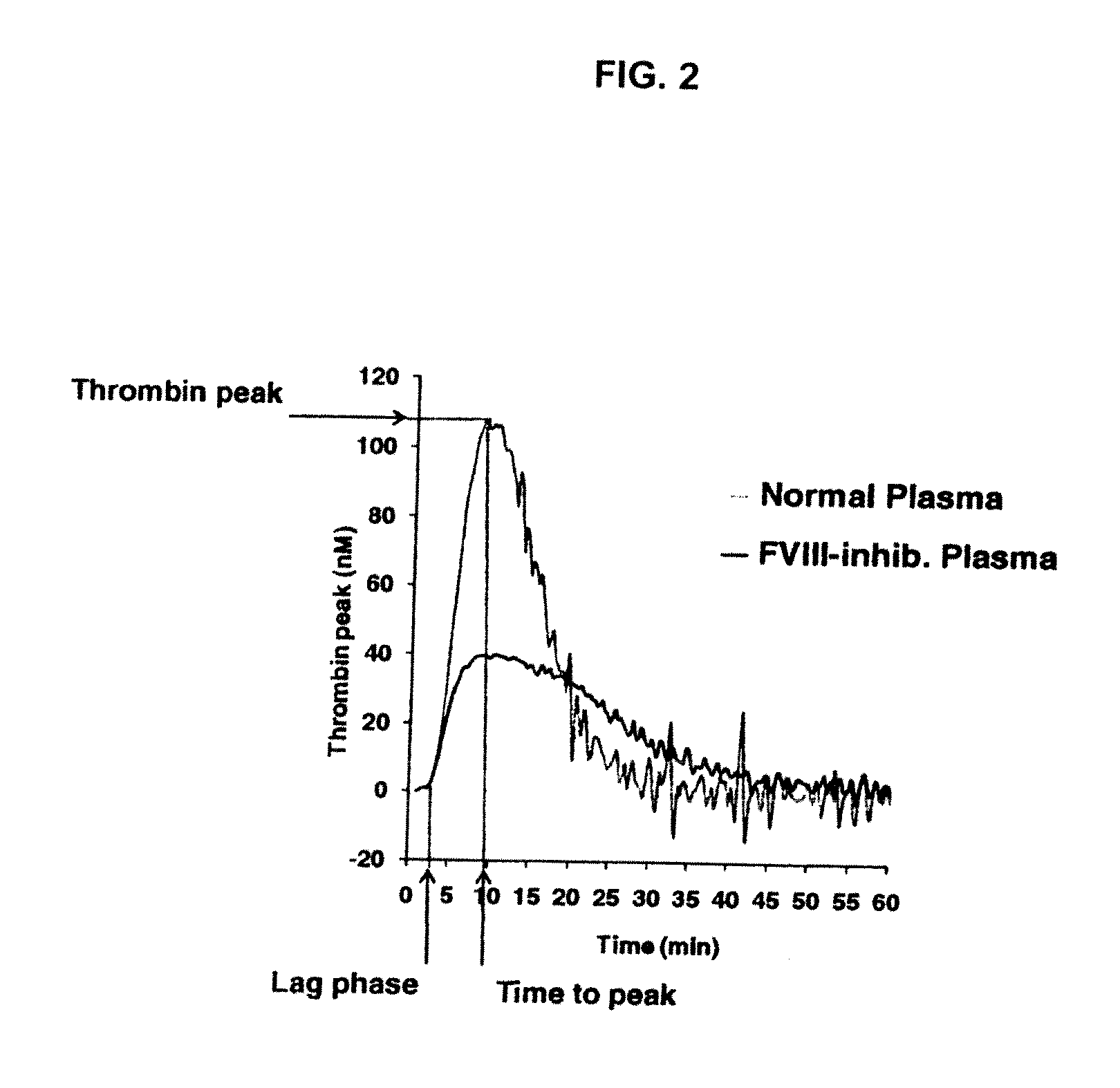
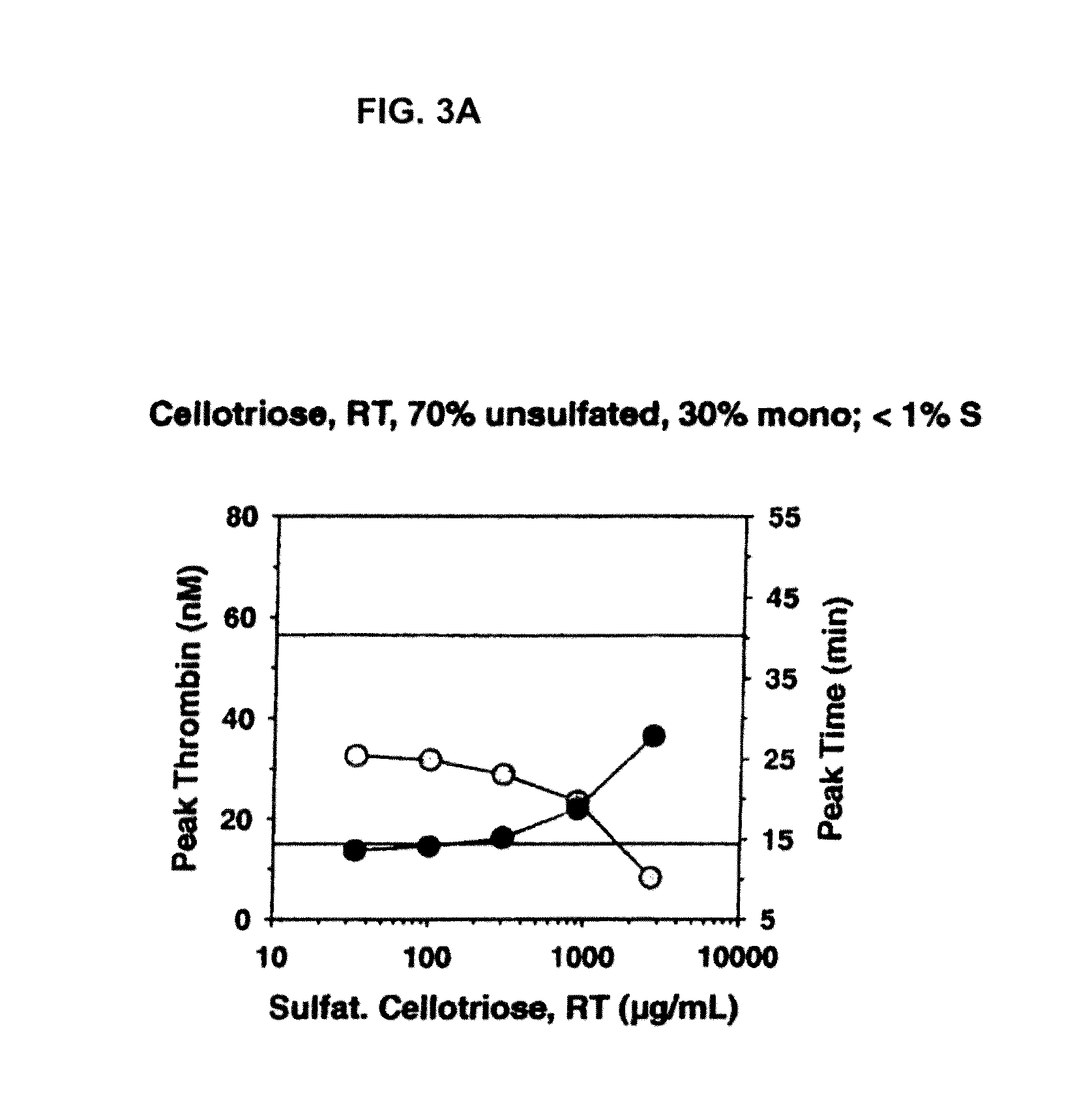
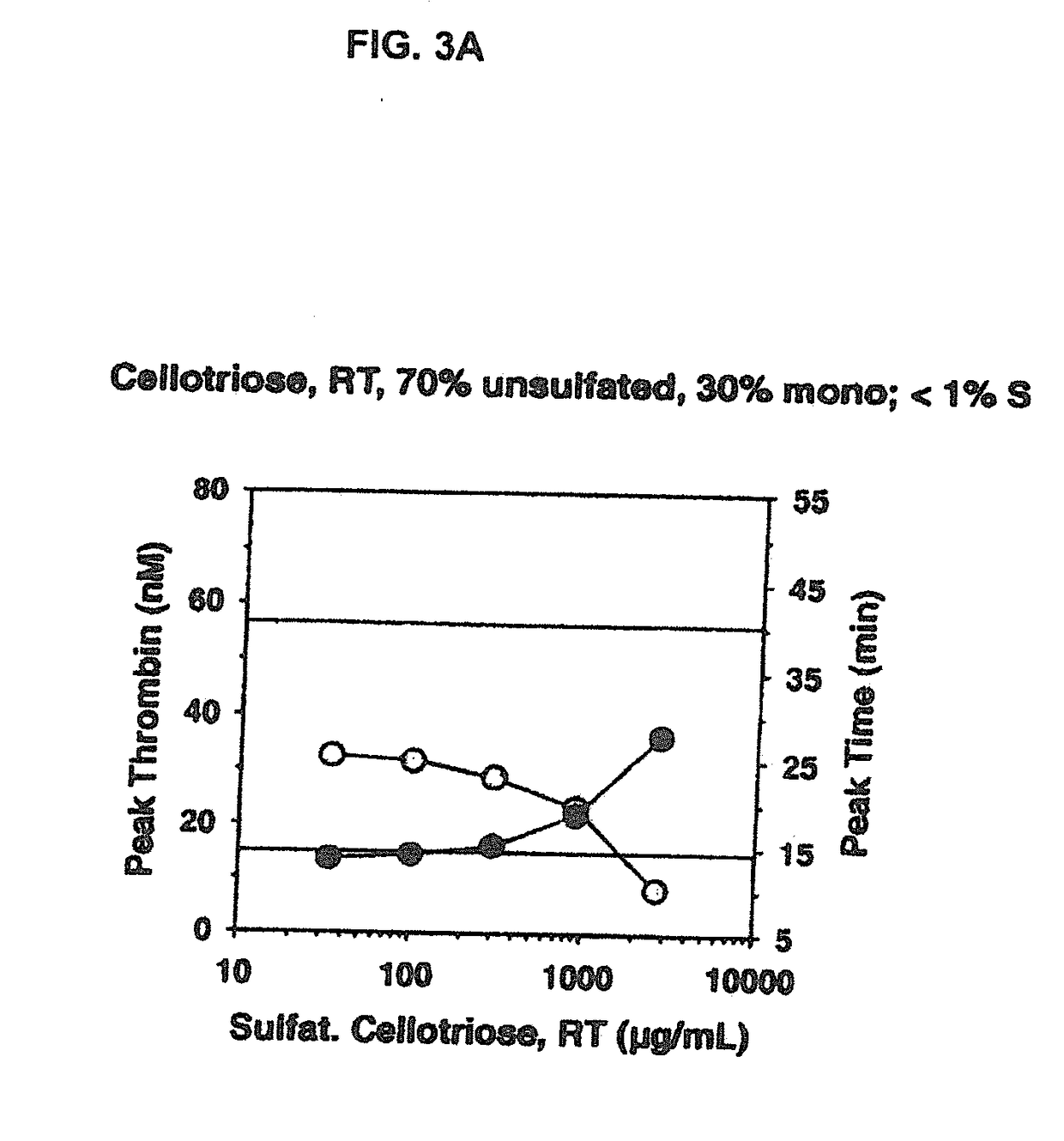
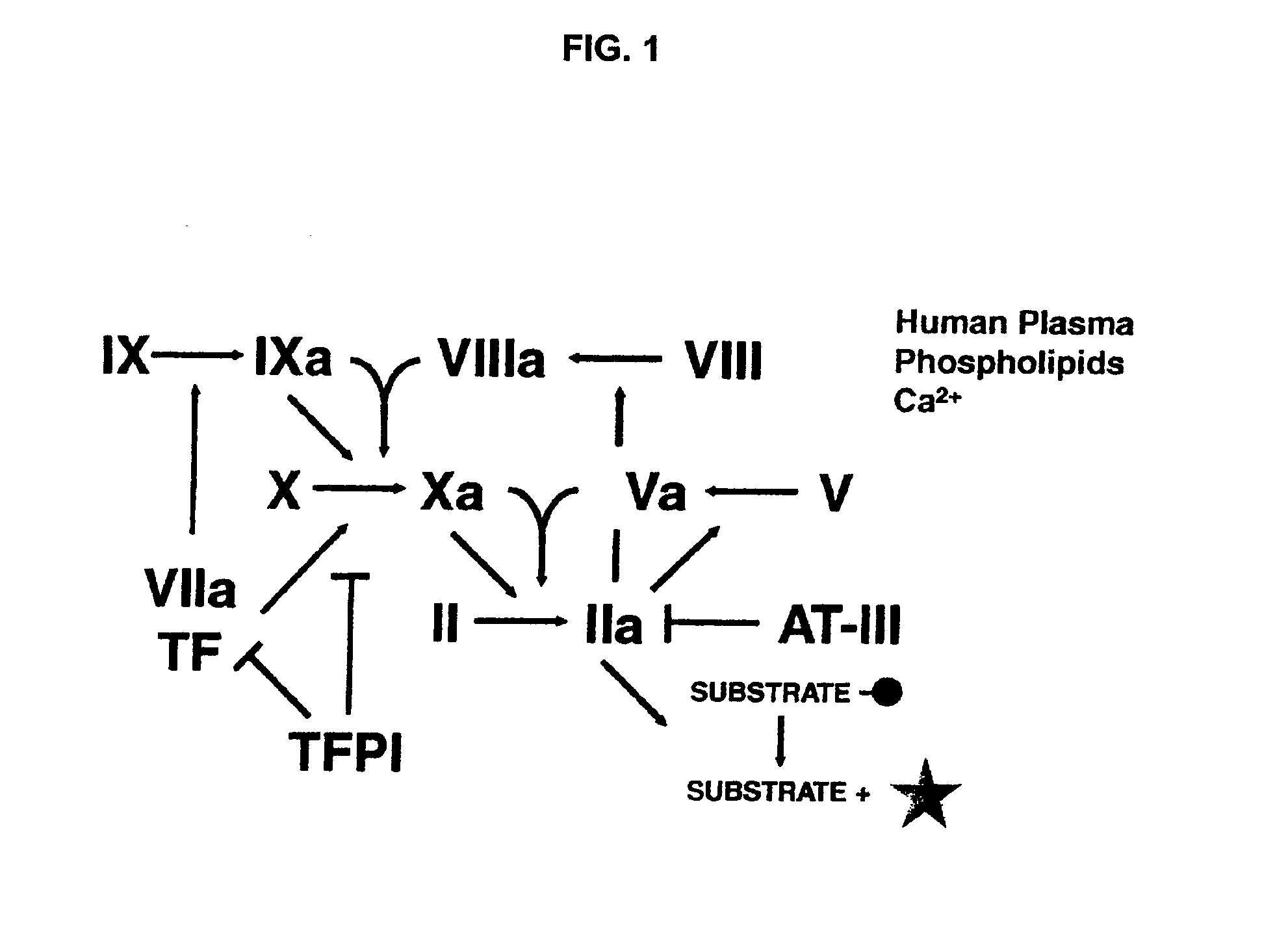
Non-anticoagulant sulfated or sulfonated polysaccharides
InactiveUS20130209444A1Offsetting effectShorten clotting timeOrganic active ingredientsBiocideSulfationMedicine
The present invention provides non-anticoagulant sulfated or sulfonated polysaccharides (NASPs), which accelerate the blood clotting process. Also provided are pharmaceutical formulations comprising a NASP of the invention in conjunction with a pharmaceutically acceptable excipient and, in various embodiments, these formulations are unit dosage formulations. The invention provides a NASP formulation, which is orally bioavailable. Also provided are methods for utilizing the compounds and formulations of the invention to promote blood clotting in vivo as therapeutic and prophylactic agents and in vitro as an aid to studies of the blood clotting process.
Owner:BAXALTA GMBH
Recombinant virus products and methods for inhibition of expression of myotilin
ActiveUS20140045925A1Ease of administrationEase of handlingSugar derivativesGenetic material ingredientsDna encodingGene expression
The present invention relates to RNA interference-based methods for inhibiting the expression of the myotilin gene. Recombinant adeno-associated viruses of the invention deliver DNAs encoding microRNAs that knock down the expression of myotilin. The methods have application in the treatment of muscular dystrophies such as Limb Girdle Muscular Dystrophy Type 1A.
Owner:RES INST AT NATIONWIDE CHILDRENS HOSPITAL
Inactivation resistant factor VIII
InactiveUS20030148953A1Increase secretionHigh expressionFactor VIIPeptide/protein ingredientsBinding siteNucleotide
The present invention provides novel purified and isolated nucleic acid sequences encoding procoagulant-active FVIII proteins. The nucleic acid sequences of the present invention encode amino acid sequences corresponding to known human FVIII sequences, wherein residue Phe309 is mutated. The nucleic acid sequences of the present invention also encode amino acid sequences corresponding to known human FVIII sequences, wherein the APC cleavage sites, Arg336 and Ile562, are mutated. The nucleic acid sequences of the present invention further encode amino acid sequences corresponding to known human FVIII sequences, wherein the B-domain is deleted, the von Willebrand factor binding site is deleted, a thrombin cleavage site is mutated, an amino acid sequence spacer is inserted between the A2- and A3-domains. Methods of producing the FVIII proteins of the invention, nucleotide sequences encoding such proteins, pharmaceutical compositions containing the nucleotide sequences or proteins, as well as methods of treating patients suffering from hemophilia, are also provided.
Owner:UNIV OF MICHIGAN TECH MANAGEMENT OFFICE
Extended release formulations of cannabinoids
PendingUS20200046643A1Ease of administrationUniform loadingPowder deliveryHydroxy compound active ingredientsCannabivarinPhenols
Compositions for the extended release of one or more cannabinoids, in which the compositions comprise a population of particles. Each particle may comprise the one or more cannabinoids, one or more drug-releasing agents, and a core. The APIs may be a cannabinoid, such as cannabidiol or Δ9-tetrahydrocannabinol. The core may comprise an inert material. The one or more cannabinoids may comprise Δ9-tetrahydrocannabinol, cannabidiol, or a combination thereof.
Owner:GLATT GMBH
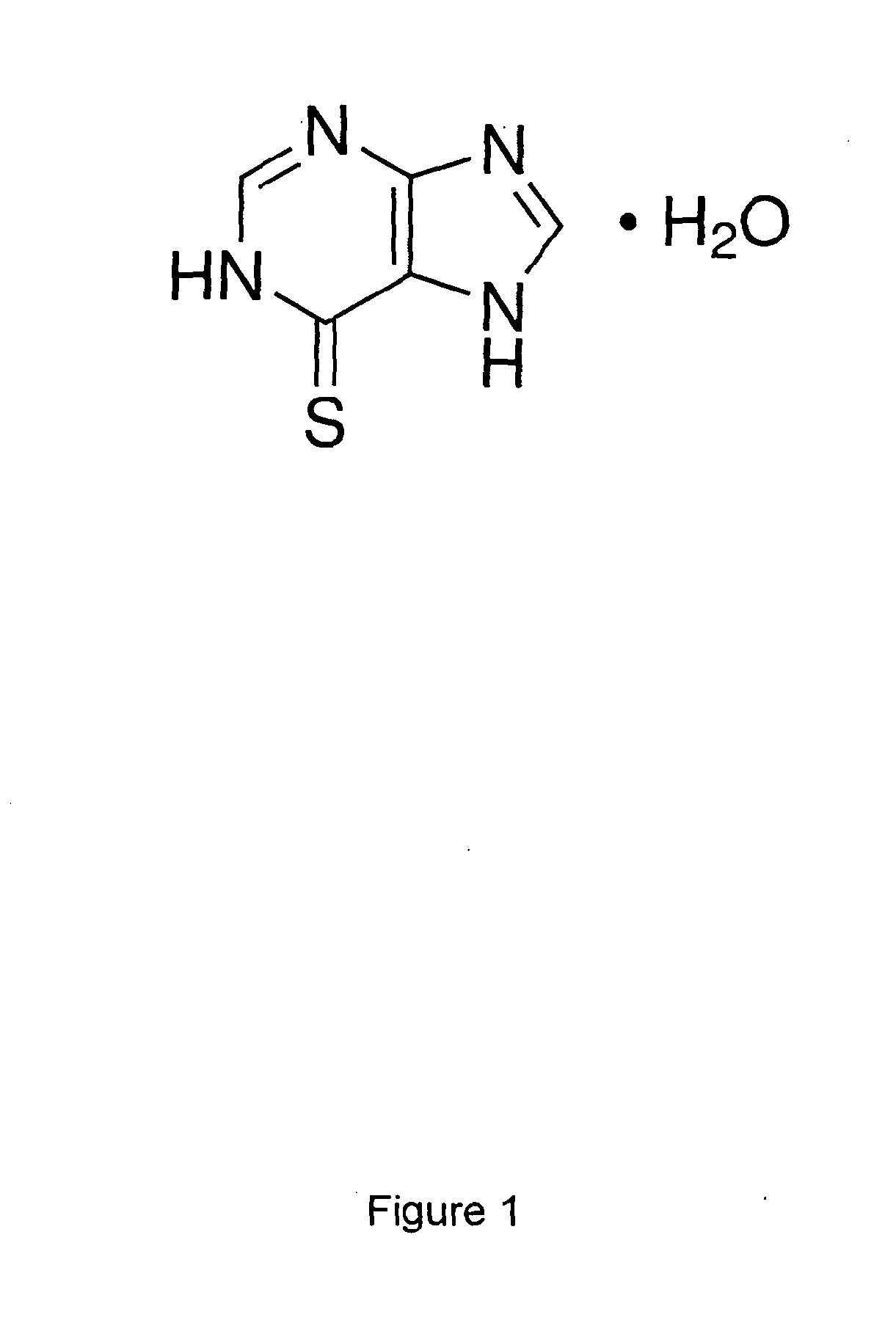
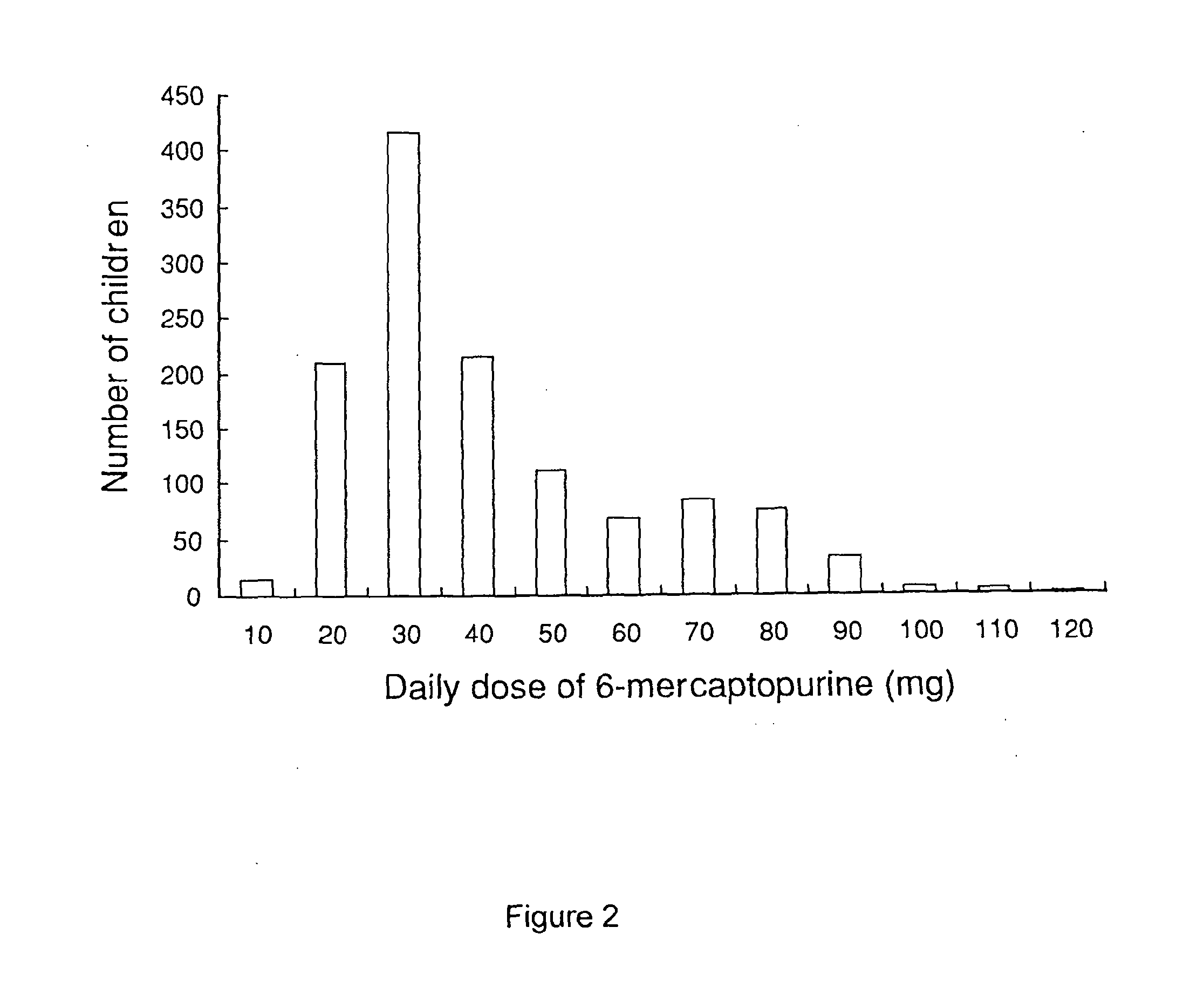
Oral Suspension
InactiveUS20140294972A1Accurate measurementImprove accuracyPowder deliveryBiocideOral suspensionsIntrathecal
A liquid pharmaceutical composition for use in the treatment of acute lymphoblastic leukaemia (ALL) comprising 6-mercaptopurine or a salt, hydrate or solvate thereof and a pharmaceutically-acceptable excipient, wherein the composition is a suspension for oral administration, a kit of parts for the accurate dosing and administration of the liquid pharmaceutical composition, and a method for the treatment of ALL in a human patient comprising administration of a therapeutically effective amount of the liquid pharmaceutical composition.
Owner:NOVA BIO PHARMA TECH LTD
Blend compositions for oral administration as a rapidly dissolving powder and/or suspension
ActiveUS10471006B2Great tasteEase of adherenceOrganic active ingredientsPowder deliveryOrganic acidOral medication
Disclosed is a dry powder oral formulation that includes an active pharmaceutical ingredient (API), a lecithin powder, a galactomannan, one or more sweetening agents, one or more flavoring agents and an organic acid in a pharmaceutically acceptable preparation. Also disclosed are an excipient composition in absence of an API and methods of making and using the formulations and compositions. Also disclosed is a chewable, swallowable, and / or orally disintegrating tablet comprising an active pharmaceutical ingredient (API), a lecithin powder, a galactomannan, one or more sweetening agents, one or more flavoring agents and an organic acid in a pharmaceutically acceptable preparation.
Owner:MARENDA PHARMA LLC
Compositions and methods for treatment and management of pain
InactiveUS20130266673A1Improved pain managementEase of administrationBiocideOrganic active ingredientsTurmeric extractFrankincense extract
Disclosed herein are compositions and methods for the treatment and management of pain in a subject comprising or consisting of topically or orally administering a therapeutic effective amount of a composition. The composition is made up of minute amounts of herbal extracts and compound mixtures to a subject. The composition includes turmeric extract, Boswellia extract, ginger extract, holy basil extract, rosemary extract, white willow extract and alpha lipolic acid each in minute amounts.
Owner:GHORBANI REZA
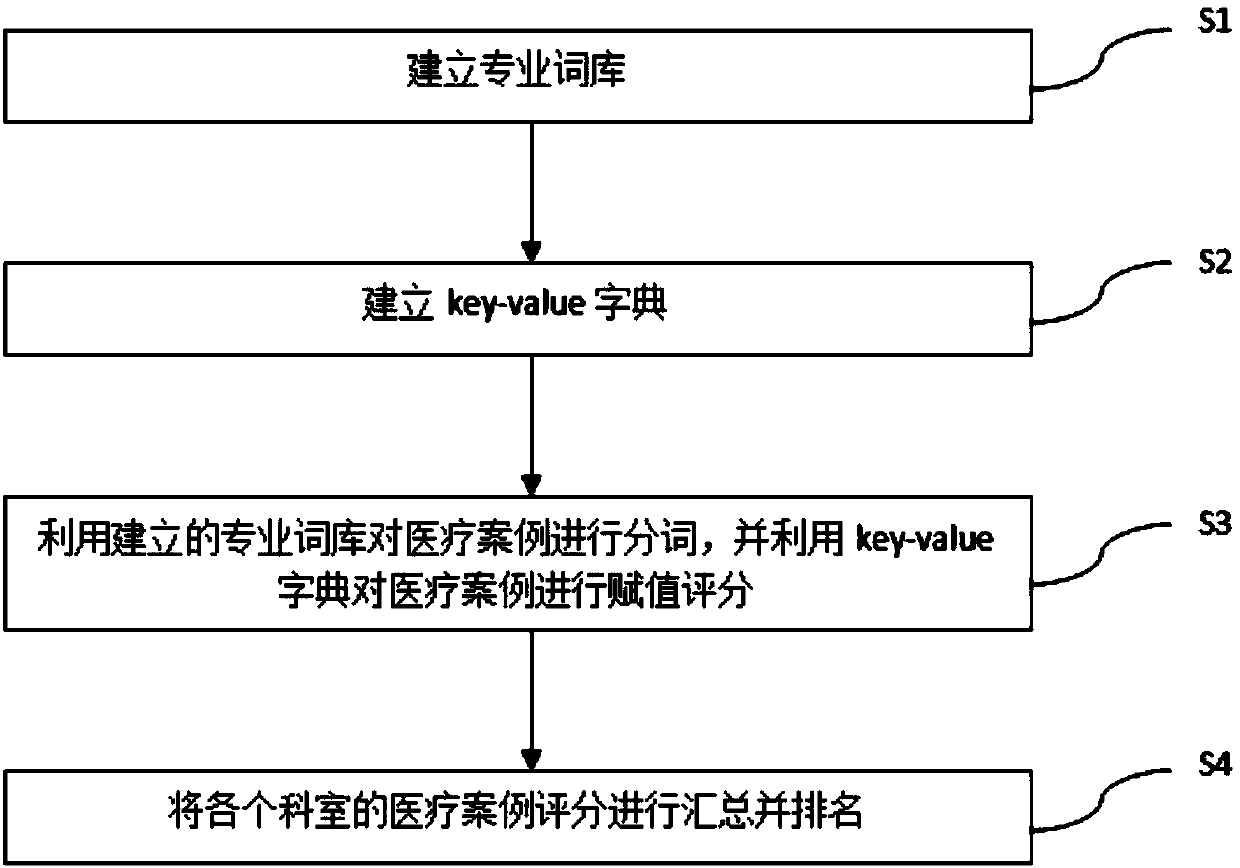
Method and system for medical performance quantification
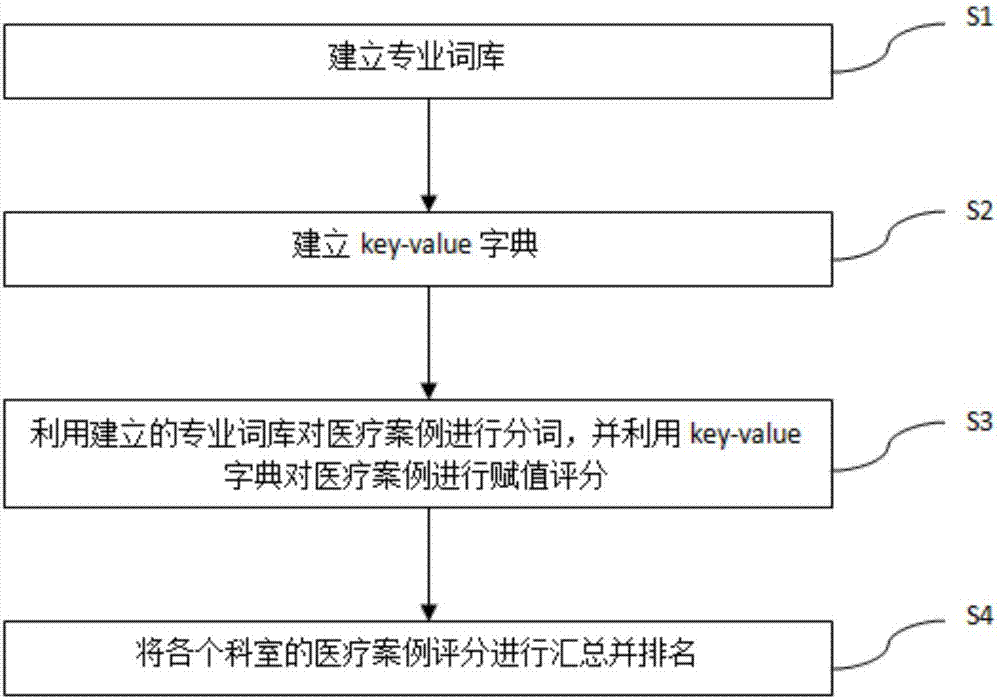
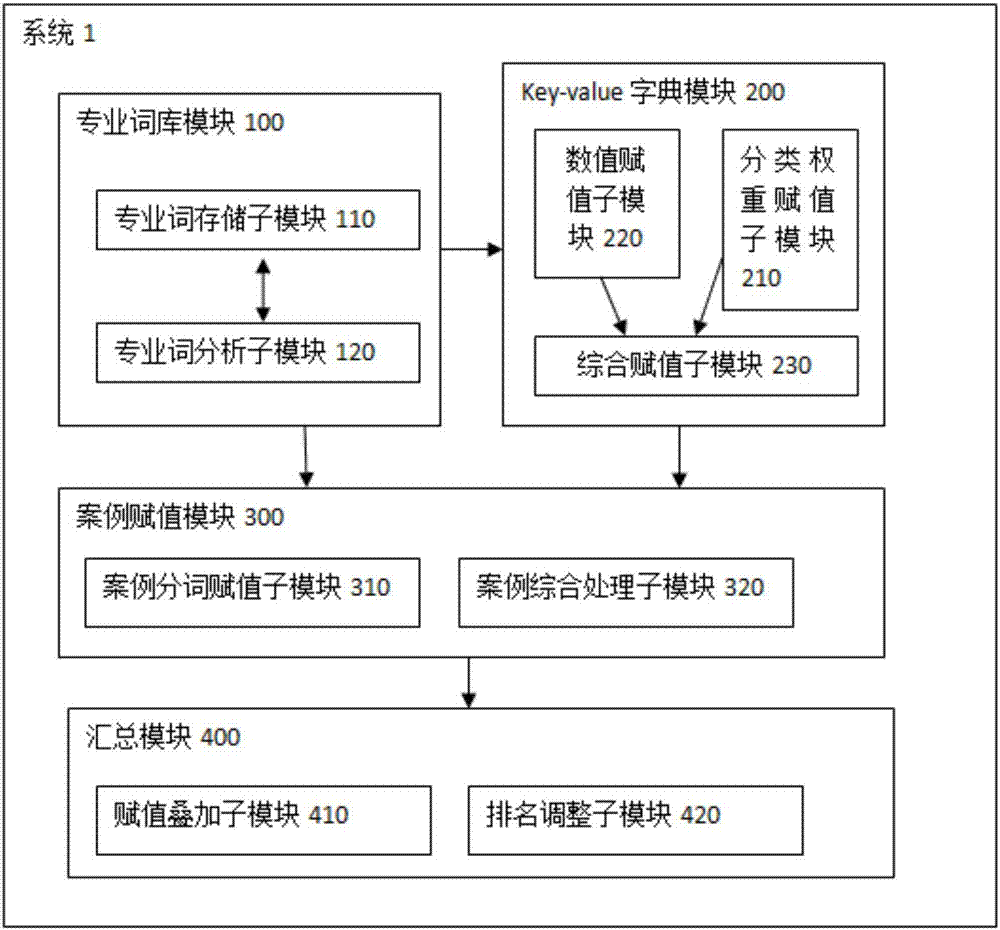
InactiveCN107273671AQuantitative results are accurate and objectiveThe quantification process is shortNatural language data processingSpecial data processing applicationsQuantitative ResultData mining
The purpose of the invention is to provide a method and a system for medical performance quantification, which make the medical performance quantification more convenient and objective. The method includes the following steps that: S1,professional thesaurus is established; S2, key-value dictionary is established, and specialized words in the professional thesaurus are assigned; S3,medical cases are segmented using the established professional thesaurus, and the medical cases are assigned scores using the key-value dictionary; S4,medical cases scores are summarized and ranked in each department. Compared with the prior art, the advantages of the method is that the system can effectively use medical data to quantify the medical performance, the quantitative results are more accurate and objective, and the quantitative processes are shorter and more efficient. The system is convenient for administrative departments of major hospitals and related units to manage the operation.
Owner:江苏金琉璃科技有限公司
Antibacterial Quinoline Derivatives
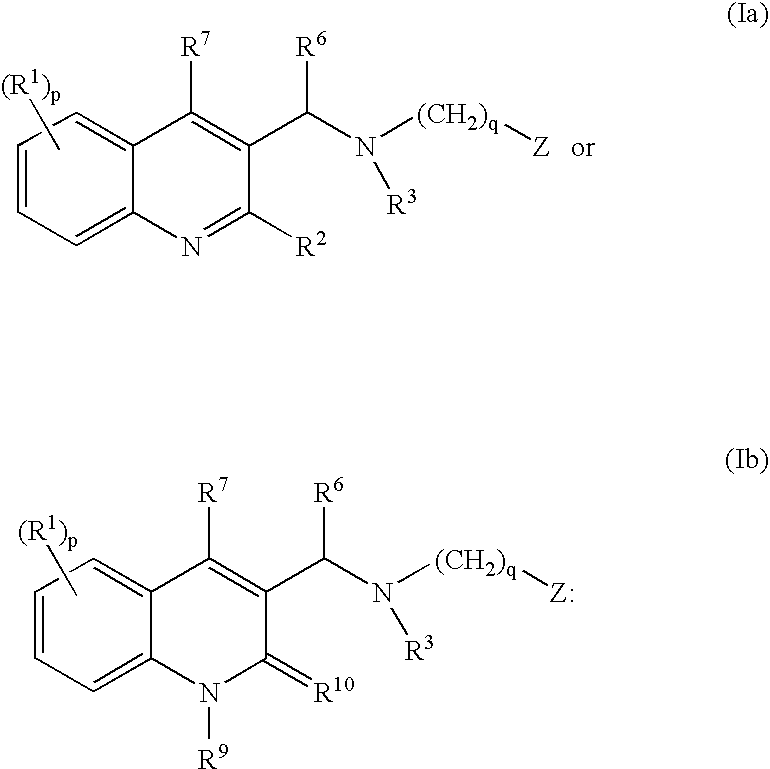
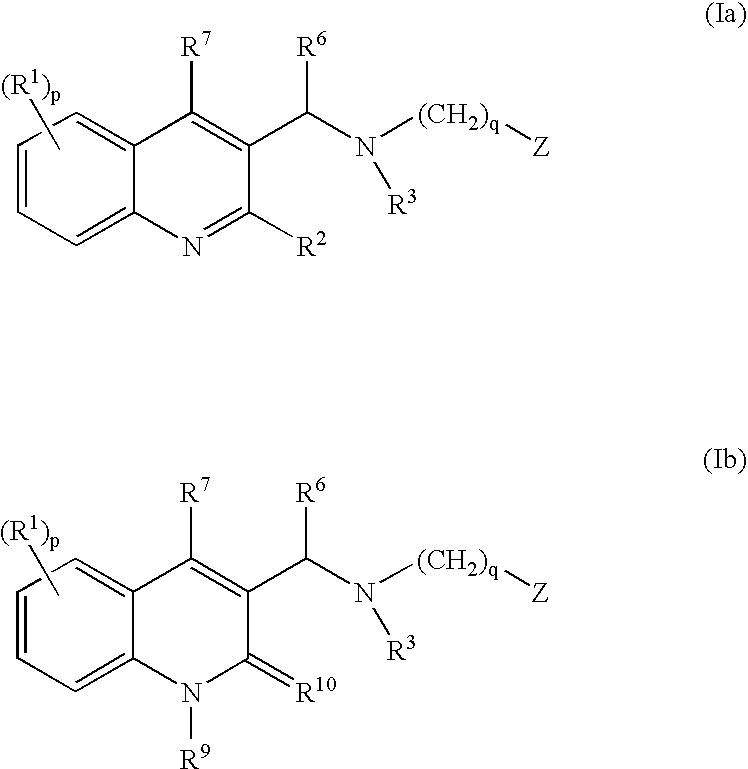
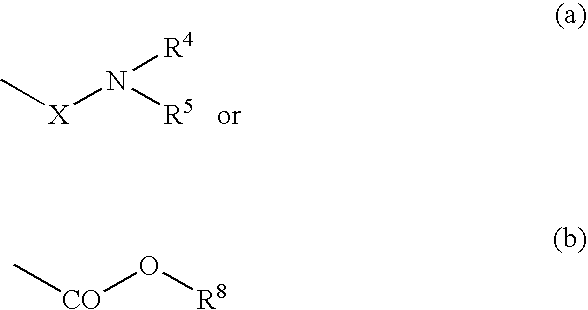
ActiveUS20080182855A1Ease of administrationImprove uniformityAntibacterial agentsBiocideMycobacterium marinumQuinoline
The present invention relates to novel substituted quinoline derivatives according to the general Formula (Ia) or Formula (Ib):the pharmaceutically acceptable acid or base addition salts thereof, the quaternary amines thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. The claimed compounds are useful for the treatment of a bacterial disease including a mycobacterial disease, particularly those diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis, M. bovis, M. avium and M. marinum. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the claimed compounds, the use of the claimed compounds or compositions for the manufacture of a medicament for the treatment of bacterial diseases and a process for preparing the claimed compounds.
Owner:JANSSEN PHARMA NV
Glycolipid containing compositions for use in the treatment of tumours
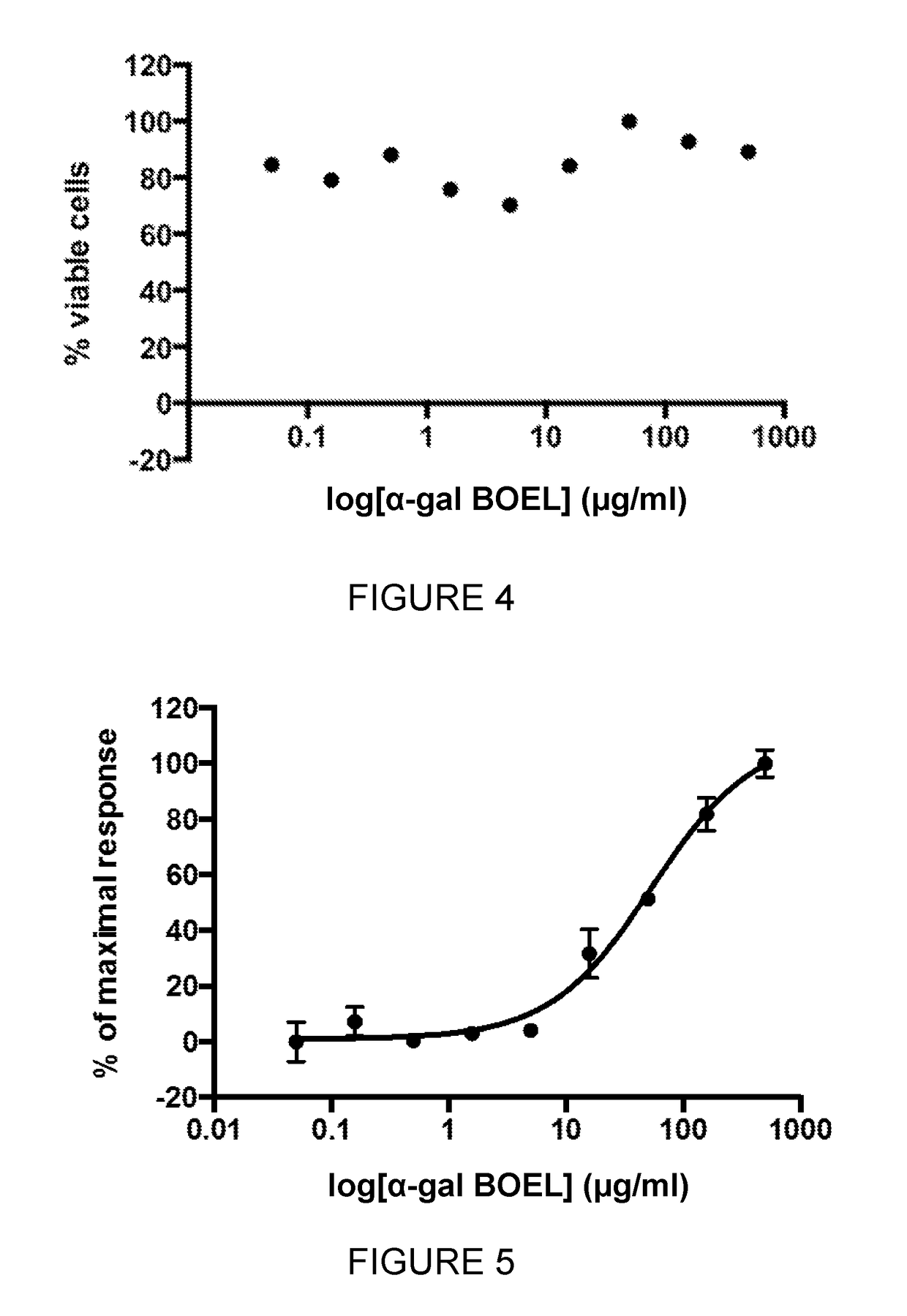
ActiveUS20170266214A1Ease formationImprove solubilityOrganic active ingredientsPharmaceutical delivery mechanismAntigenAntibody
The invention relates to pharmaceutical compositions comprising α-Gal BOEL for use in treating patients with tumors. The invention also relates to methods of treating tumours using said compositions. The invention discloses that following intratumoral injection of α-Gal BOEL, binding of the natural anti-Gal antibody to de novo expressed tumoural α-Gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumour cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumour specific T cells thereby converting the treated tumour lesions into in situ autologous tumour vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumour resection. In addition to the regression and / or destruction of the treated tumour, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumour. The invention further teaches the enhancement of anti-tumour α-Gal BOEL treatment by the use of antibodies that inhibit the activity of immunological checkpoints molecules.
Owner:AGALIMMUNE
Blend compositions for oral administration as a rapidly dissolving powder and/or suspension
ActiveUS20190008766A1Improve tasteGreat tasteOrganic active ingredientsPowder deliveryOrganic acidIntrathecal
Disclosed is a dry powder oral formulation that includes an active pharmaceutical ingredient (API), a lecithin powder, a galactomannan, one or more sweetening agents, one or more flavoring agents and an organic acid in a pharmaceutically acceptable preparation. Also disclosed are an excipient composition in absence of an API and methods of making and using the formulations and compositions. Also disclosed is a chewable, swallowable, and / or orally disintegrating tablet comprising an active pharmaceutical ingredient (API), a lecithin powder, a galactomannan, one or more sweetening agents, one or more flavoring agents and an organic acid in a pharmaceutically acceptable preparation.
Owner:MARENDA PHARMA LLC
Glycolipid containing compositions for use in the treatment of tumors
ActiveUS10092586B2Ease of administrationBenefits of easeOrganic active ingredientsPharmaceutical delivery mechanismAntigenDrainage lymph nodes
The invention relates to pharmaceutical compositions comprising α-Gal BOEL for use in treating patients with tumors. The invention also relates to methods of treating tumors using said compositions. The invention discloses that following intratumoral injection of α-Gal BOEL, binding of the natural anti-Gal antibody to de novo expressed tumoral α-Gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor. The invention further teaches the enhancement of anti-tumor α-Gal BOEL treatment by the use of antibodies that inhibit the activity of immunological checkpoints molecules.
Owner:AGALIMMUNE
Non-anticoagulant sulfated or sulfonated polysaccharides
InactiveUS20170360823A1Improves patient careEase of administrationOrganic active ingredientsSugar derivativesAnti coagulationPharmaceutical medicine
The present invention provides non-anticoagulant sulfated or sulfonated polysaccharides (NASPs), which accelerate the blood clotting process. Also provided are pharmaceutical formulations comprising a NASP of the invention in conjunction with a pharmaceutically acceptable excipient and, in various embodiments, these formulations are unit dosage formulations. The invention provides a NASP formulation, which is orally bioavailable. Also provided are methods for utilizing the compounds and formulations of the invention to promote blood clotting in vivo as therapeutic and prophylactic agents and in vitro as an aid to studies of the blood clotting process.
Owner:BAXALTA GMBH
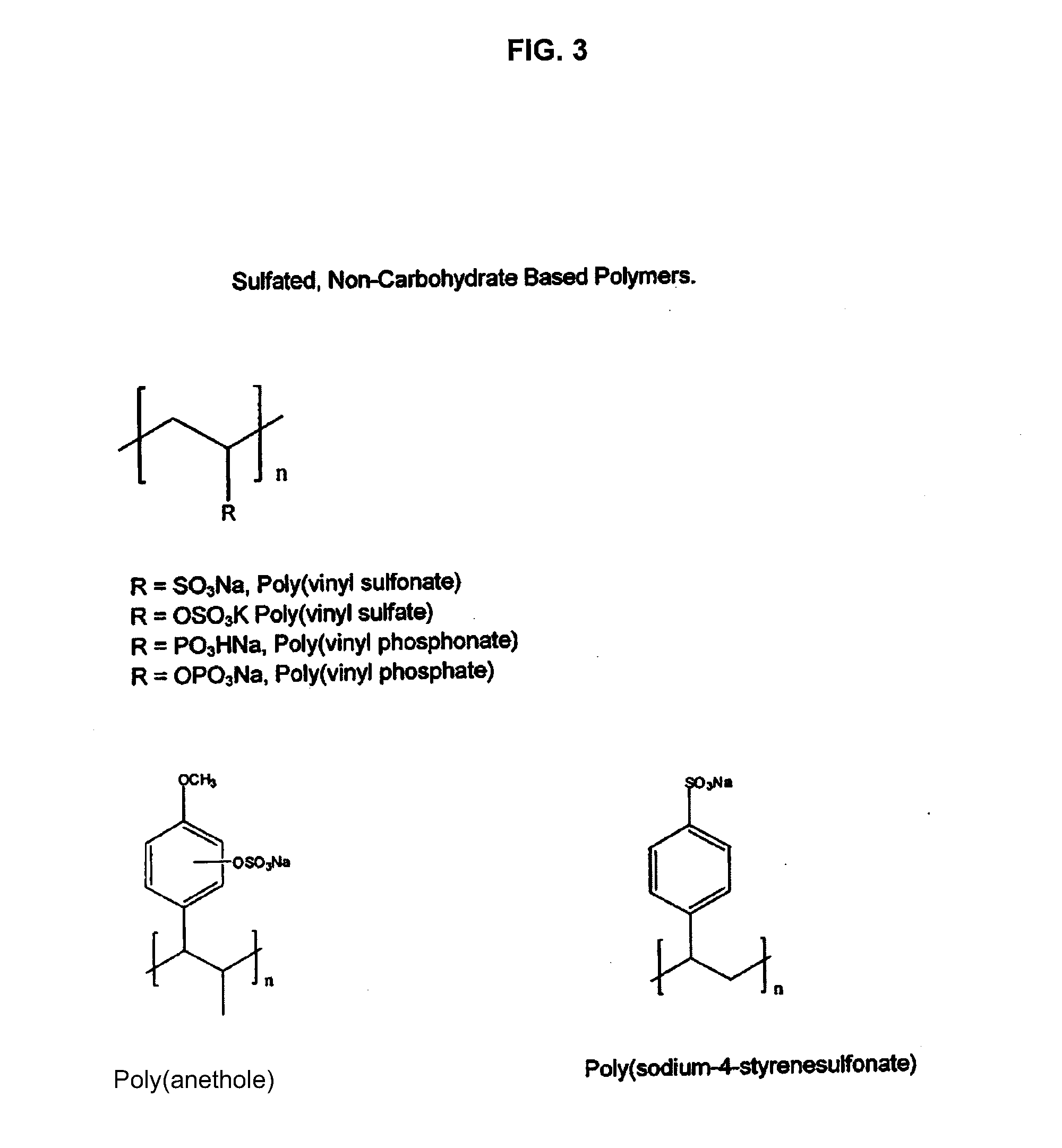
Non-anticoagulant sulfated or sulfonated synthetic polymers
InactiveUS20130202550A1Shorten clotting timeReverses anticoagulant effectPeptide/protein ingredientsPharmaceutical delivery mechanismSulfationSulfate
The present invention provides pharmaceutical formulations including a non-anticoagulant, non-saccharide polymer that with at least one sulfate or sulfonate moiety. The pharmaceutical formulations of the invention are of use to improve blood clotting in a subject. Also provided are useful analytical methods utilizing these polymers to query the dynamics of blood clotting in vitro.
Owner:BAXALTA GMBH
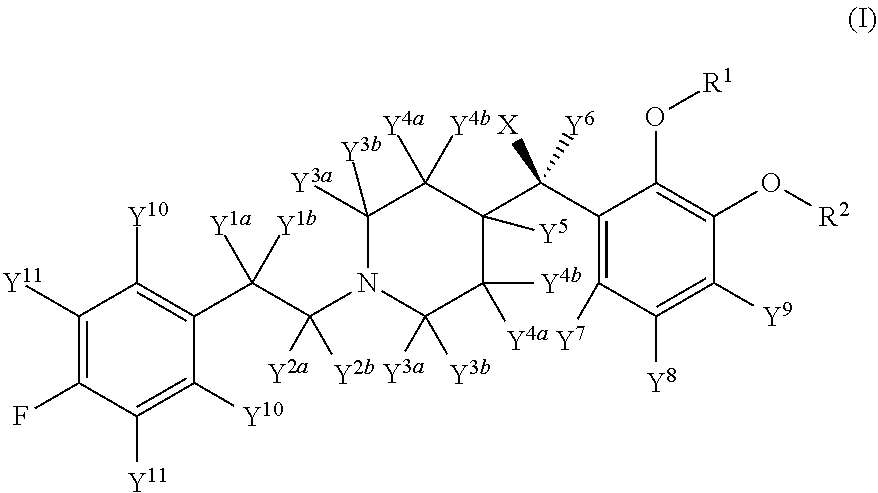
Deuterated Forms And Derivatives Of Volinanserin
PendingUS20220106272A1Minimizing toxic side effectReduce expensesHormone peptidesOrganic active ingredientsSchizo-affective typeThromboembolic disorder
Deuterated forms of volinanserin according to structural formula (I), and their pharmaceutically acceptable salts, pharmaceutical compositions containing these compounds, and methods of treatment or prevention using these compounds or pharmaceutical compositions are described. The compounds are useful for treating or preventing a disease or condition selected from psychosis, schizophrenia, schizoaffective disorder, Parkinson's disease, Lewy body dementia, sleep disorder (including insomnia), agitation, mood disorder (including depression), thromboembolic disorder, autism, and attention deficit hyperactivity disorder.
Owner:TERRAN BIOSCIENCES INC
Heterocyclic compounds as antibacterials
ActiveUS20190134046A1Ease of administrationImprove uniformityAntibacterial agentsOrganic active ingredientsTuberculosisChemistry
The present invention relates to the following compoundswherein the integers are as defined in the description, and where the compounds may be useful as medicaments, for instance for use in the treatment of tuberculosis.
Owner:JANSSEN SCI IRELAND UC
Substituted indoline derivatives as dengue viral replication inhibitors
ActiveUS20200054606A1Ease of administrationImprove uniformityOrganic active ingredientsOrganic chemistryPharmaceutical drugIndoline
The present invention concerns substituted indoline derivatives, methods to prevent or treat dengue viral infections by using said compounds and also relates to said compounds for use as a medicine, more preferably for use as a medicine to treat or prevent dengue viral infections. The present invention furthermore relates to pharmaceutical compositions or combination preparations of the compounds, to the compositions or preparations for use as a medicine, more preferably for the prevention or treatment of dengue viral infections. The invention also relates to processes for preparation of the compounds.
Owner:JANSSEN PHARMA INC +1
A method and system for realizing medical performance quantification
InactiveCN107273671BQuantitative results are accurate and objectiveThe quantification process is shortNatural language data processingSpecial data processing applicationsData miningMedical treatment
The purpose of the invention is to provide a method and a system for medical performance quantification, which make the medical performance quantification more convenient and objective. The method includes the following steps that: S1,professional thesaurus is established; S2, key-value dictionary is established, and specialized words in the professional thesaurus are assigned; S3,medical cases are segmented using the established professional thesaurus, and the medical cases are assigned scores using the key-value dictionary; S4,medical cases scores are summarized and ranked in each department. Compared with the prior art, the advantages of the method is that the system can effectively use medical data to quantify the medical performance, the quantitative results are more accurate and objective, and the quantitative processes are shorter and more efficient. The system is convenient for administrative departments of major hospitals and related units to manage the operation.
Owner:江苏金琉璃科技有限公司
Non-anticoagulant sulfated or sulfonated synthetic polymers
InactiveUS20170281671A1Reduce molecular weightImproves patient carePeptide/protein ingredientsPharmaceutical delivery mechanismSulfationSulfate
The present invention provides pharmaceutical formulations including a non-anticoagulant, non-saccharide polymer that with at least one sulfate or sulfonate moiety. The pharmaceutical formulations of the invention are of use to improve blood clotting in a subject. Also provided are useful analytical methods utilizing these polymers to query the dynamics of blood clotting in vitro.
Owner:BAXALTA GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com