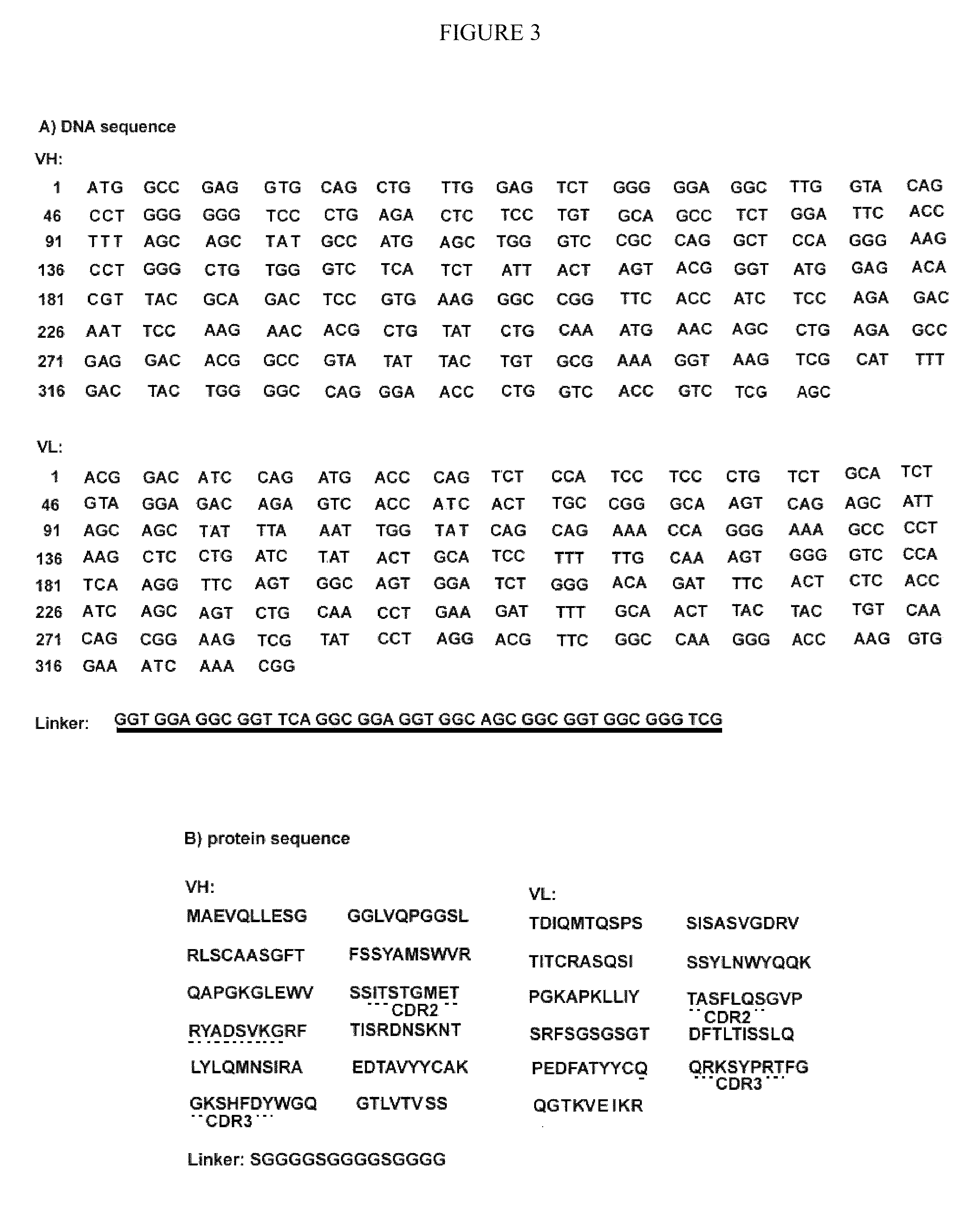
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Thromboembolic disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
About Thrombotic/Thromboembolic Disorder: Thromboembolic Disorder is a condition in which the formation of a blood clot (thrombus) inside one of the blood vessels is carried by the blood from the site of origin to block another vessel.
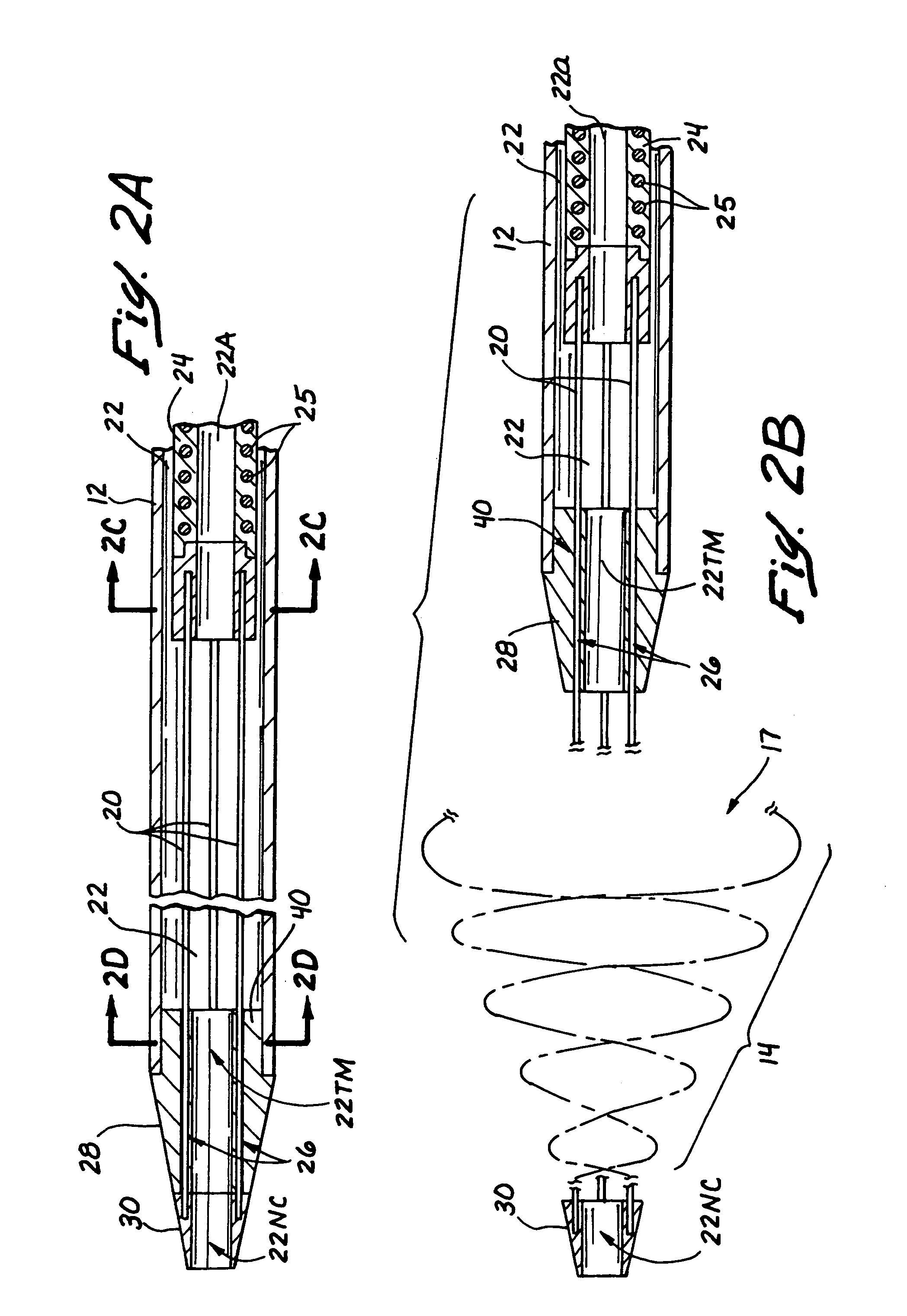
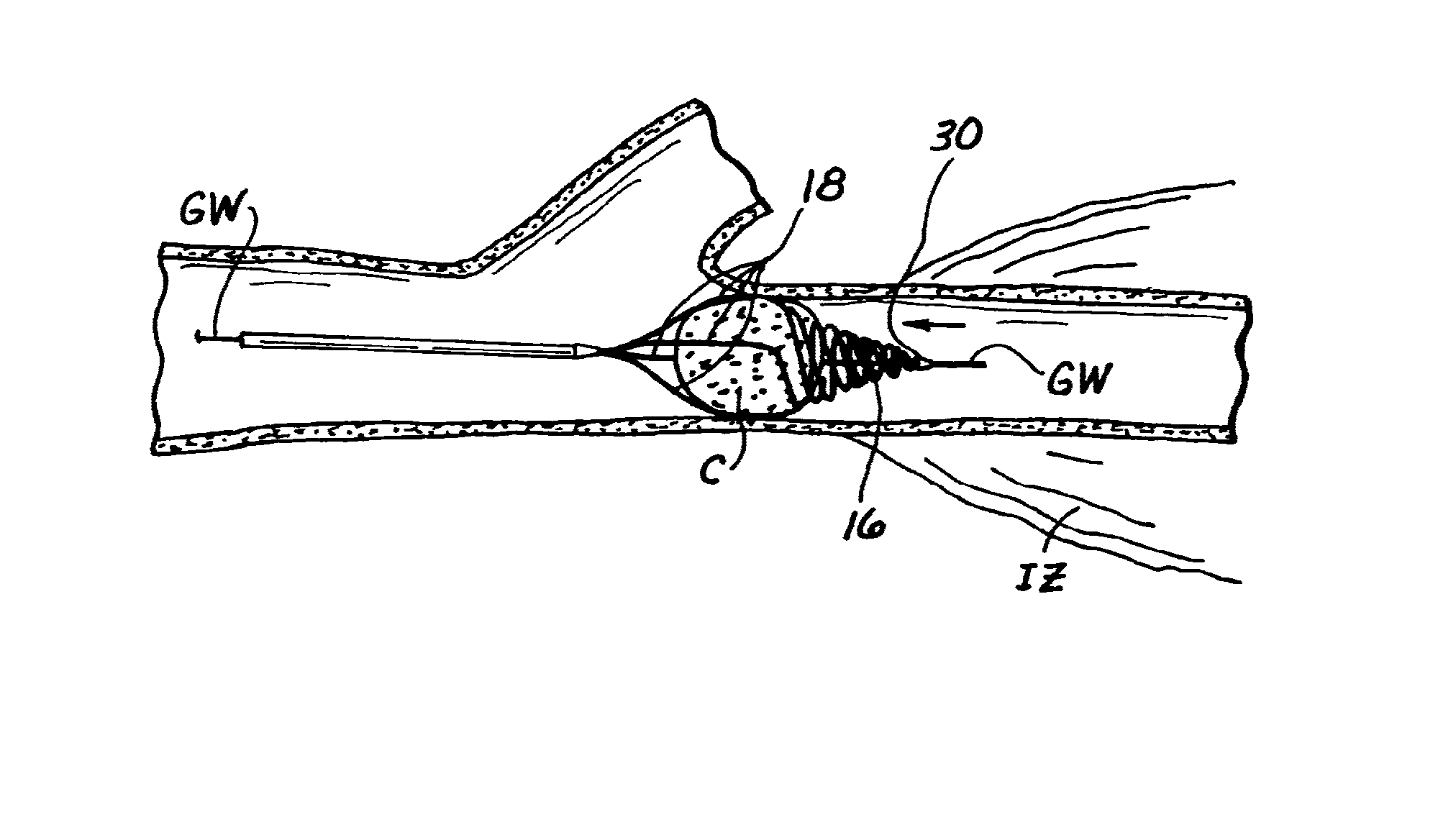
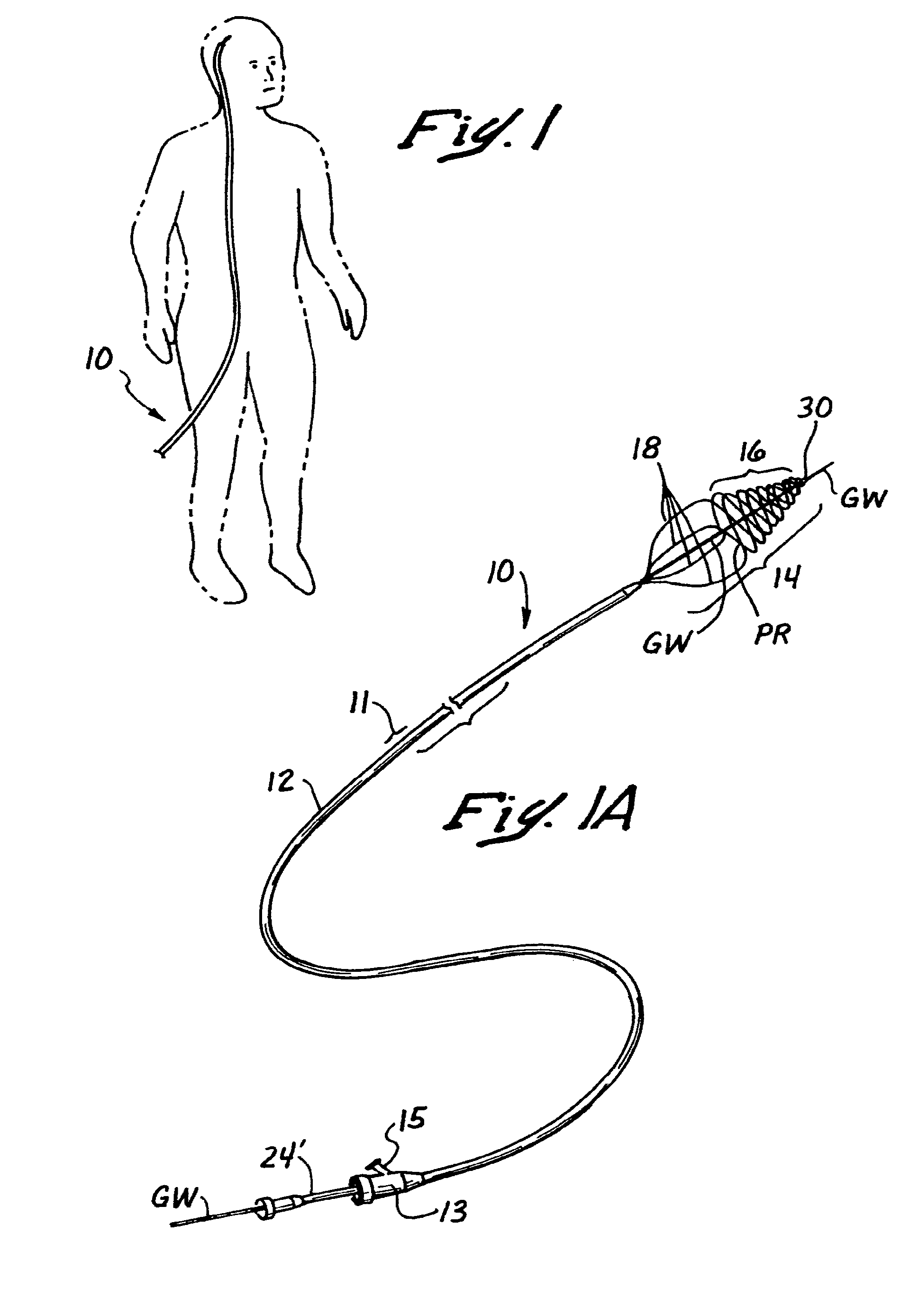
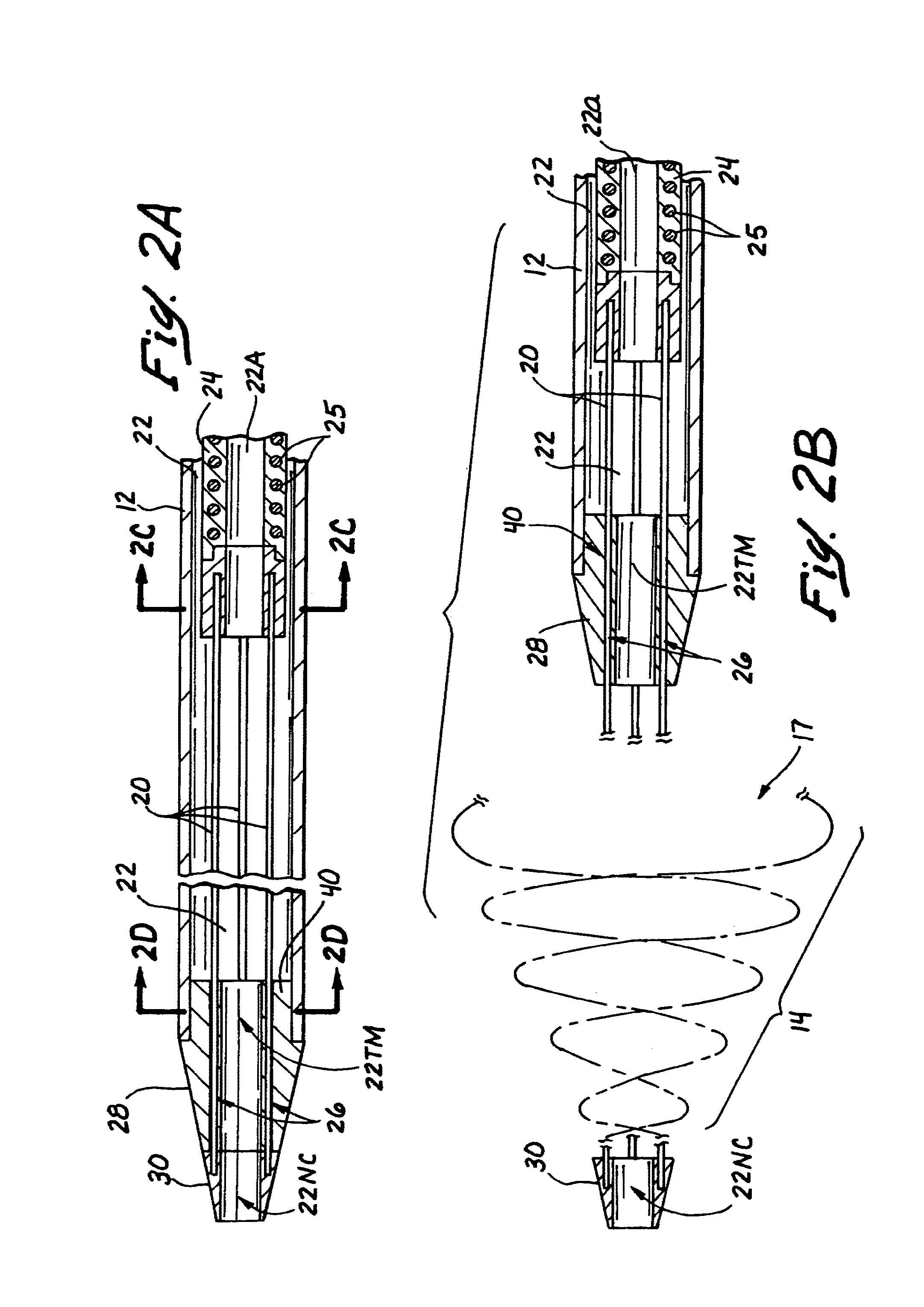
Embolectomy catheters and methods for treating stroke and other small vessel thromboembolic disorders
InactiveUS7691121B2Prevent and minimize severityEasy extractionStentsDilatorsThrombosis embolismThromboembolic disorder
Embolectomy catheters, rapid exchange microcatheters, systems and methods for removing clots or other obstructive matter (e.g., thrombus, thromboemboli, embolic fragments of atherosclerotic plaque, foreign objects, etc.) from blood vessels. This invention is particularly useable for percutaneous removal of thromboemboli or other obstructive matter from small blood vessels of the brain, during an evolving stroke or period of cerebral ischemia. In some embodiments, the embolectomy catheters of this invention are advanceable with or over a guidewire which has been pre-inserted through or around the clot. Also, in some embodiments, the embolectomy catheters include clot removal devices which are deployable from the catheter after the catheter has been advanced at least partially through the clot. The clot removal device may included a deployable wire nest that is designed to prevent a blood clot from passing therethrough. The delivery catheter may include telescoping inner and outer tubes, with the clot removal device being radially constrained by the outer tube. Retraction of the outer tube removes the constraint on the clot removal device and permits it to expand to its deployed configuration. An infusion guidewire is particularly useful in conjunction with the embolectomy catheter, and permits infusion of medicaments or visualization fluids distal to the clot.
Owner:MICROVENTION INC
Embolectomy Catheters And Methods For Treating Stroke And Other Small Vessel Thromboembolic Disorders
InactiveUS20080015541A1Prevent and minimize severityEasy extractionStentsBalloon catheterThrombusThromboembolic disorder
Embolectomy catheters, rapid exchange microcatheters, systems and methods for removing clots or other obstructive matter (e.g., thrombus, thromboemboli, embolic fragments of atherosclerotic plaque, foreign objects, etc.) from blood vessels. This invention is particularly useable for percutaneous removal of thromboemboli or other obstructive matter from small blood vessels of the brain, during an evolving stroke or period of cerebral ischemia. In some embodiments, the embolectomy catheters of this invention are advanceable with or over a guidewire which has been pre-inserted through or around the clot. Also, in some embodiments, the embolectomy catheters include clot removal devices which are deployable from the catheter after the catheter has been advanced at least partially through the clot. The clot removal device may include a deployable wire nest that is designed to prevent a blood clot from passing therethrough. The delivery catheter may include telescoping inner and outer tubes, with the clot removal device being radially constrained by the outer tube. Retraction of the outer tube removes the constraint on the clot removal device and permits it to expand to its deployed configuration. An infusion guidewire is particularly useful in conjunction with the embolectomy catheter, and permits infusion of medicaments or visualization fluids distal to the clot.
Owner:MICROVENTION INC
(Hetero)aryl-bicyclic heteroaryl derivatives, their preparation and their use as protease inhibitors
The present invention provides novel compounds of the Formula (I): A-B, its prodrug forms, or pharmaceutically acceptable salts thereof, wherein A represents a saturated, unsaturated, or a partially unsaturated bicyclic heterocyclic ring structure, and B represents an aryl or a heteroaryl group. Preferred compounds of the present invention comprise a benzimidazole or indole nucleus. The compounds of this invention are inhibitors of serine proteases, Urokinase (uPA), Factor Xa (FXa), and / or Factor VIIa (FVIIa), and have utility as anti cancer agents and / or as anticoagulants for the treatment or prevention of thromboembolic disorders in mammals.
Owner:AXYX PHARMA INC
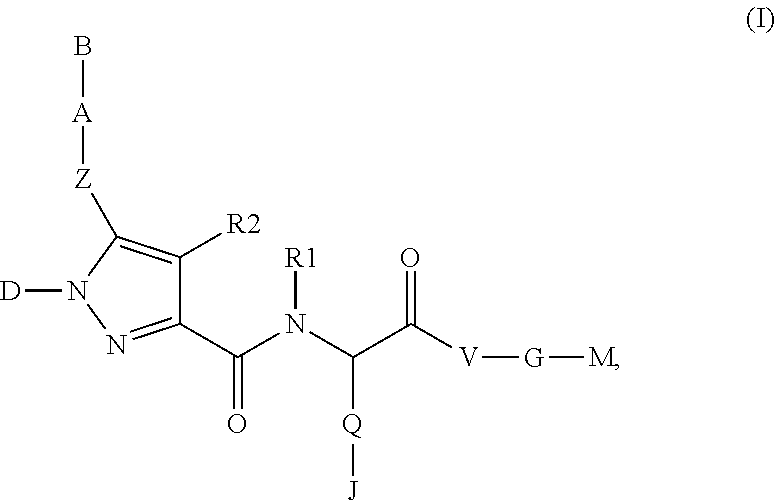
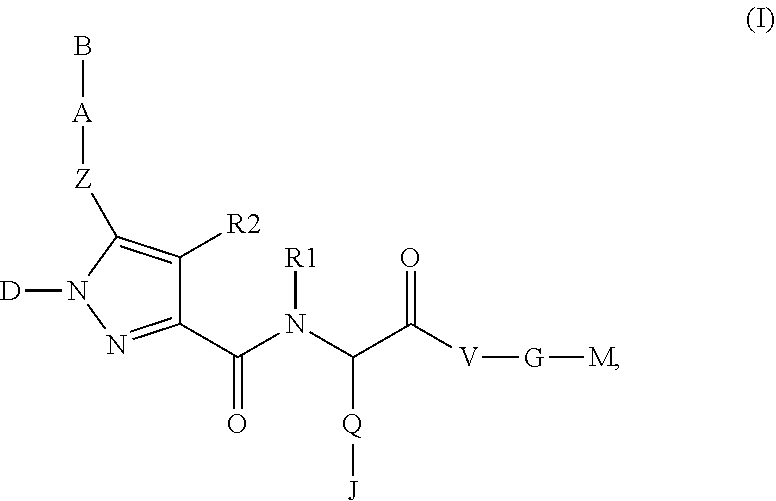
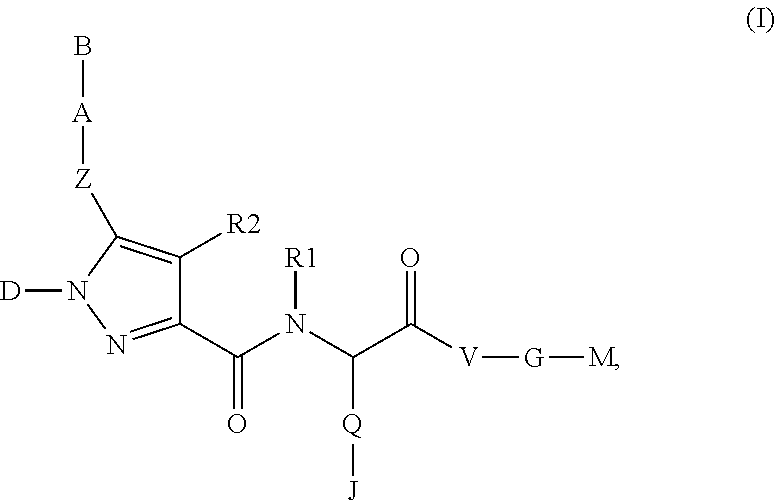
Beta-Aminoacid-Derivatives As Factor Xa Inhibitors
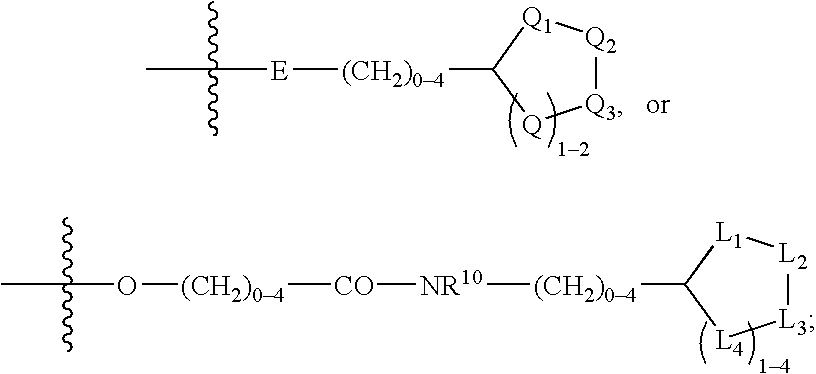
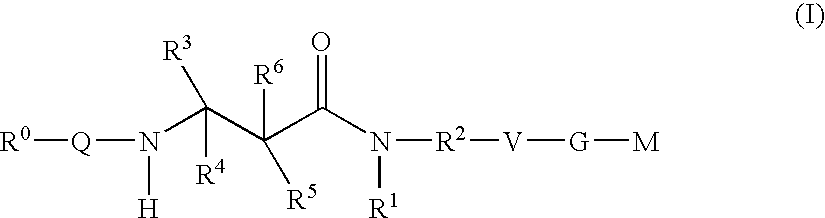
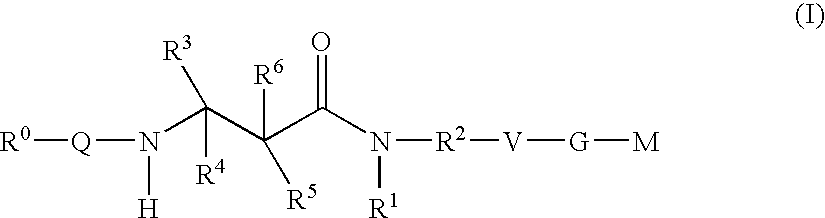
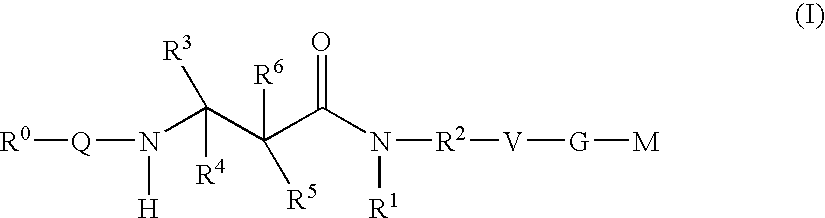
The present invention relates to compounds of the formula I, in which R0; R1; R2; R3; R4; R5, R, Q; V, G and M have the meanings indicated in the claims. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders like thromboemboic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and / or factor VIIa (FVIIa), and can in general be applied in conditions in which an undesired activity of factor Xa and / or factor VIIa is present or for the cure or prevention of which an inhibition of factor Xa and / or factor VIIa is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI AVENTIS DEUTSCHLAND GMBH
Pyrazole derivatives as Anti-platelet and Anti-thrombotic agents
Owner:PFIZER INC
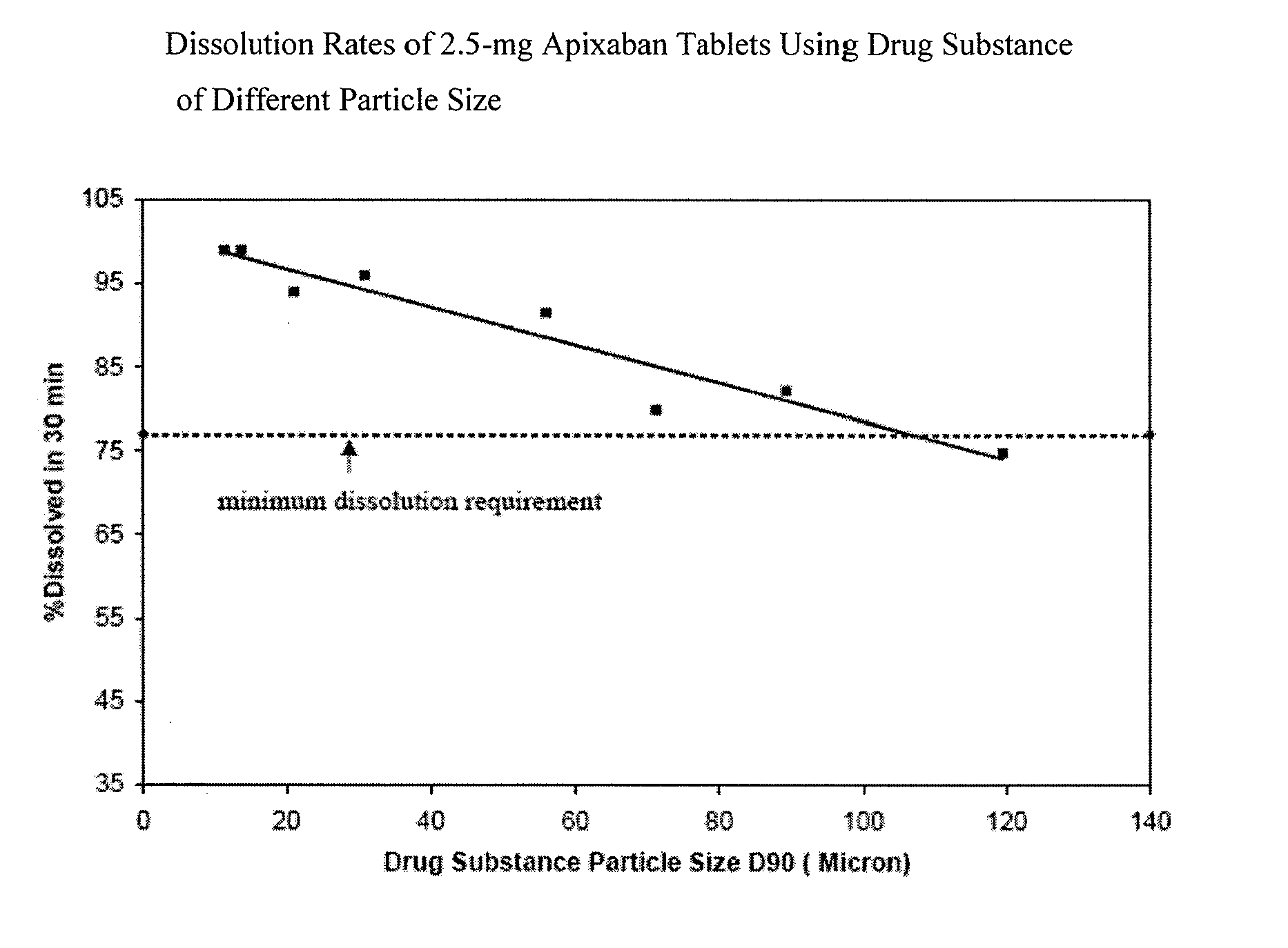
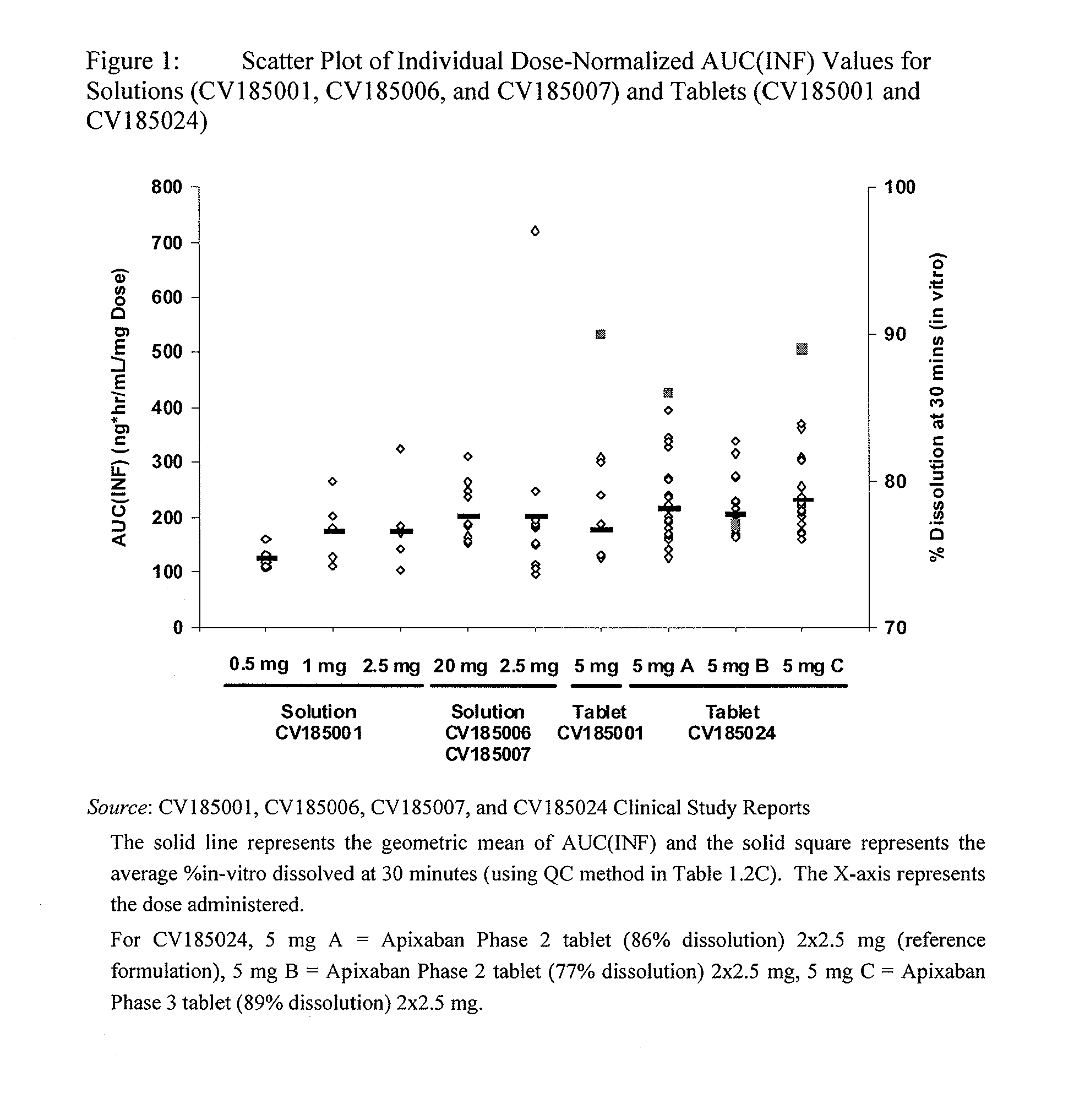
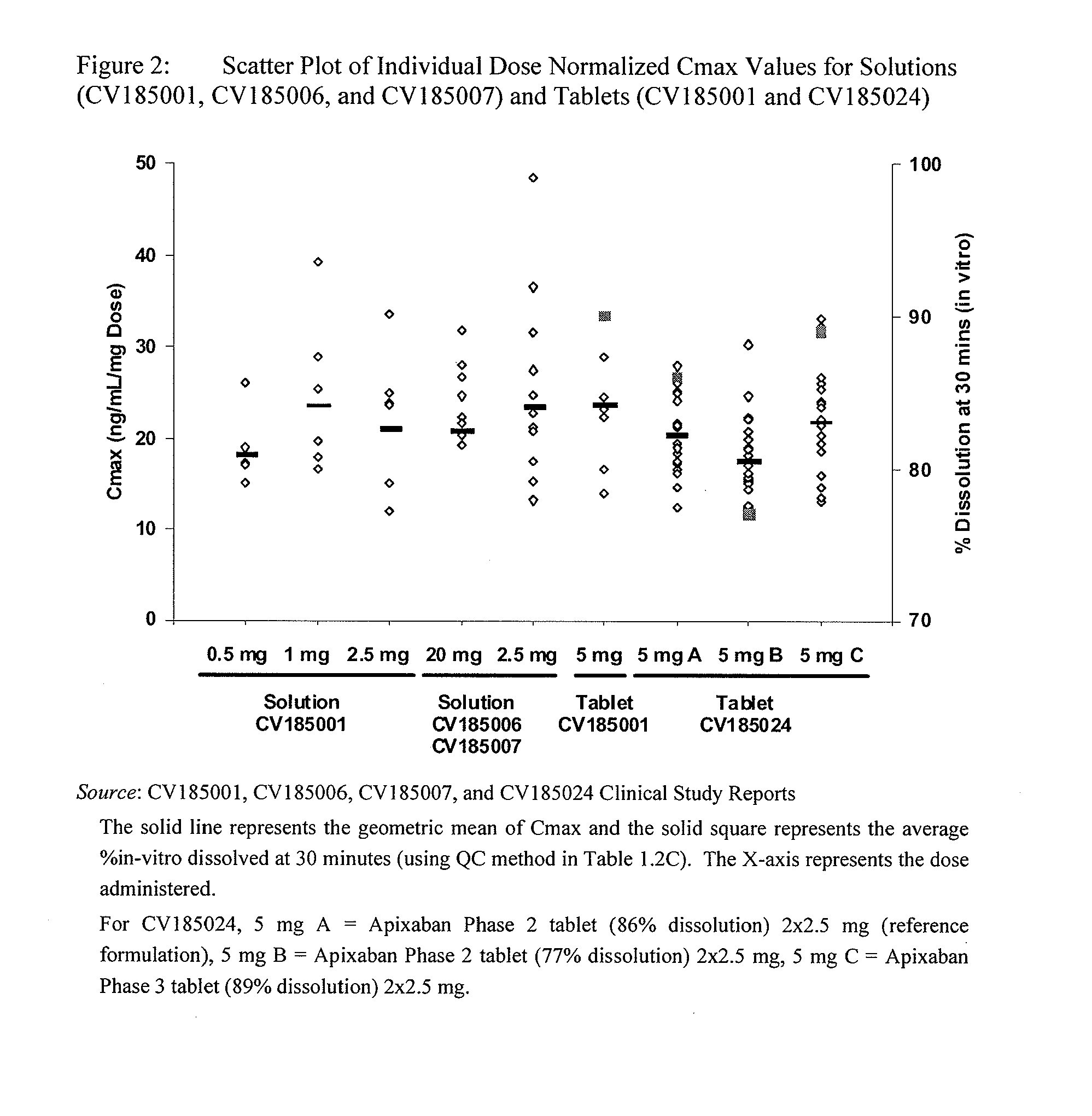
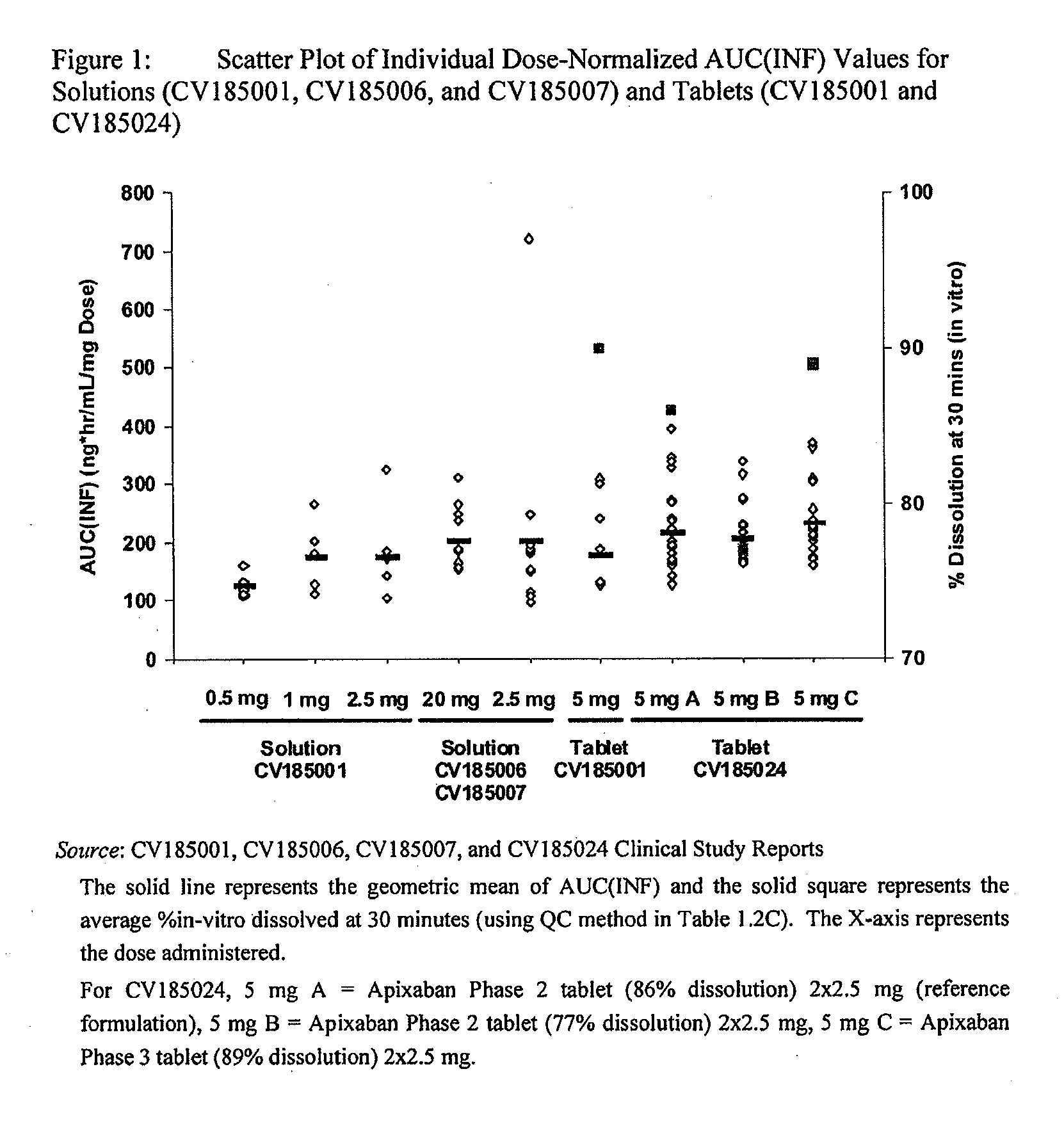
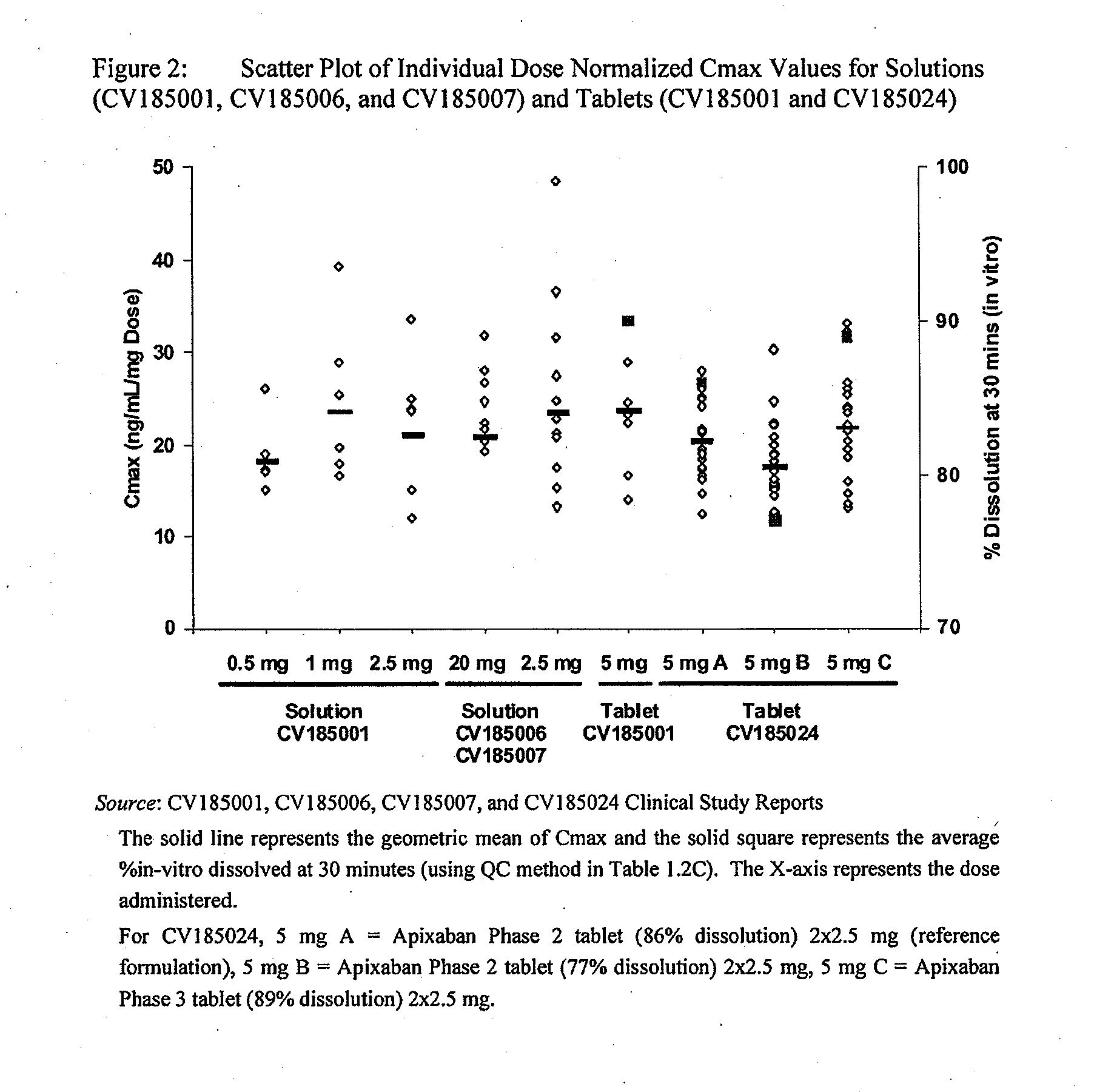
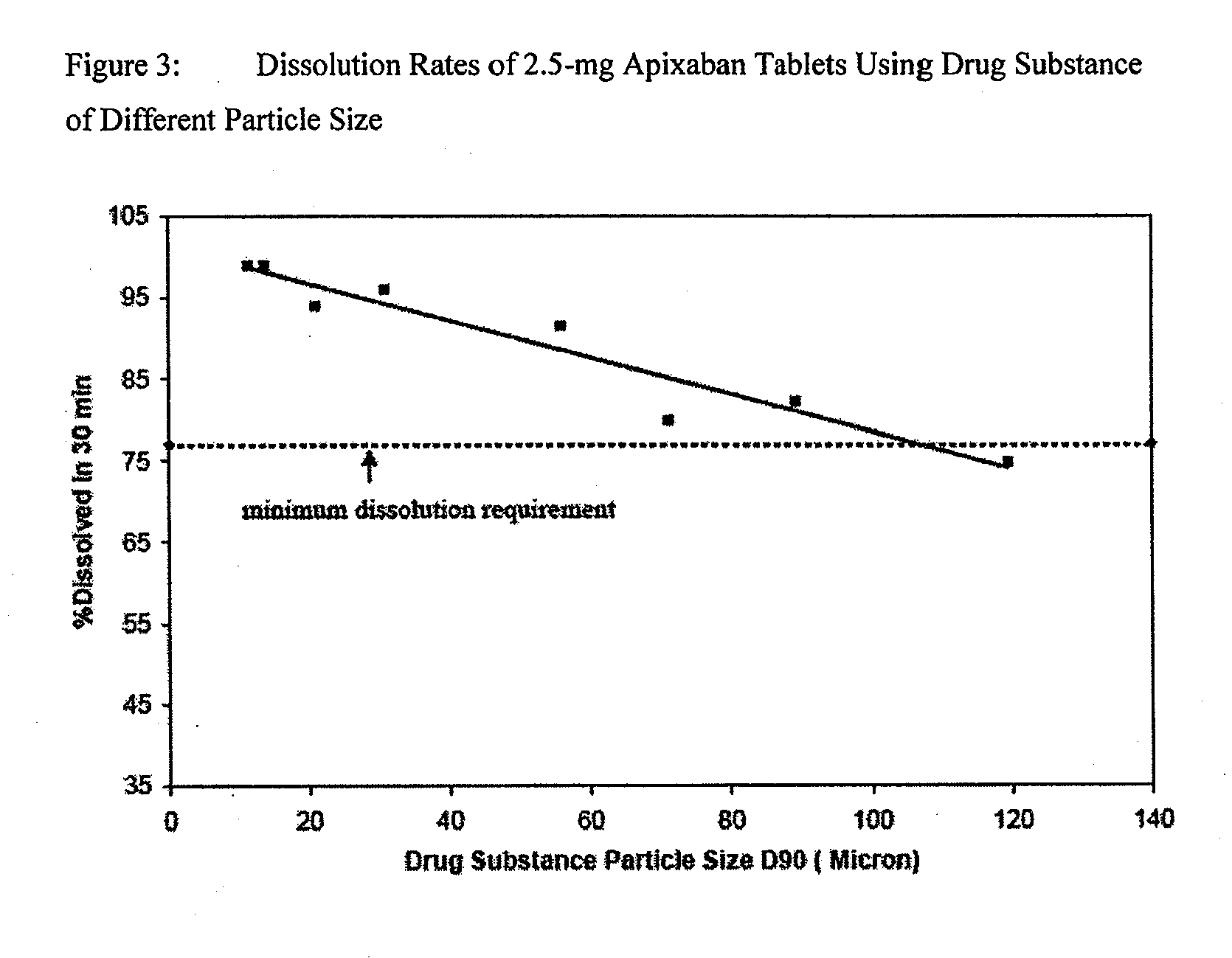
Apixaban formulations
ActiveUS20130045245A1Facilitate consistent in vivo dissolutionImprove solubilityPowder deliveryBiocideThromboembolic disorderPharmacology
Compositions comprising crystalline apixaban particles having a D90 equal to or less than 89 μm, and a pharmaceutically acceptable carrier, are substantially bioequivalent and can be used to for the treatment and / or prophylaxis of thromboembolic disorders.
Owner:BRISTOL MYERS SQUIBB CO +1
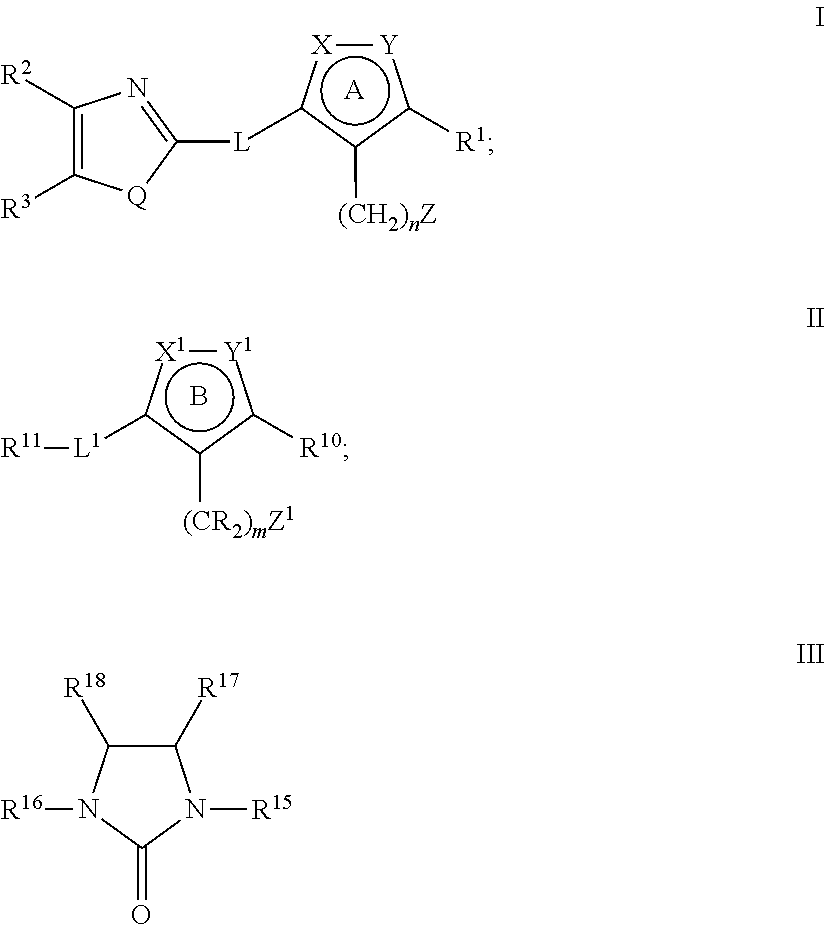
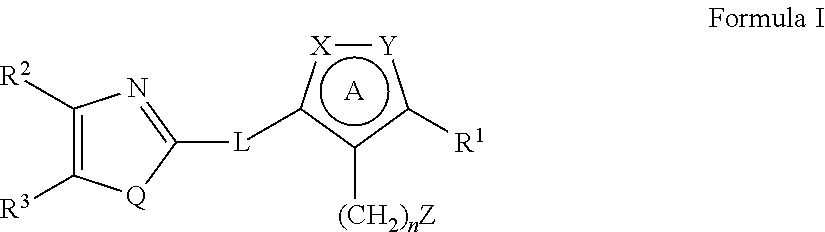
Heterocyclic compounds as factor ixa inhibitors
The present invention relates to novel heterocyclic compounds of Formulae I-III; as disclosed herein or a pharmaceutically acceptable salt, solvate, ester, prodrug or stereoisomer thereof. Also disclosed are pharmaceutical compositions comprising said compounds, and methods for using said compounds for treating or preventing a thromboembolic disorder.
Owner:MERCK SHARP & DOHME LLC
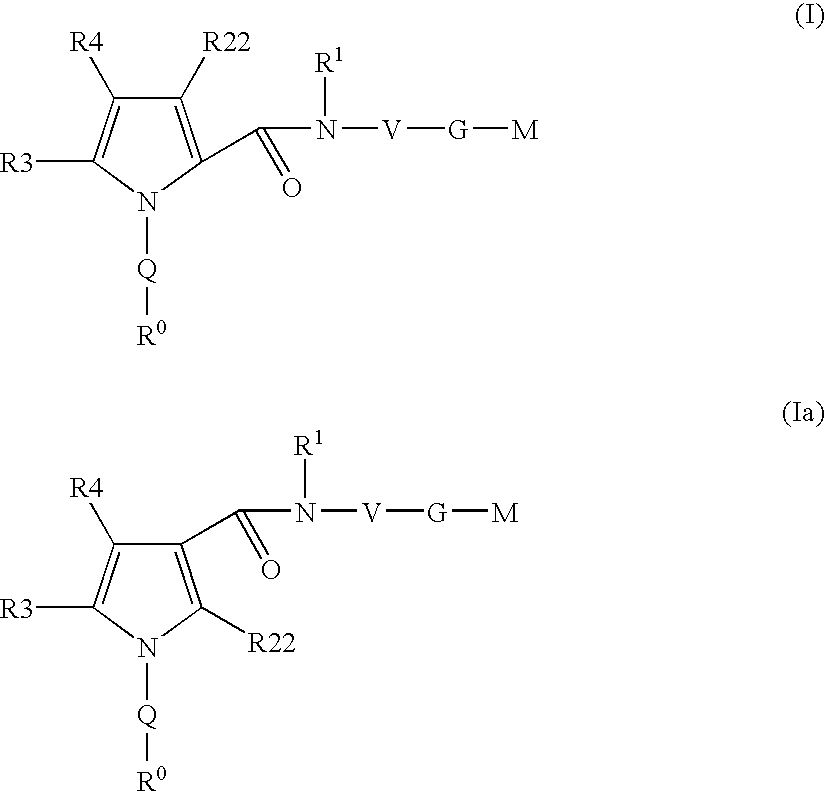
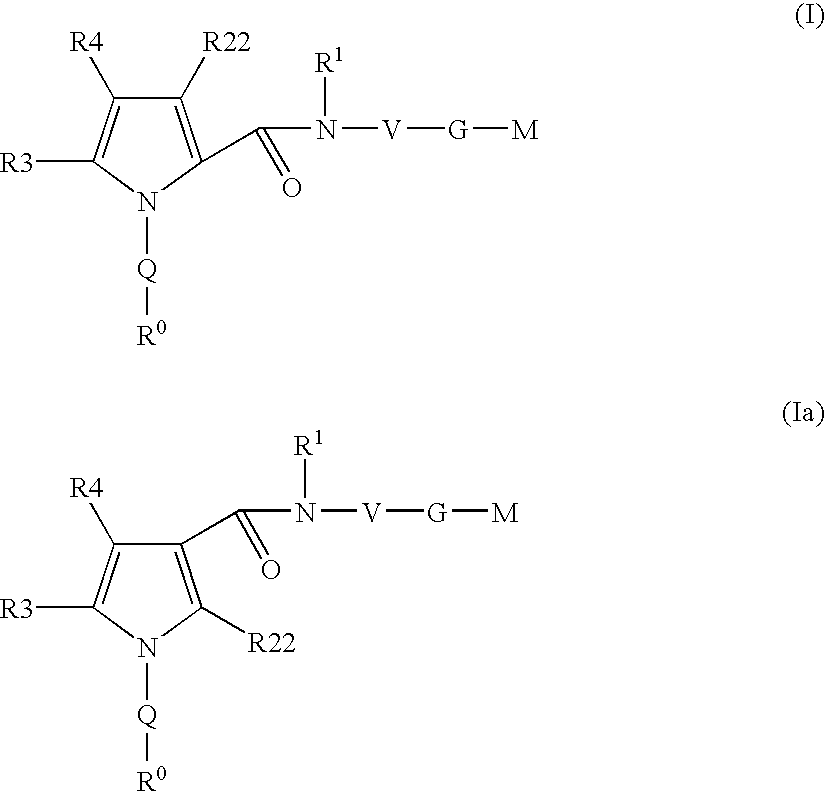
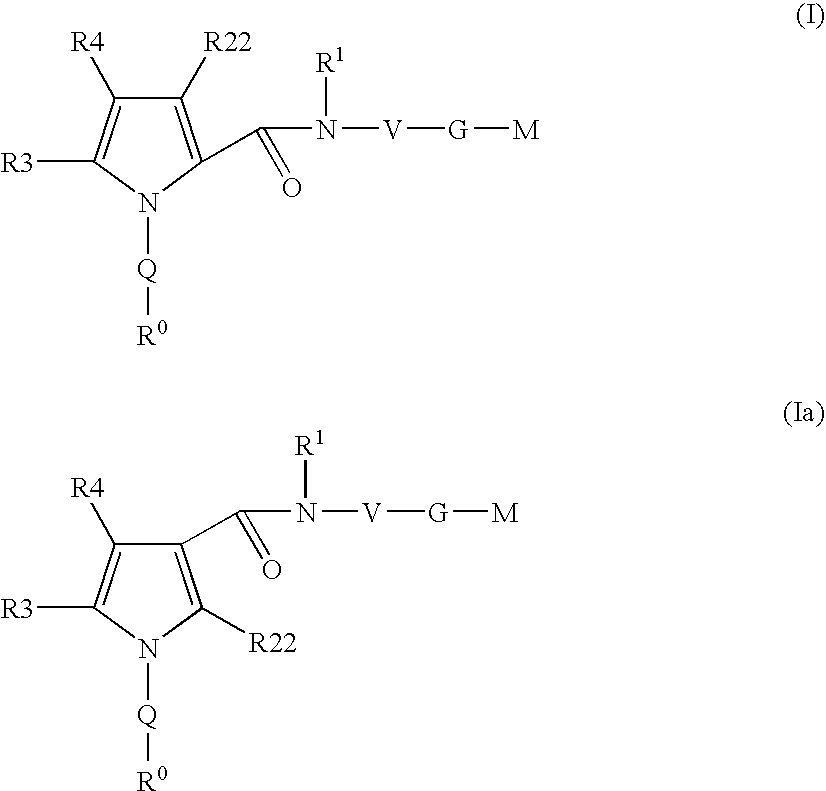
Pyrrole-derivatives as factor Xa inhibitors
The present invention relates to compounds of the formulae I and Ia,wherein R0; R1; R3; R4; R22, Q; V, G and M have the meanings indicated in the claims. The compounds of the formulae I and Ia are valuable pharmacologically active compounds. They exhibit a strong antithrombotic effect and are suitable for the therapy and prophylaxis of cardiovascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and / or factor VIIa (FVIIa), and can in general be applied in conditions in which an undesired activity of factor Xa and / or factor VIIa is present or for the cure or prevention of which an inhibition of factor Xa and / or factor VIIa is intended. The invention furthermore relates to processes for the preparation of compounds of the formulae I and Ia, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI AVENTIS DEUT GMBH
Therapeutic agents for inducing platelet fragmentation and treating thromboembolic disorders
ActiveUS20110045008A1Restore blood flowInhibit bindingPeptide/protein ingredientsAntibody mimetics/scaffoldsMedicineThromboembolic disorder
The present invention is directed to a therapeutic agent comprising a GPIIIa(49-66) specific targeting agent and a thrombi-specific homing agent. Also disclosed is the use of the therapeutic agent in carrying out a method of treating thromboembolic disorders and a method of inducing platelet fragmentation.
Owner:NEW YORK UNIV
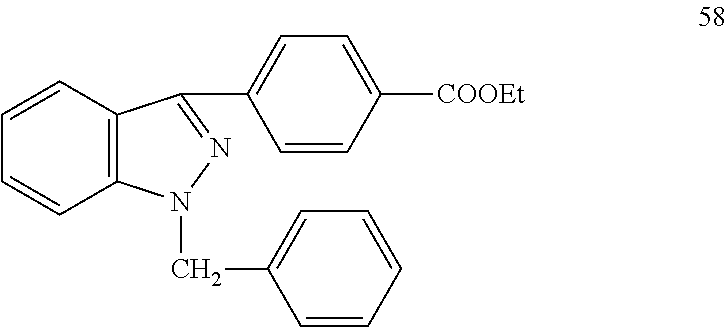
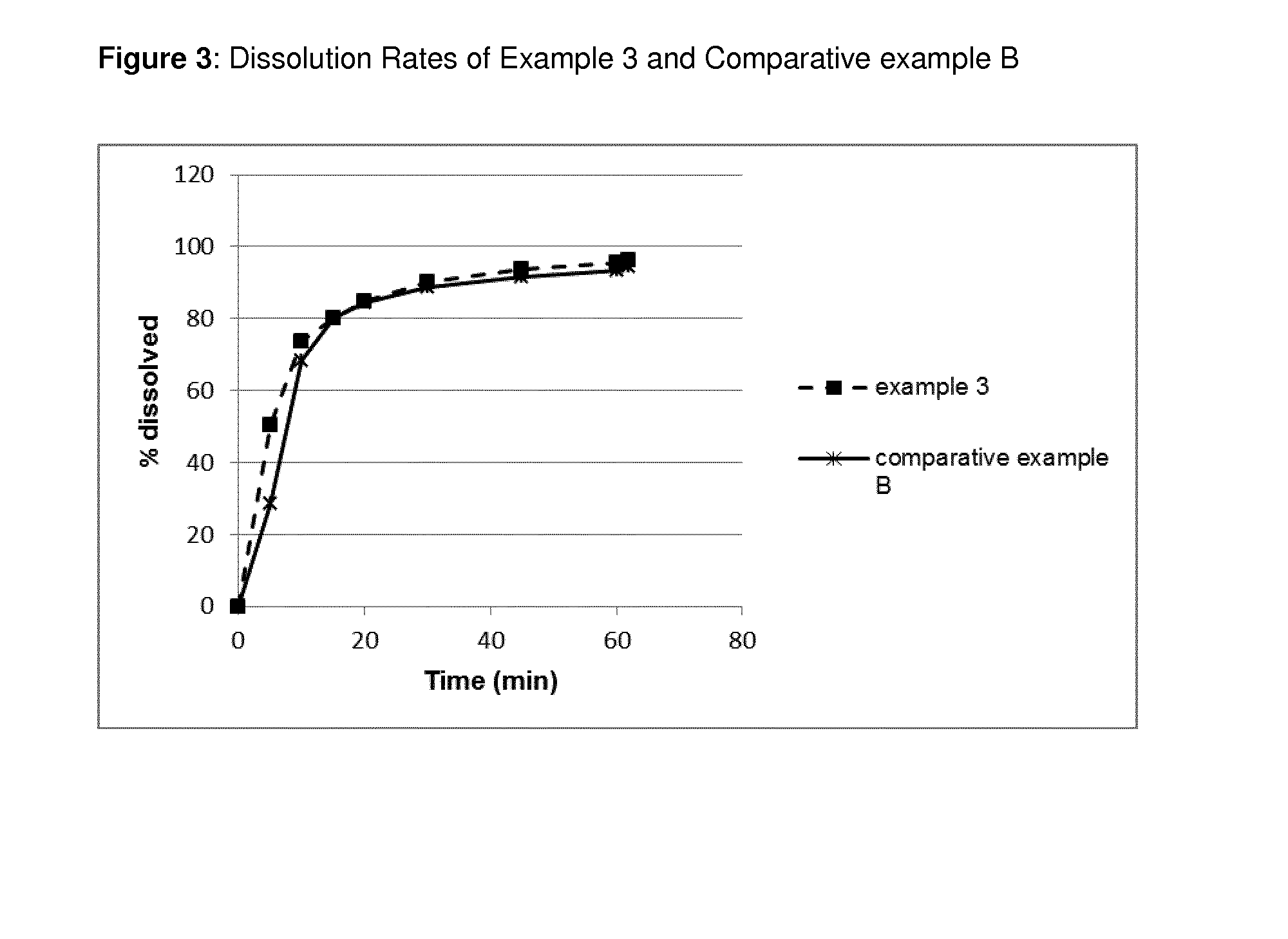
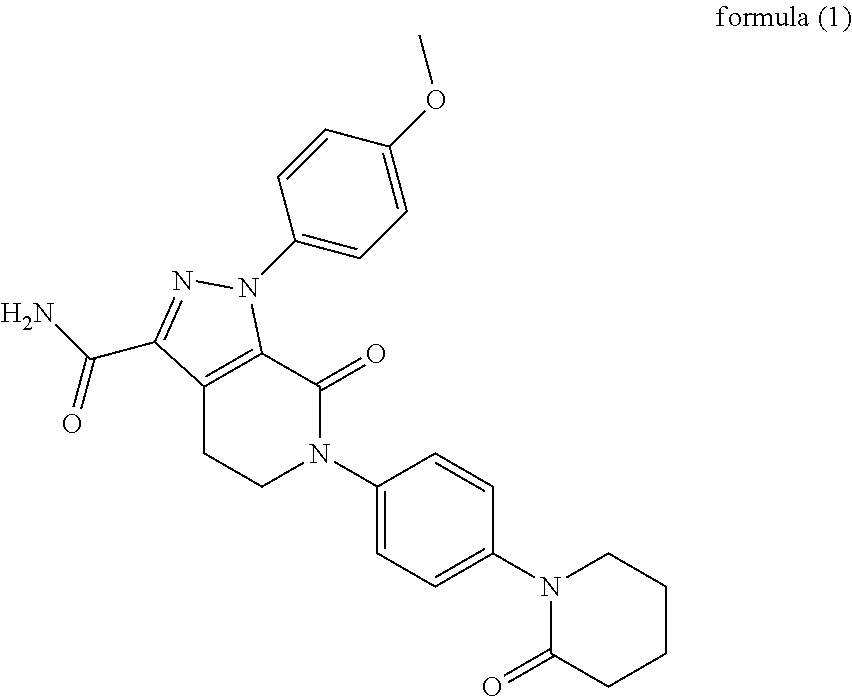
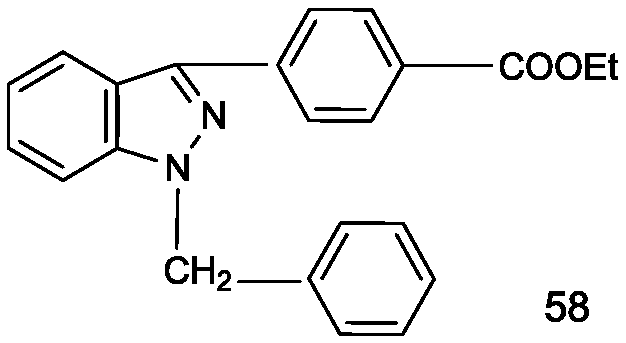
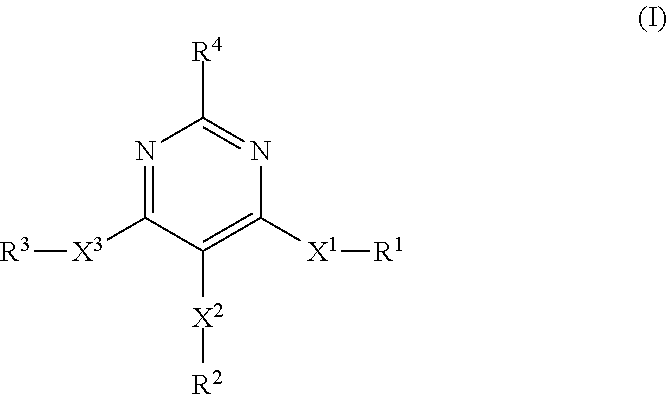
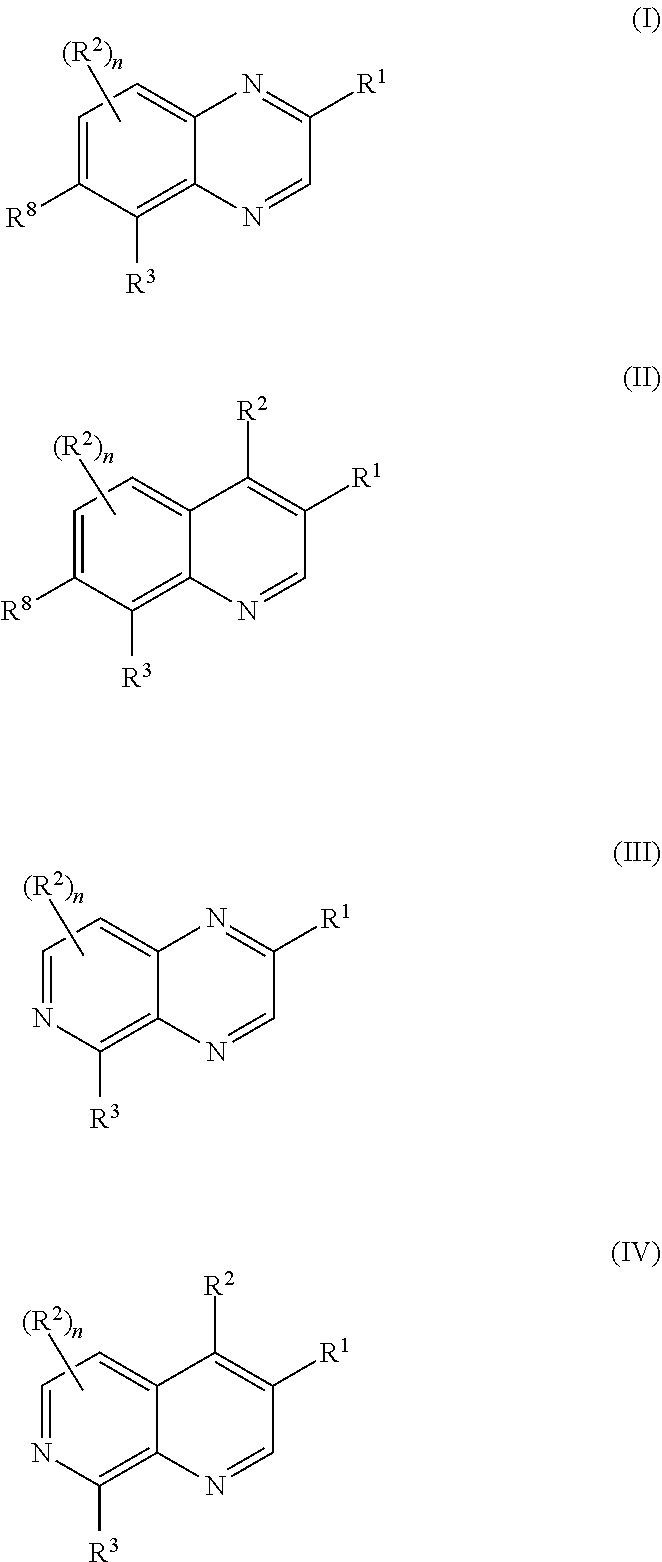
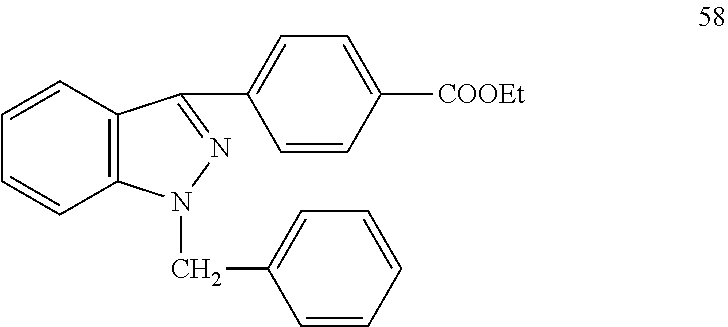
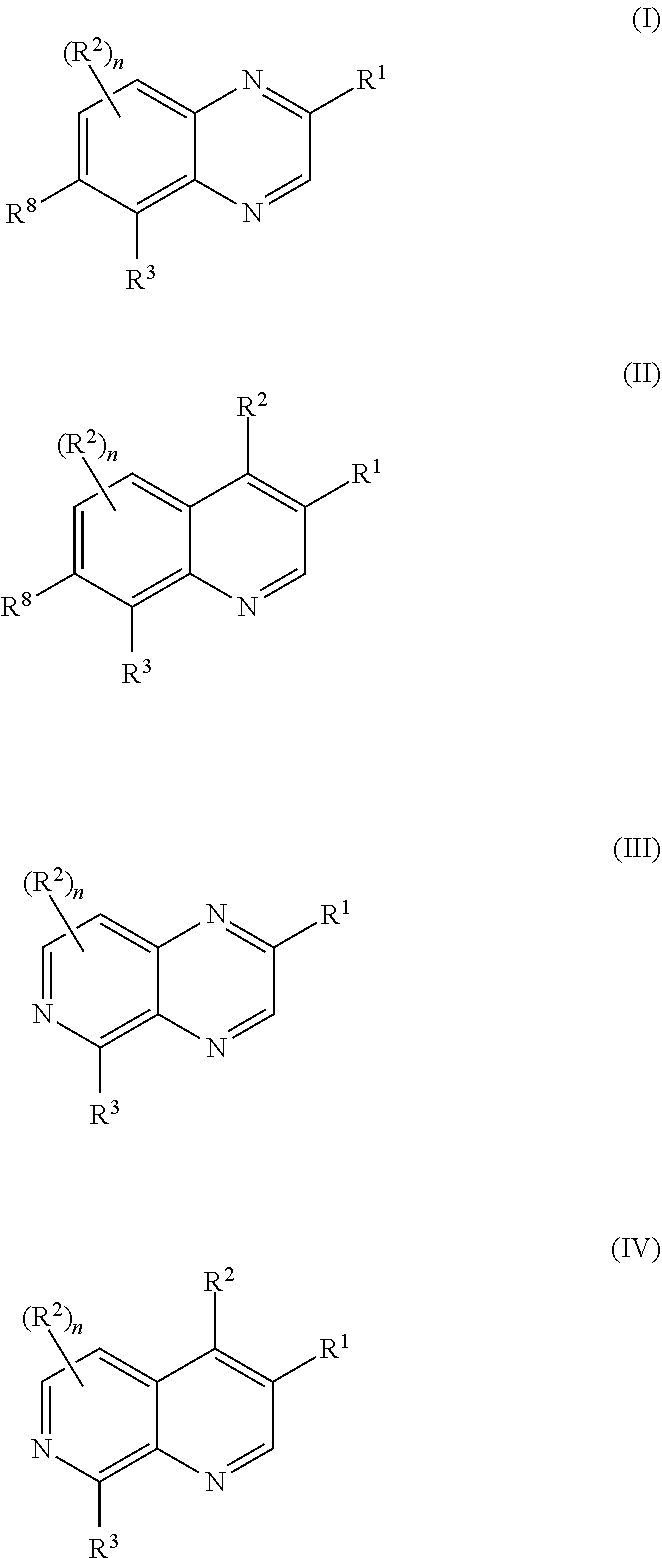
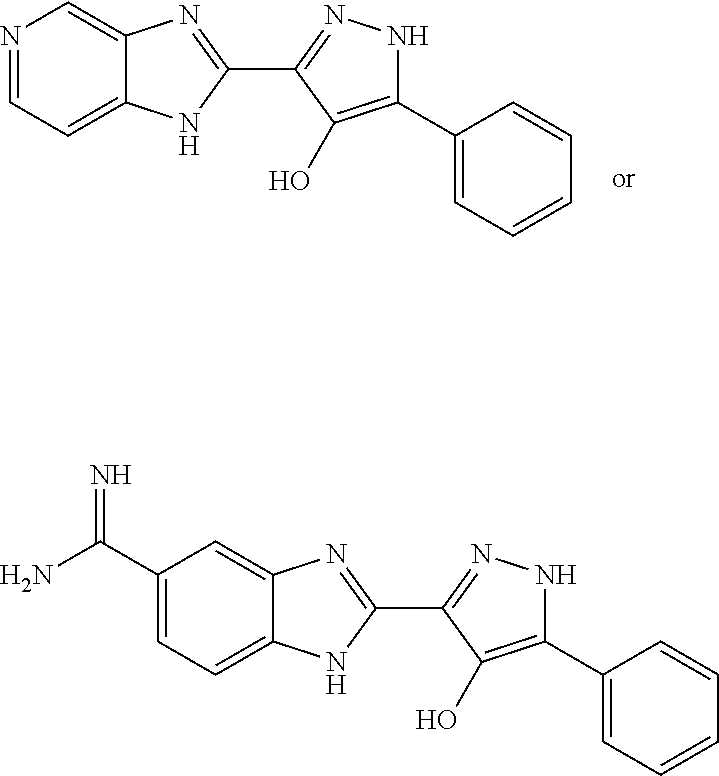
Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof
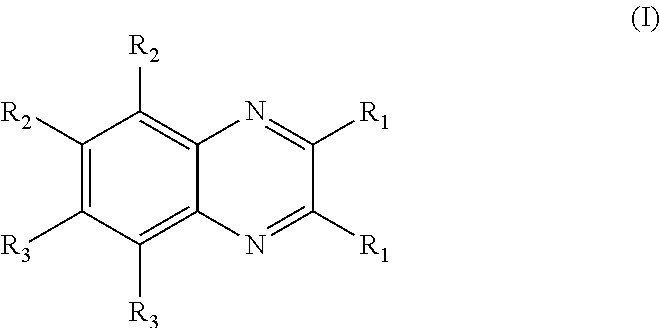
Belonging to the technical field of medicine, the invention relates to a 4, 5-dihydro-1H-pyrazolo[3, 4-c]pyridine-7-one containing derivative shown as general formula I, and pharmaceutically acceptable salt, hydrate or prodrug thereof, wherein the substituents A, R1 and R2 have meanings given in the specification. The preparation also relates to a preparation method of the general formula I compound and its pharmaceutically acceptable salt or prodrug, medicinal compositions containing the compound and application of the compound as an Xa factor inhibitor, especially application in preparation of drugs for treatment and / or prevention of thromboembolic diseases. (formula I).
Owner:SHENYANG PHARMA UNIVERSITY
Imidazothiadiazole and imidazopyridazine derivatives as protease activated receptor 4 (PAR4) inhibitors for treating platelet aggregation
ActiveUS20150119390A1Inhibits platelet aggregationBiocideOrganic chemistryThromboembolic disorderThrombus
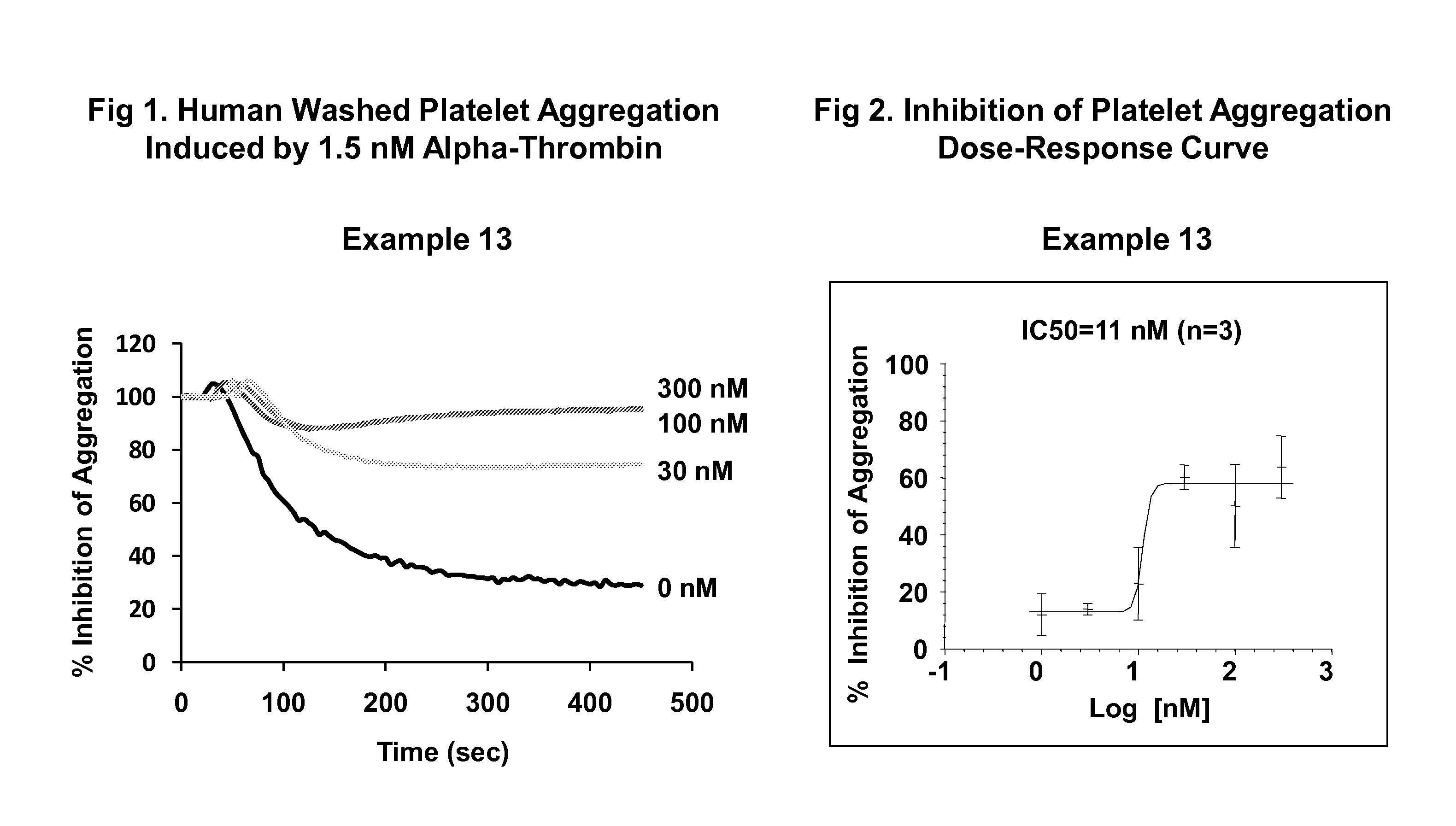
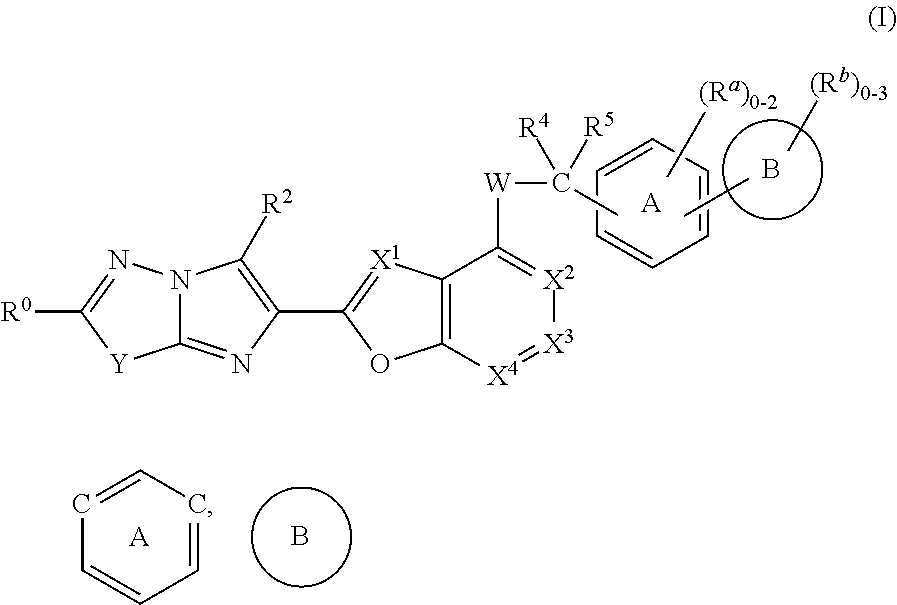
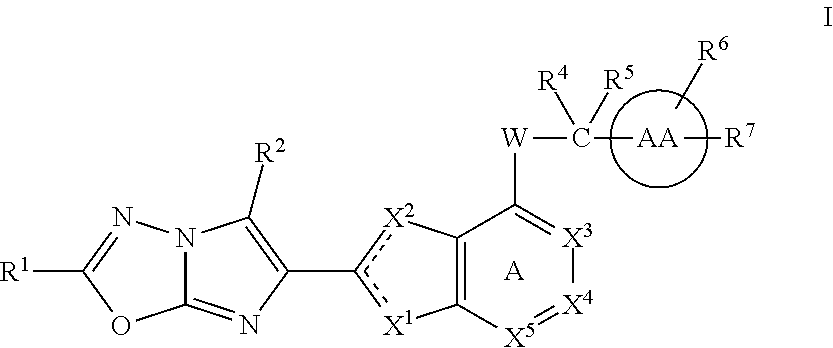
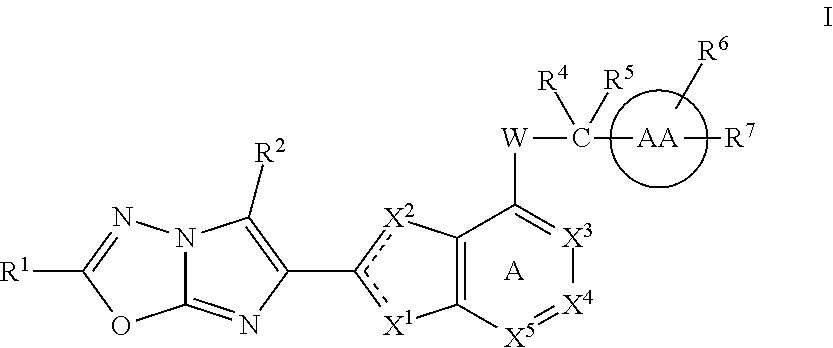
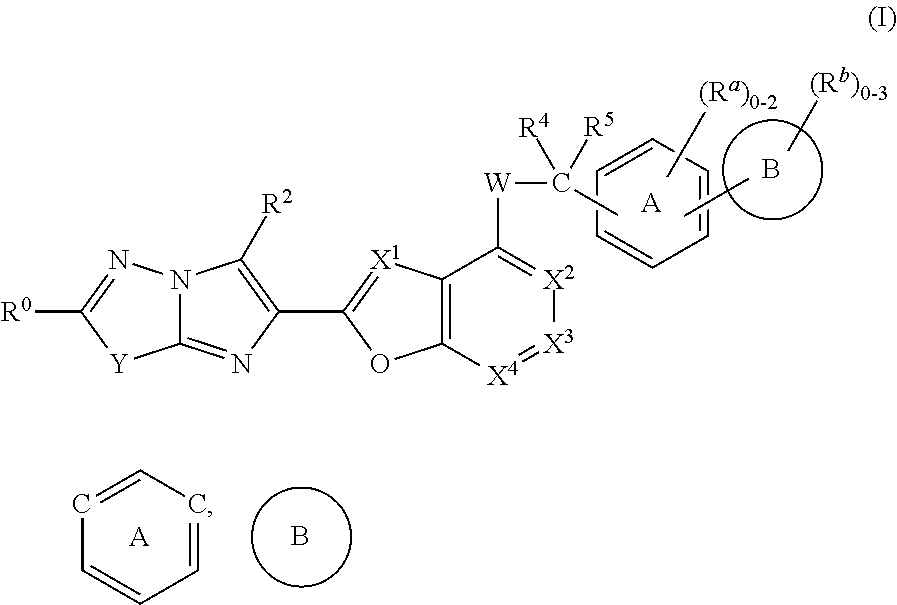
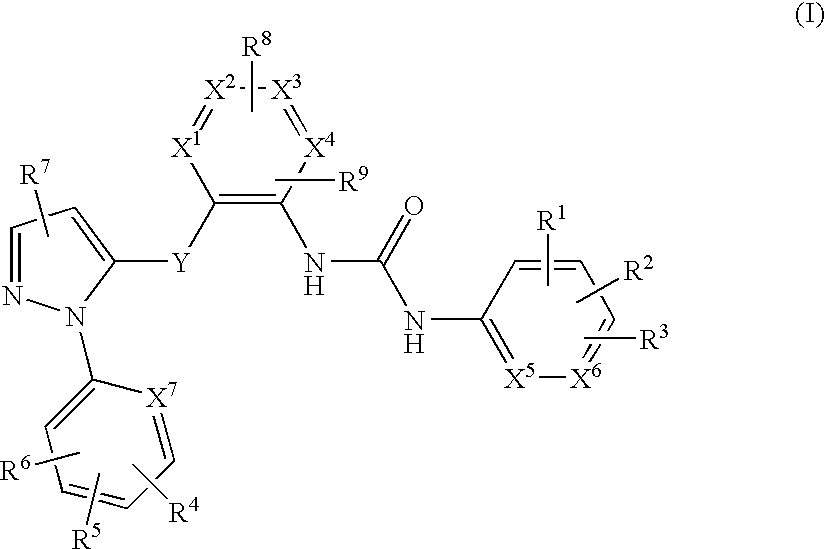
The present invention provides imidazothiadiazole compounds of Formula (I); Wherein W, Y, R0, R2, R4, Ra, Rb, X1, X2, X3 and X4 are as defined herein, or a stereoisomer, tautomer, pharmaceutically acceptable salt, prodrug ester or solvate form thereof, wherein all of the variables are as defined herein. These compounds are inhibitors of platelet aggregation and thus can be used as medicaments for treating or preventing thromboembolic disorders.
Owner:UNIV DE MONTREAL +1
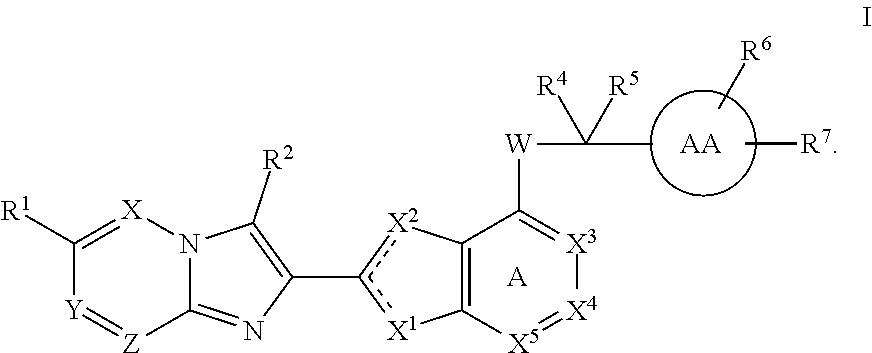
Imidazotriazine and imidazodiazine compounds
The present invention provides thiazole compounds of Formula Iwherein X, Y, Z, X1, X2, X3, X4, X5, R1, R2, R4, R5, R6, R7, and W, are as defined herein, or a stereoisomer, tautomer, pharmaceutically acceptable salt, prodrug, ester or solvate form thereof, wherein all of the variables are as defined herein. These compounds are inhibitors of platelet aggregation and thus can be used as medicaments for treating or preventing thromboembolic disorders.
Owner:UNIV DE MONTREAL
Therapeutic agents for inducing platelet fragmentation and treating thromboembolic disorders
ActiveUS8753631B2Peptide/protein ingredientsAntibody mimetics/scaffoldsMedicineThromboembolic disorder
Owner:NEW YORK UNIV
Pyrrole derivatives as P2Y12 antagonists
Owner:SANOFI SA
Imidazooxadiazole compounds
ActiveUS9617279B1Inhibitor usedOrganic active ingredientsOrganic chemistryThiazoleThromboembolic disorder
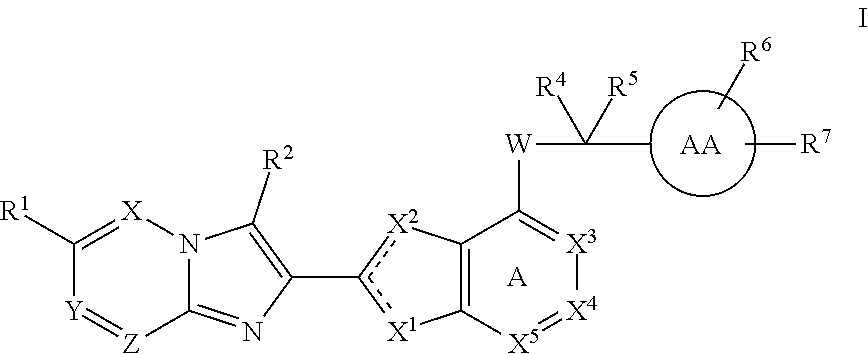
The present invention provides thiazole compounds of Formula Iwherein X1, X2, X3, X4, X5, R1, R2, R4, R5, R6, R7, and W, and ring AA, are as defined herein, or a stereoisomer, tautomer, pharmaceutically acceptable salt, prodrugs, or esters or solvate form thereof, wherein all of the variables are as defined herein. These compounds are inhibitors of platelet aggregation and thus can be used as medicaments for treating or preventing thromboembolic disorders.
Owner:BRISTOL MYERS SQUIBB CO
Pharmaceutical Composition Comprising Apixaban
The present invention relates to a pharmaceutical composition comprising apixaban, in particular to a pharmaceutical composition comprising apixaban and a polymer having low viscosity as binder, and to a process for its preparation. The pharmaceutical composition is particularly useful as a medicament, especially for the treatment or prevention of a thromboembolic disorder.
Owner:SANDOZ AG
Pyrrole derivatives as p2y12 antagonists
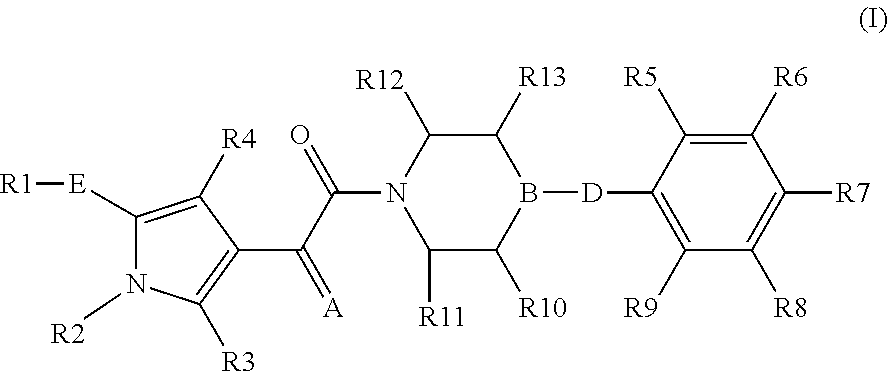
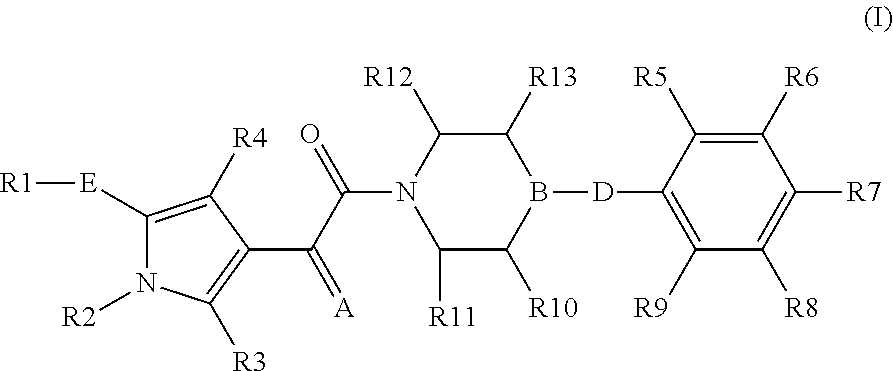
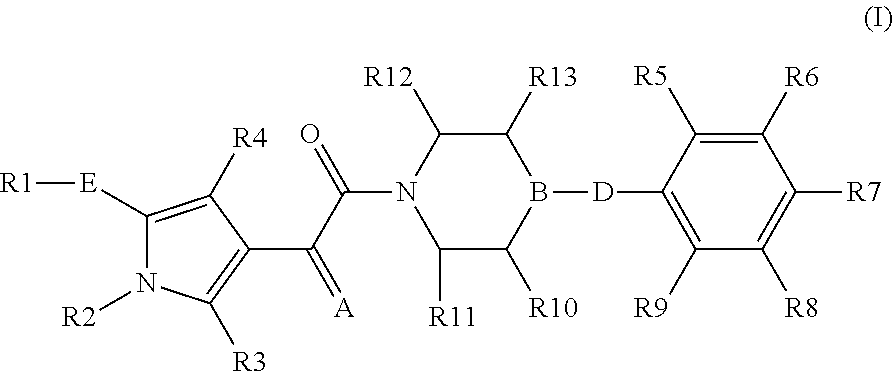
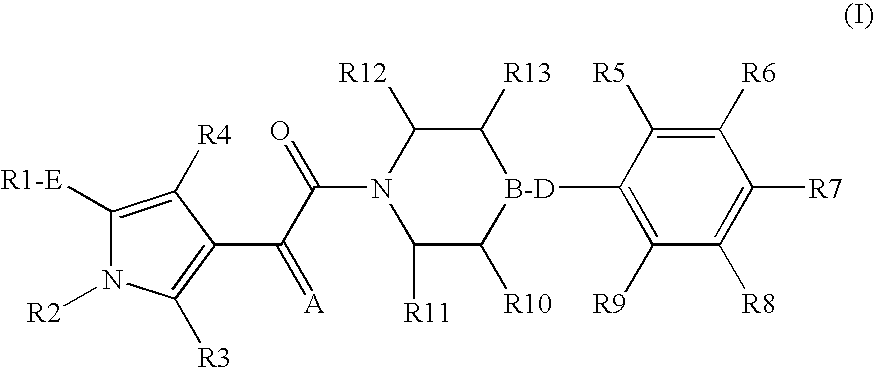
The present invention relates to compounds of the formula I,in which R1; R2; R3; R4; R5; R6; R7; R8; R9; R10; R11; R12; R13; A; B, D and E have the meanings indicated in the claims. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong anti-aggregating effect on platelets and thus an anti-thrombotic effect and are suitable e.g. for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible antagonists of the platelet ADP receptor P2Y12, and can in general be applied in conditions in which an undesired activation of the platelet ADP receptor P2Y12 is present or for the cure or prevention of which an inhibition of the platelet ADP receptor P2Y12 is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI SA
Von willebrand factor specific binding agents and uses thereof
InactiveUS20120321640A1Prevent thrombosisReduce brain damageImmunoglobulins against blood coagulation factorsAntibody mimetics/scaffoldsDiseaseThrombus
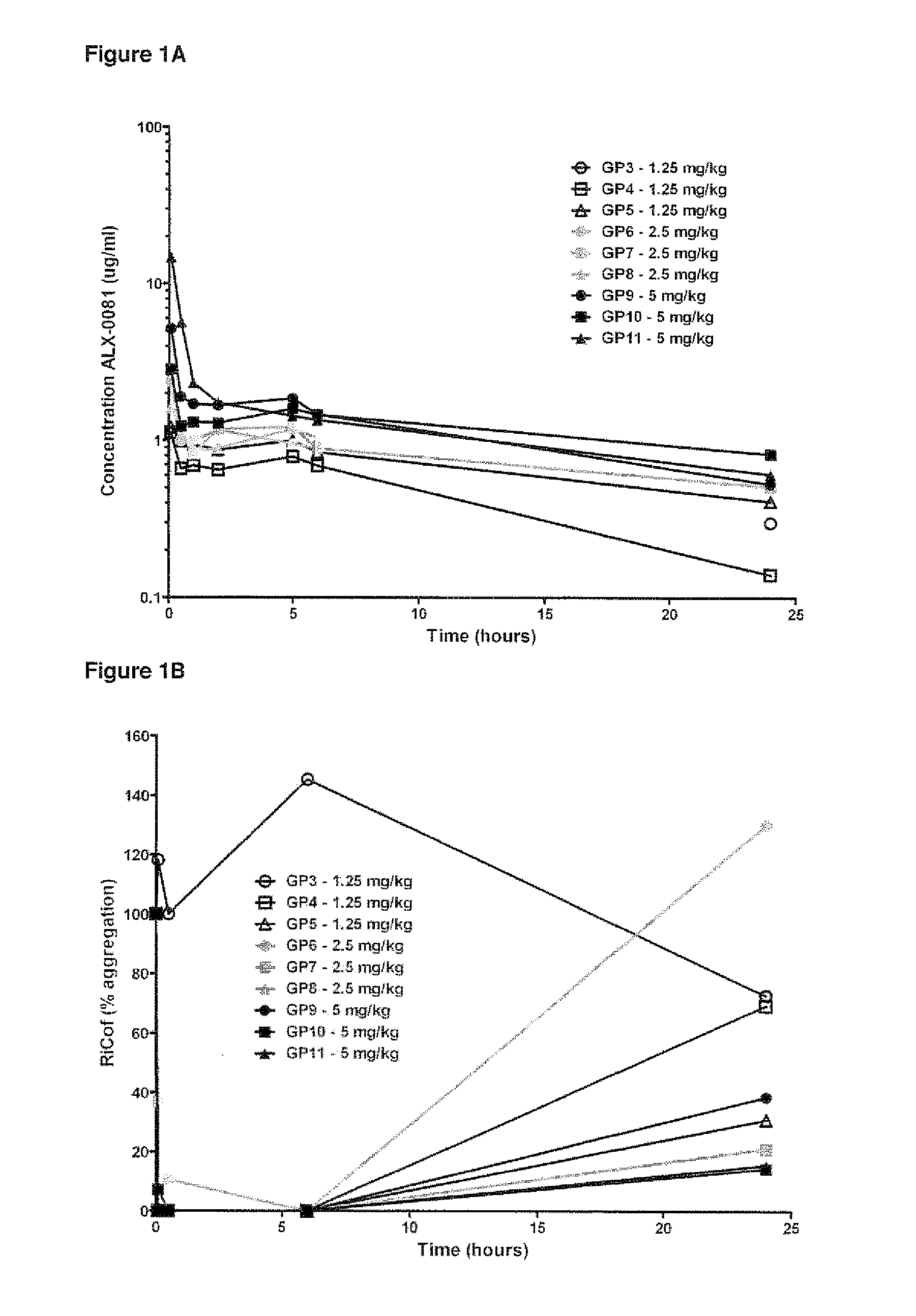
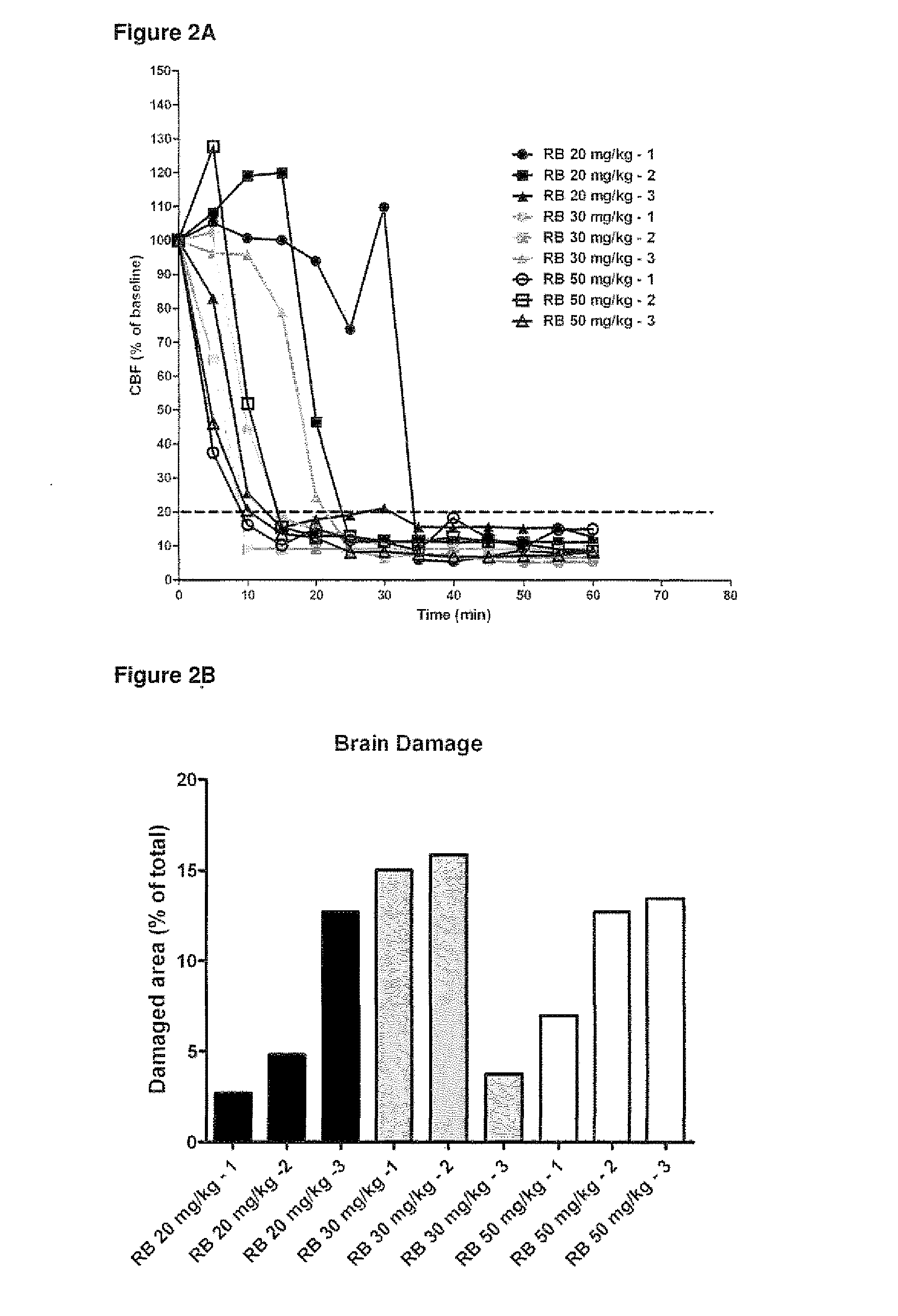
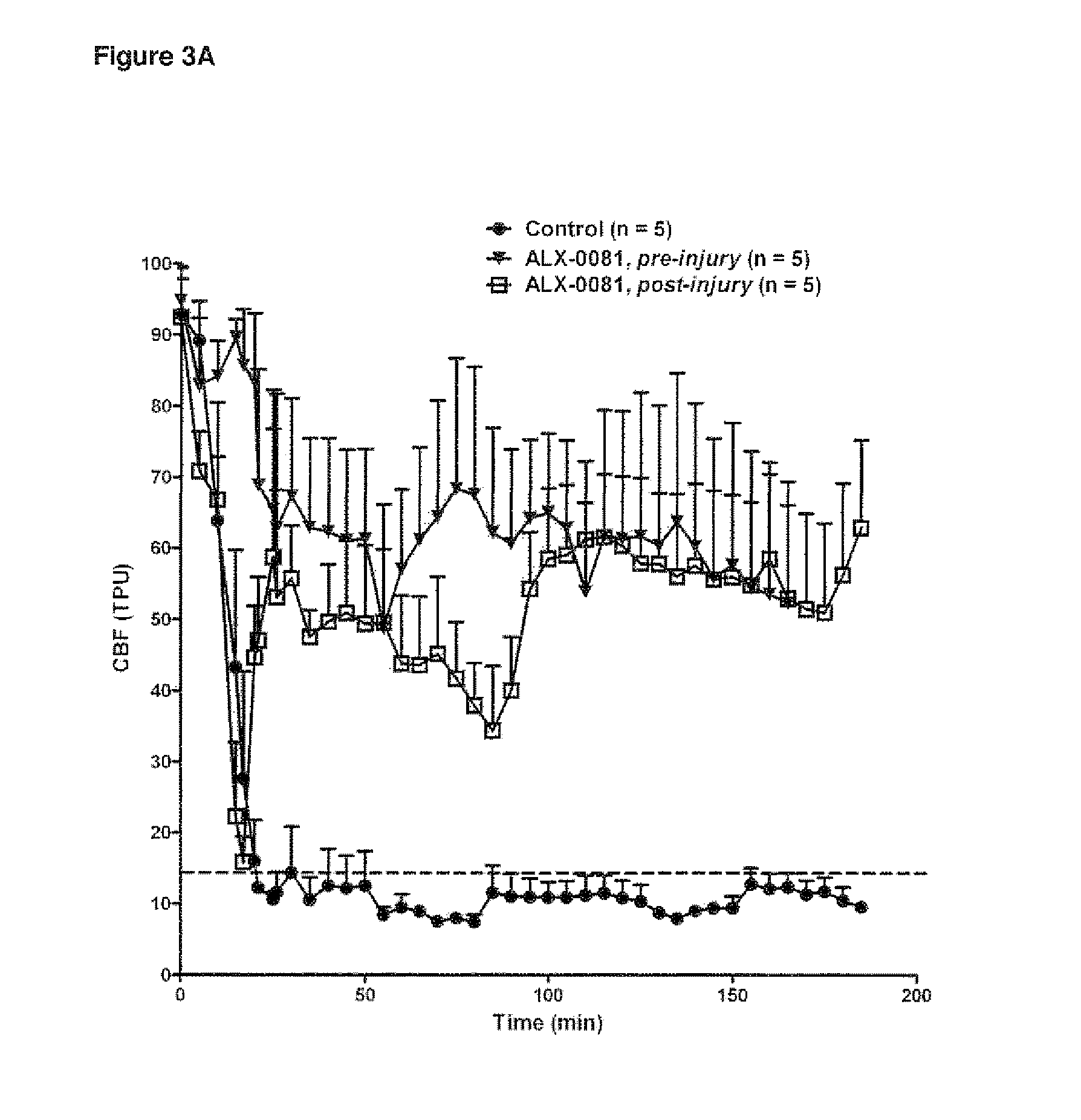
The invention provides new uses, compositions and methods of administration for specific binding agents to von Wiliebrand Factor (vWF) in patients with thromboembolic disorders and in particular new combined uses with thrombolytic agents such as tissue plasminogen activator in patients with thromboembolic disorders such as e.g. ischemic stroke. Furthermore, a new group of vWF binding agents and an improved Middle Cerebral Artery Thrombosis Model in guinea pigs to study the effects of stroke such as ischemia (oxygen and glucose depriviation) and hemorrhage (bleeding), in particular hemorrhage, are provided.
Owner:ABLYNX NV
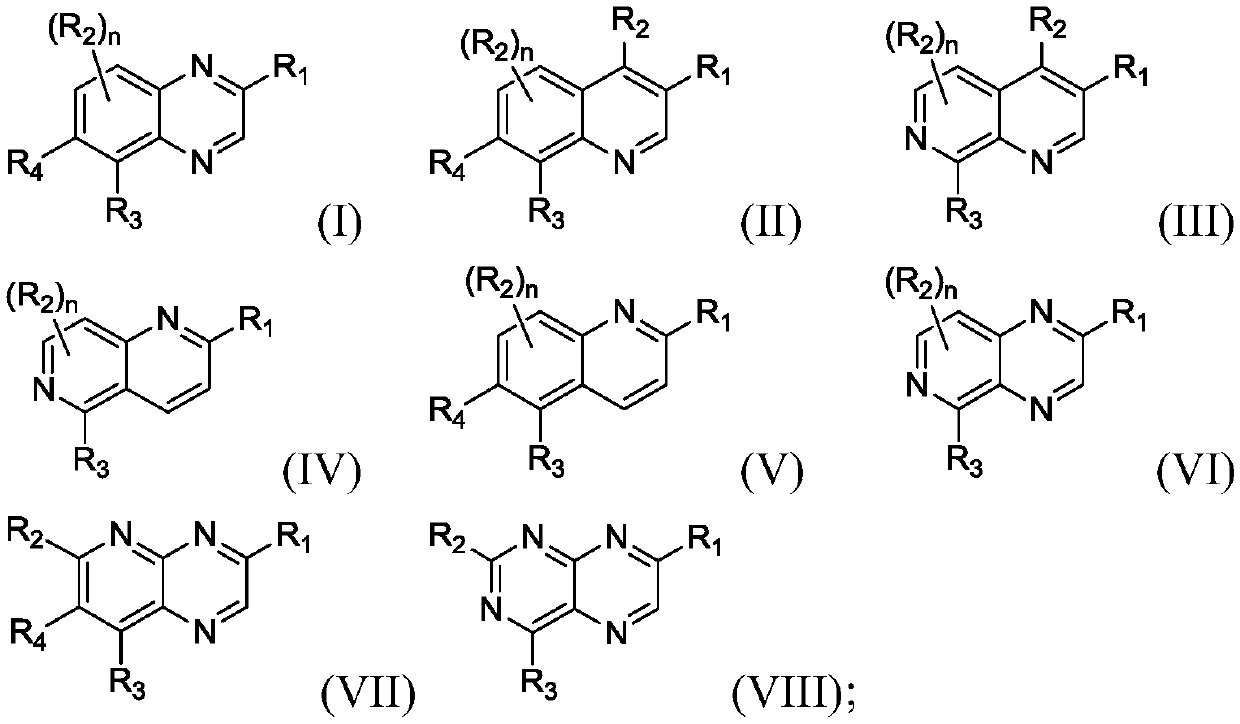
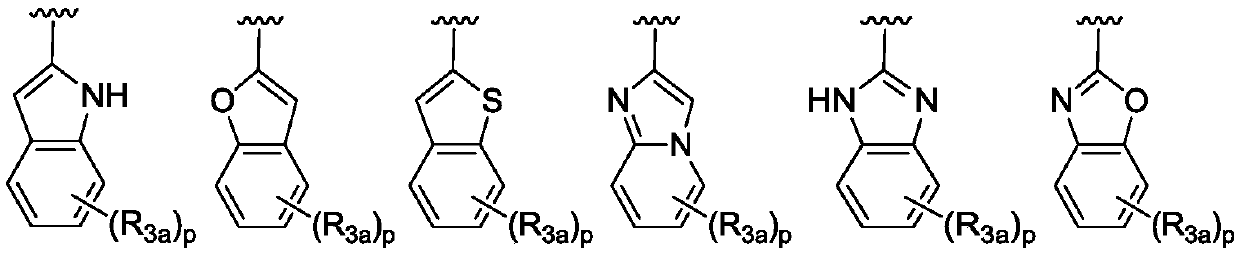
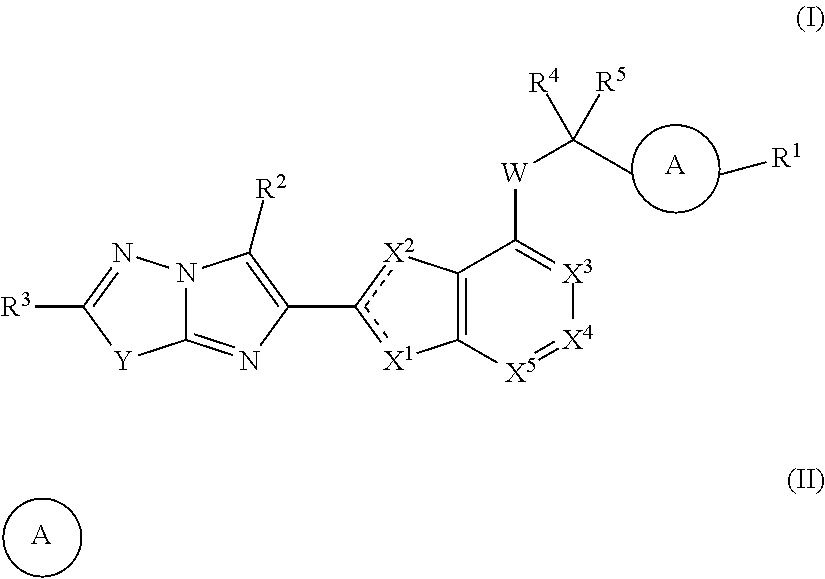
Bicyclic heteroaryl substituted compounds
Disclosed are compounds of Formula (I) to (VIII); or a stereoisomer, a tautomer, a pharmaceutically acceptable salt, a solvate or a prodrug thereof, wherein R3 is a bicyclic heteroaryl group substituted with zero to three R3a; and R1, R2, R3a, R4, and n are defined herein. Also disclosed are methods of using the compounds as PAR4 inhibitors, and pharmaceutical compositions comprising the compounds. These compounds are useful in inhibiting or preventing platelet aggregation, and are useful for the treatment of thromboembolic disorders or the primary prophylaxis of thromboembolic disorders.
Owner:BRISTOL MYERS SQUIBB CO
Pyrazole-carboxamide derivatives as P2Y12 antagonists
The present invention relates to compounds of the formula I,whereinR1; R2; Z; A; B; D; Q; J; V; G and M have the meanings indicated in the claims. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong anti-aggregating effect on platelets and thus an anti-thrombotic effect and are suitable, e.g., for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible antagonists of the platelet ADP receptor P2Y12, and can in general be applied in conditions in which an undesired activation of the platelet ADP receptor P2Y12 is present or for the cure or prevention of which an inhibition of the platelet ADP receptor P2Y12 is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI SA
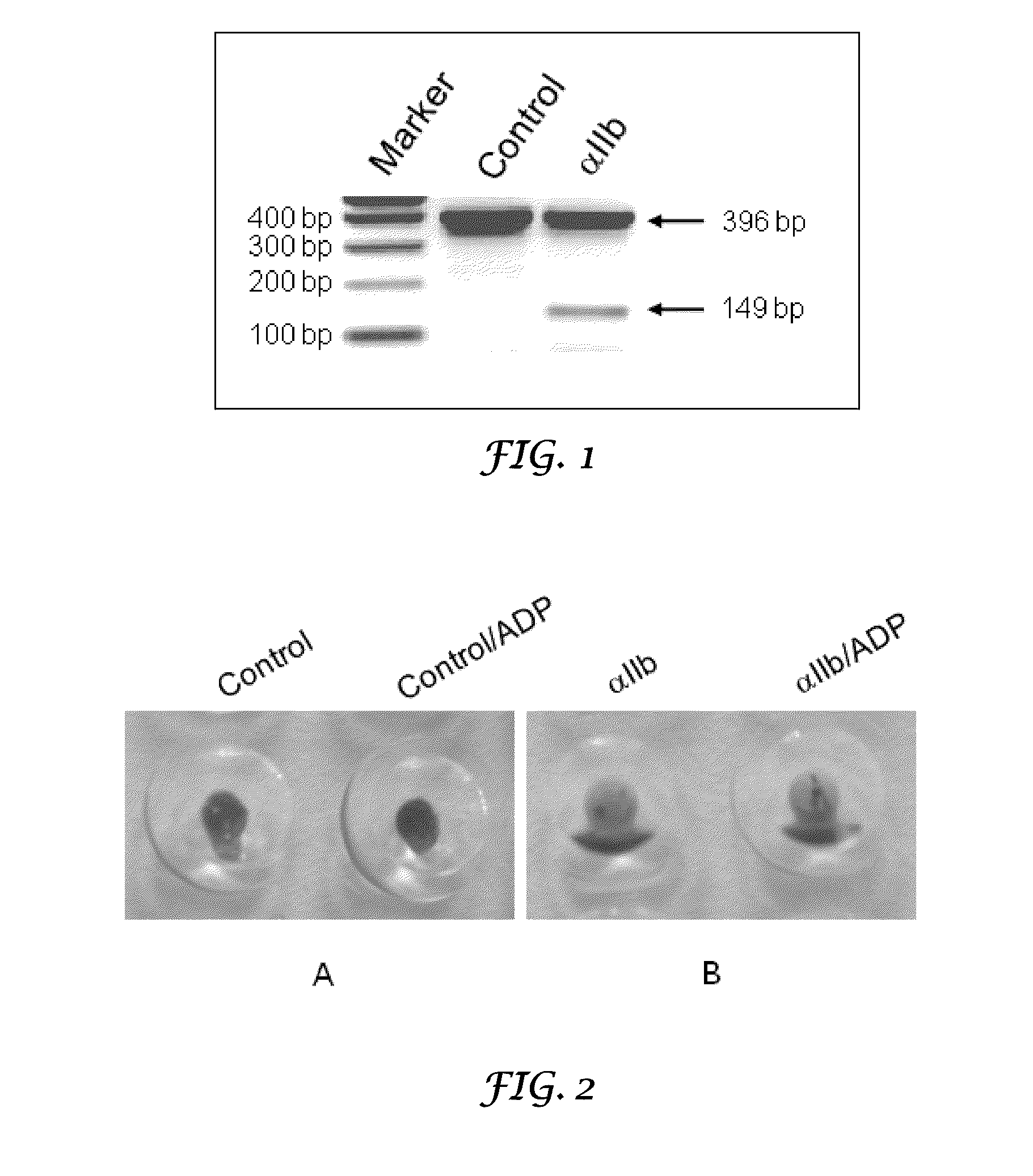
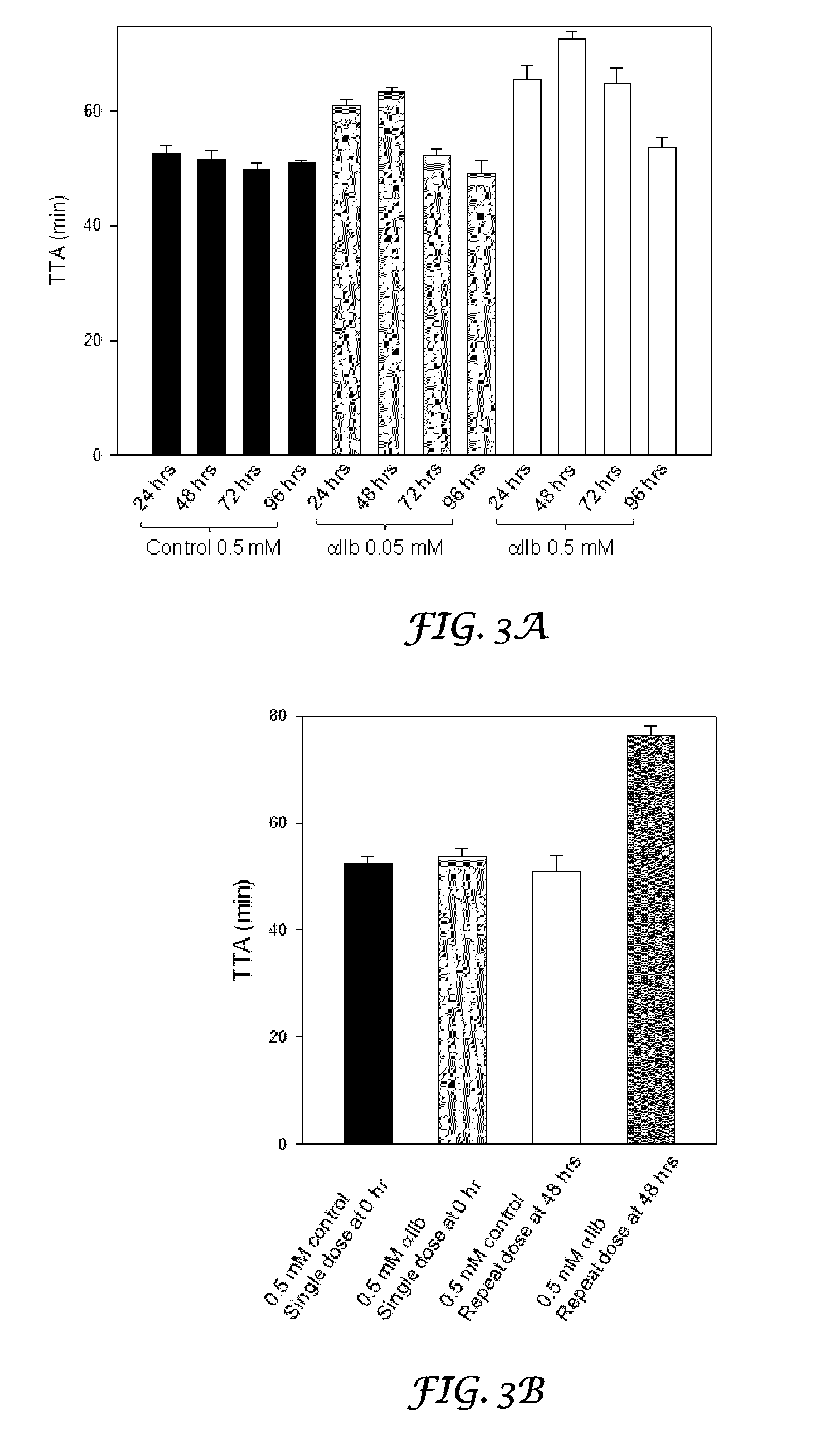
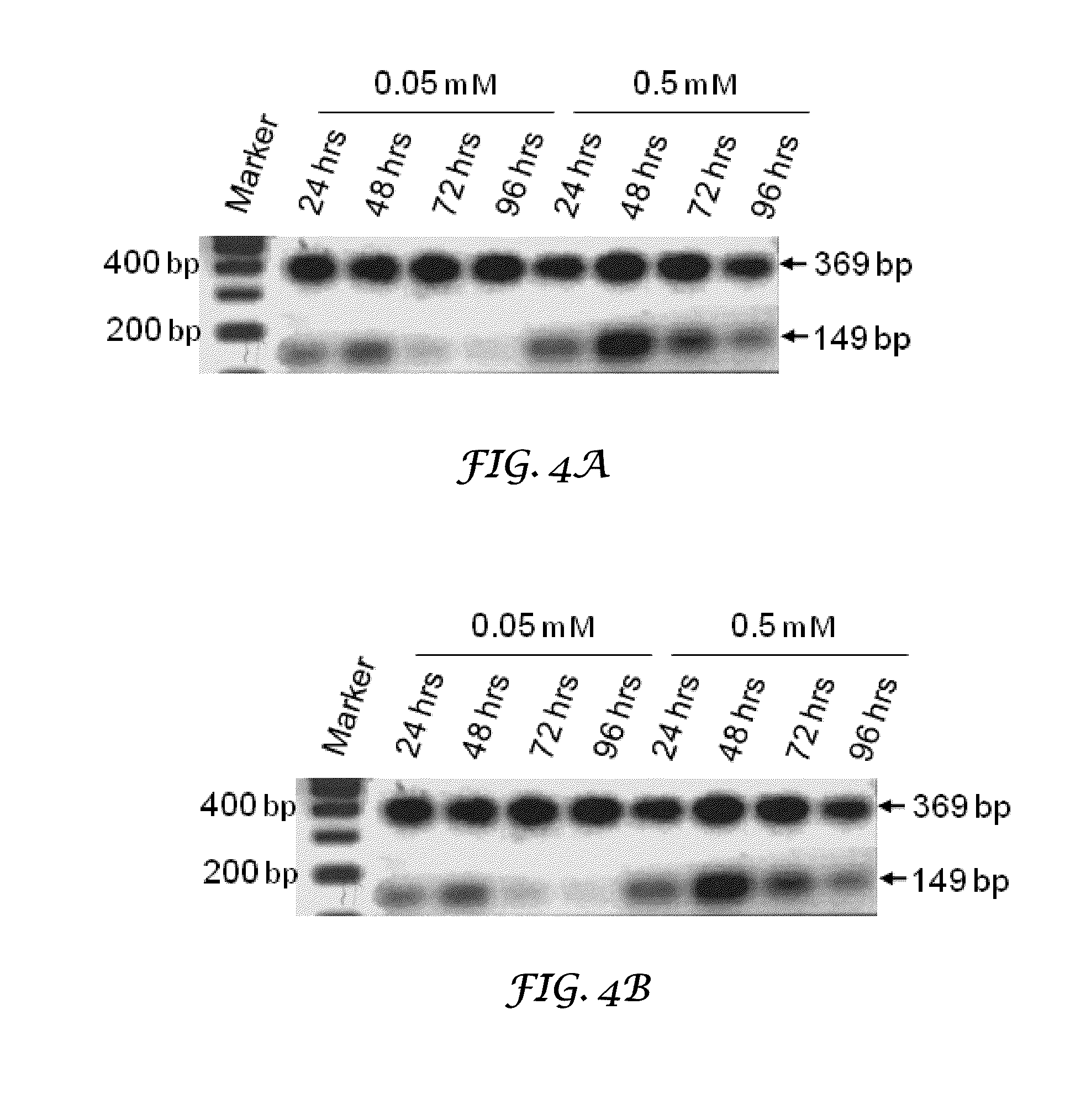
Thrombocyte Inhibition Via Vivo-Morpholino Knockdown of Alpha IIB
ActiveUS20110190287A1Alleviate and cure pathological effectModulating gene expressionOrganic active ingredientsOrganic chemistryThromboembolic disorderThrombus
Novel compounds comprising a guanidine-rich head covalently coupled to one or more oligonucleotide antisense sequences which are useful to modulate blood coagulation by affecting the expression of integrin αIIb or β3 are described herein. This invention also includes pharmaceutical compositions containing these compounds, with or without other therapeutic agents, and to methods of using these compounds as inhibitor of platelet aggregation, as thrombolytics, and / or for the treatment of other thromboembolic disorders. Vivo-MOs, which include eight guanidine groups dendrimerically arranged in the guanidine-rich head and two synthetic antisense morpholino oligonucleotides, are representative compounds of the present invention.
Owner:UNIVERSITY OF NORTH TEXAS
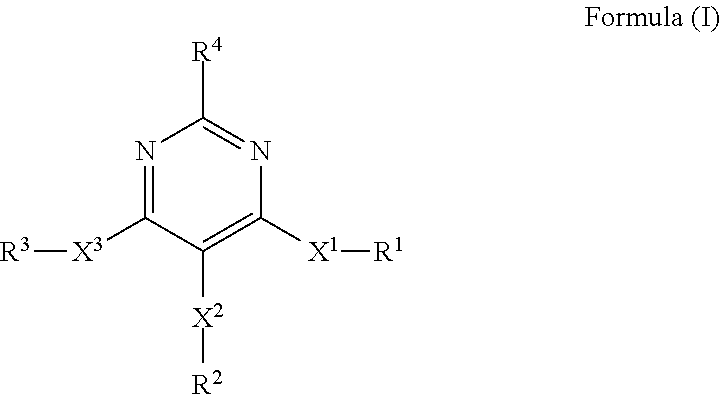
4, 5, 6-trisubstituted pyrimidine derivatives as factor IXa inhibitors
ActiveUS8609676B2Avoid adjustmentBiocideOrganic active ingredientsThromboembolic disorderStereoisomerism
The present invention relates to novel heterocyclic compounds of Formulae (I): (Chemical formula should be inserted here as it appears on abstract in paper form) Formula (I) as disclosed herein, or a pharmaceutically acceptable salt, solvate, ester, prodrug or stereoisomer thereof. Also disclosed are pharmaceutical compositions comprising said compounds, and methods for using said compounds for treating or preventing a thromboembolic disorder.
Owner:MERCK SHARP & DOHME LLC
Imidazopyridazine and imidazothiadiazole compounds
ActiveUS10214544B2Organic active ingredientsOrganic chemistry methodsPyridazineThromboembolic disorder
Owner:UNIV DE MONTREAL
Aryl substituted bicyclic heteroaryl compounds
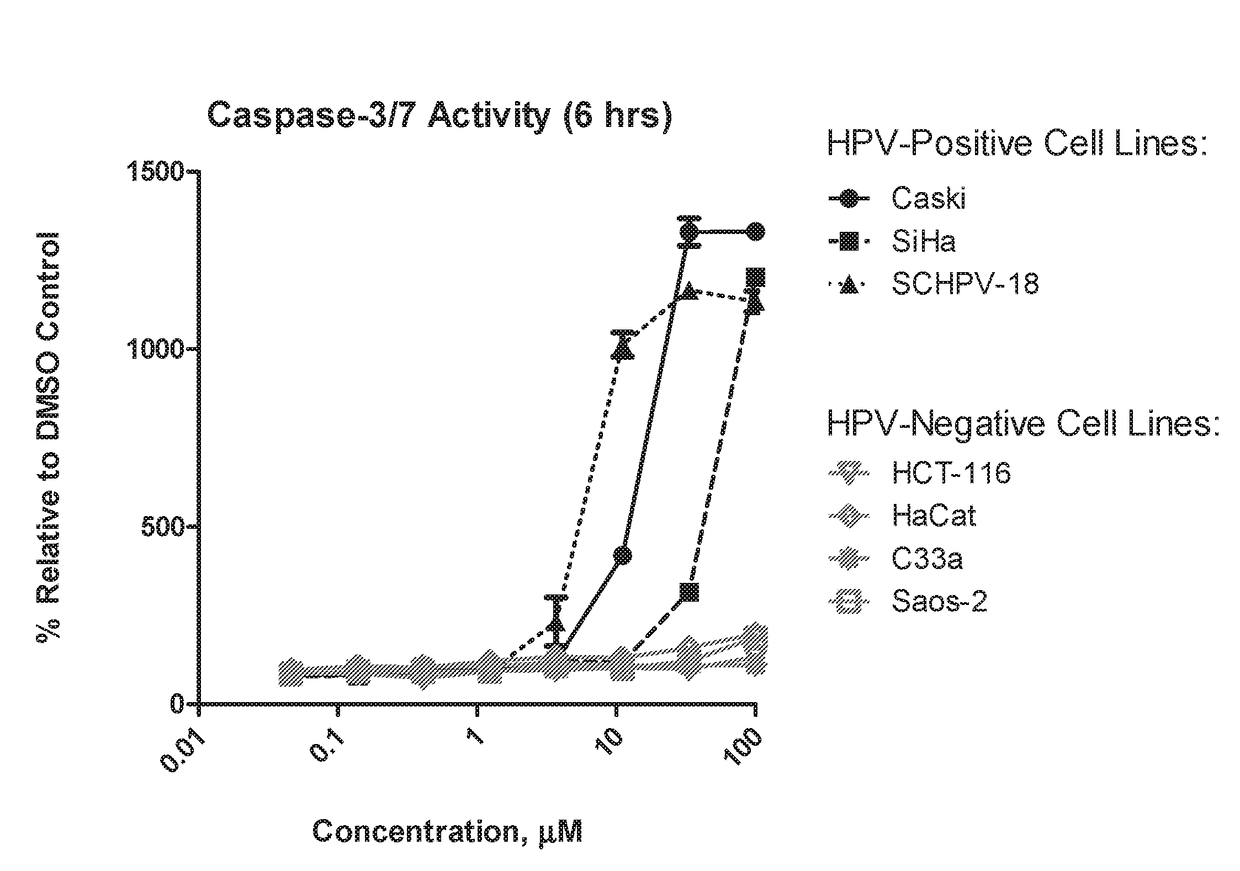
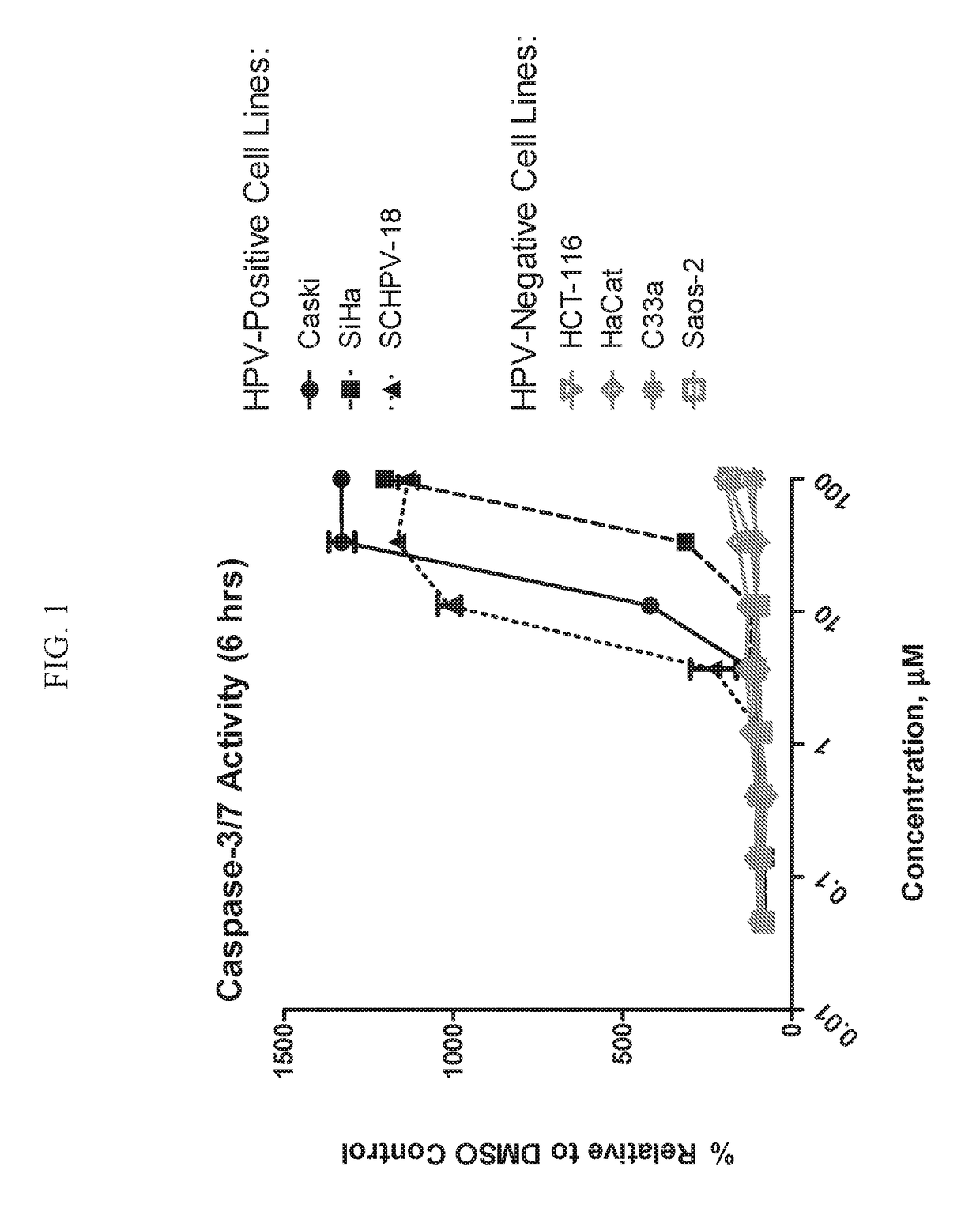
ActiveUS20180214445A1Reduce probabilityHigh riskOrganic active ingredientsOrganic chemistryArylHuman papillomavirus
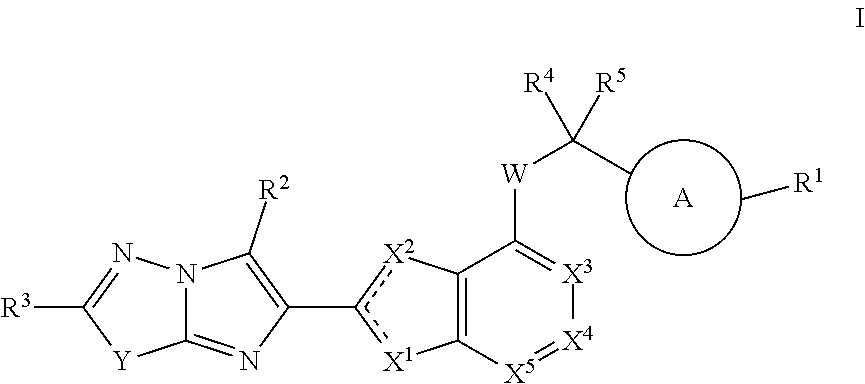
Disclosed is a compound of Formula (I) or a stereoisomer, tautomer, pharmaceutically acceptable salt, solvate or prodrug thereof, wherein one R3 is H and the other R3 is an aryl group substituted with zero to 3 R3a; and R1, R2, and R3a are defined herein. Also disclosed are methods of using such compounds as PAR4 inhibitors for the inhibition or prevention of platelet aggregation, and the treatment of a thromboembolic disorder or the primary prophylaxis of a thromboembolic disorder. Also disclosed are methods of using such compounds for the treatment of human papillomavirus. Additionally, pharmaceutical compositions comprising at least one compound of Formula (I) are disclosed.
Owner:BRISTOL MYERS SQUIBB CO
Apixaban formulations
ActiveUS9326945B2Improve solubilityPowder deliveryOrganic active ingredientsThromboembolic disorderPharmacology
Compositions comprising crystalline apixaban particles having a D90 equal to or less than 89 μm, and a pharmaceutically acceptable carrier, are substantially bioequivalent and can be used to for the treatment and / or prophylaxis of thromboembolic disorders.
Owner:BRISTOL MYERS SQUIBB CO +1
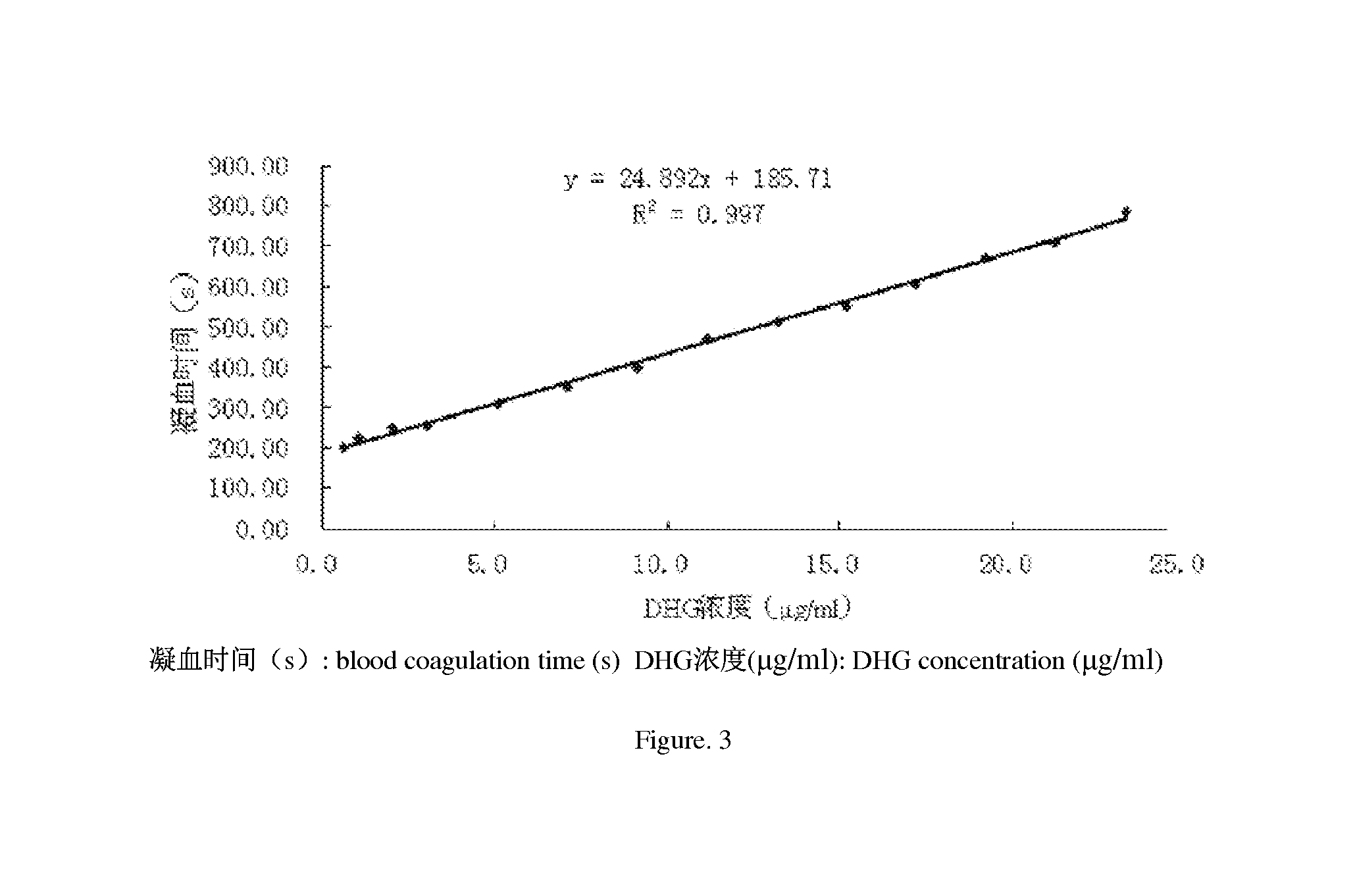
Application of depolymerized holothurian gylcosaminoglycans (DHG) in preparation of drug for prevention and treatment of thromboembolic diseases
ActiveUS20150051165A1Improve securitySolve the problem of safe useBiocideCarbohydrate active ingredientsSide effectThrombus
The present invention discloses an application of depolymerized holothurian glycosaminoglycans (DHG) in preparation of a drug for the prevention and treatment of thromboembolic diseases. The DHG is more than one type of DHG with weight-average molecular weights between 26,000 and 45,000 Da. When being intravenously or subcutaneously injected, the drug using the DHG with weight-average molecular weights between 26,000 and 45,000 Da as an active ingredient has a significant anticoagulant effect, while at the same time, has little side effects, and is effective for use in the prevention and treatment of the thromboembolic diseases. For an injection of DHG with weight-average molecular weights between 26,000 Da and 45,000 Da, the blood coagulation time is prolonged and the anticoagulant effect is enhanced as the dosage increases; the subcutaneous administration is used and is more favorable for use in the drug, and the convenience and safety of use the drug are improved.
Owner:SHANGHAI KAIRUN BIOLOGY MEDICINE LIMITED LIABILITY +2
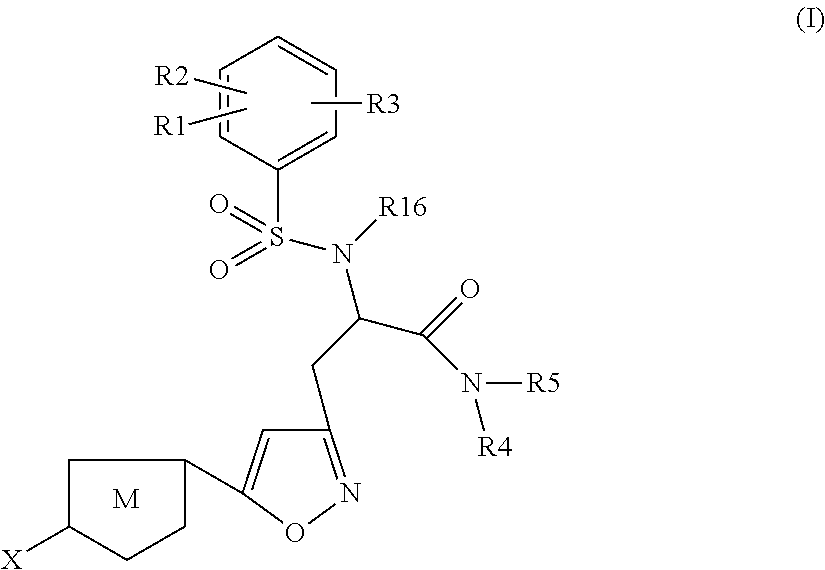
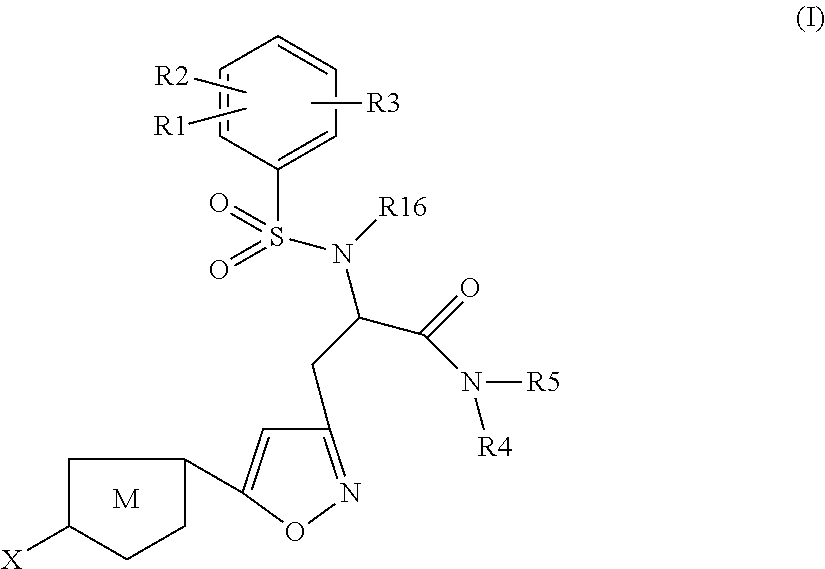
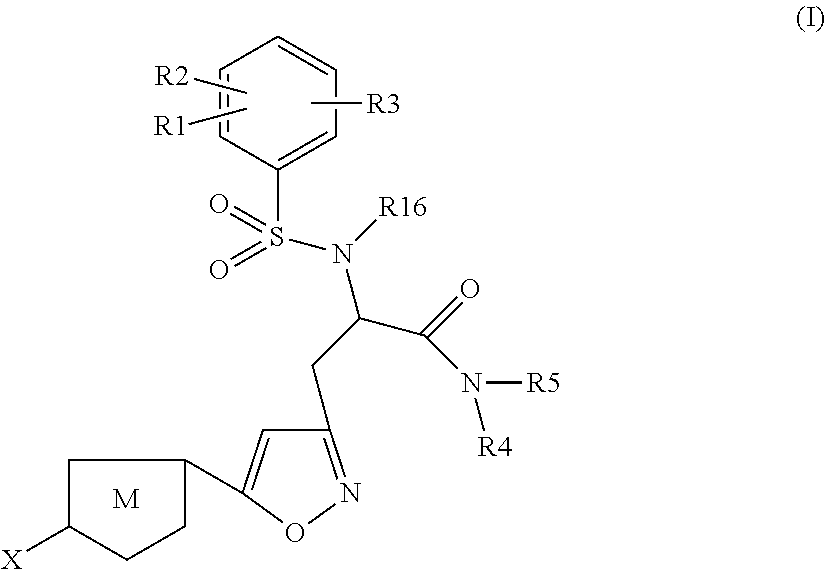
Chlorothiophene-isoxazoles as inhibitors of coagulation factors xa and thrombin
The present invention relates to compounds of the formula I,wherein R1; R2; R3; R4; R5, R16, X and M have the meanings indicated in the claims. The compounds of formula I are valuable pharmacologically active compounds. They exhibit a strong anti-thrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa and thrombin and can in general be applied in conditions in which an undesired activity of factor Xa and / or thrombin are present or for the cure or prevention of which an inhibition of factor Xa and thrombin are intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI SA
Bicyclic heteroaryl substituted compounds
Disclosed are compounds of Formula (I) to (IV): [INSERT CHEMICAL STRUCTURE HERE] or a stereoisomer, tautomer, pharmaceutically acceptable salt, solvate or prodrug thereof. Also disclosed are methods of using such compounds as PAR4 inhibitors, and pharmaceutical compositions comprising such compounds. These compounds are useful in inhibiting or preventing platelet aggregation, and are useful for the treatment of a thromboembolic disorder or the primary prophylaxis of a thromboembolic disorder.
Owner:BRISTOL MYERS SQUIBB CO
Imidazothiadiazole and imidazopyridazine derivatives as protease activated receptor 4 (PAR4) inhibitors for treating platelet aggregation
The present invention provides imidazothiadiazole compounds of Formula (I); Wherein W, Y, R0, R2, R4, Ra, Rb, X1, X2, X3 and X4 are as defined herein, or a stereoisomer, tautomer, pharmaceutically acceptable salt, prodrug ester or solvate form thereof, wherein all of the variables are as defined herein. These compounds are inhibitors of platelet aggregation and thus can be used as medicaments for treating or preventing thromboembolic disorders.
Owner:UNIV DE MONTREAL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

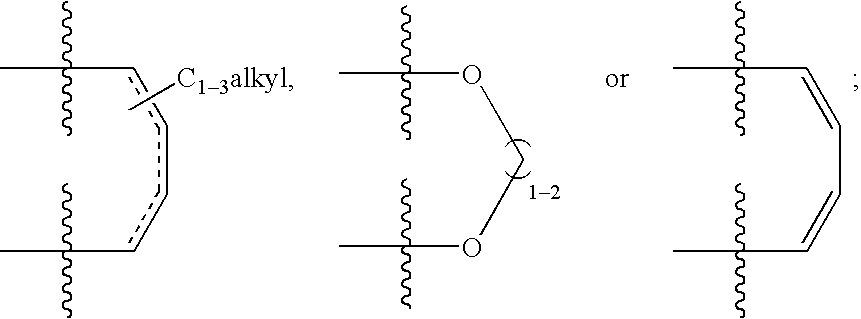


![Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ba01bb1d-6279-47d7-a61b-2f65d761156a/BDA0000629594000000021.PNG)
![Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ba01bb1d-6279-47d7-a61b-2f65d761156a/BDA0000629594000000022.PNG)
![Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ba01bb1d-6279-47d7-a61b-2f65d761156a/BDA0000629594000000032.PNG)