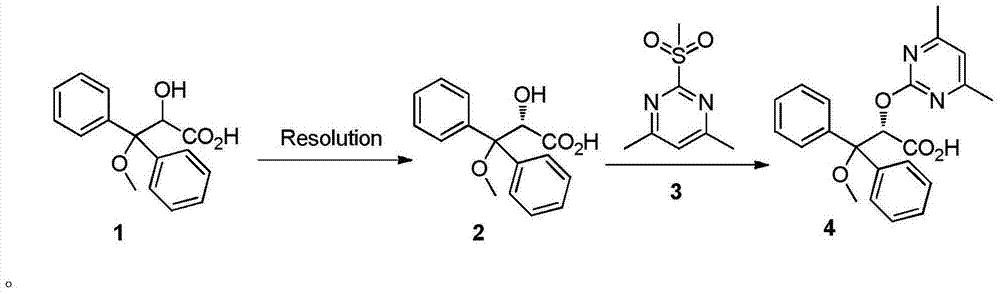
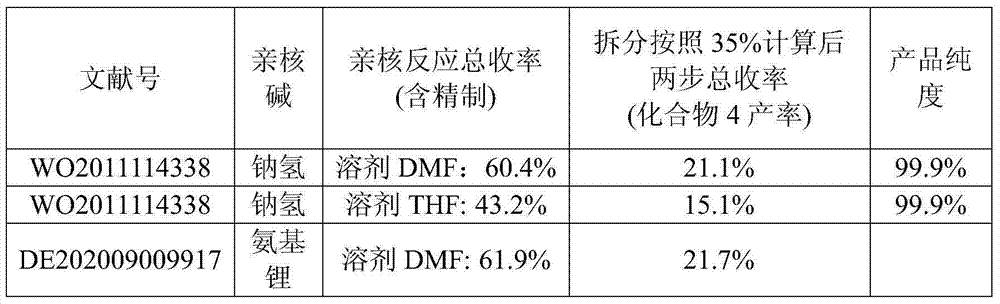
Synthetic method of ambrisentan
A synthetic method, ambrisentan technology, applied in the field of medicine and chemical industry, can solve the problems of incapable of large-scale production, complicated synthesis steps, hidden safety hazards, etc., and achieve the effects of low cost, reduced cost of process materials, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: A kind of synthetic method of ambrisentan
[0066] At room temperature (20-25°C), mix 137g of L-proline methyl ester hydrochloride in 130ml of methanol (MeOH), control the temperature not to exceed 25°C, add dropwise a solution of 44.7g of sodium methoxide and 132ml of MeOH, After the dropwise addition, add 4800ml of methyl tert-butyl ether (MTBE) and 225g of compound 1. After stirring for half an hour, evaporate about 2680ml of MTBE / MeOH mixed solvent under normal pressure. The distillation temperature is 50-55°C, and the distillation process lasts for about 2- After 3 hours, the temperature dropped naturally to 20-25°C, and the solids in the system were removed by filtration under reduced pressure. Evaporate the filtrate to about 900ml under reduced pressure (a water pump can be used to distill under reduced pressure, the temperature is generally -5 ~ 5°C), add 1000ml of water, adjust the pH of the system to acidic with concentrated hydrochloric acid (th...
Embodiment 2
[0068] At room temperature (20-25°C), mix 137g of L-proline methyl ester hydrochloride in 130ml of MeOH, control the temperature not to exceed 25°C, add a solution of 44.7g of sodium methoxide and 132ml of MeOH dropwise, and the addition is complete Then add 4800ml of MTBE and 225g of compound 1, stir for half an hour and evaporate about 2680ml of MTBE / MeOH mixed solvent under normal pressure. The distillation temperature is 50-55℃. °C, the solids in the system were removed by filtration under reduced pressure. Evaporate the filtrate to about 900ml under reduced pressure (a water pump can be used to distill under reduced pressure, the temperature is generally -5 ~ 5°C), add 1000ml of water, adjust the pH of the system to acidic with concentrated hydrochloric acid (the pH value can be controlled from 1 to 4), and let stand to separate. The water layer was removed, and the aqueous phase was back-extracted with 400 ml of MTBE. The MTBE was combined, dried over sodium sulfate, fil...
Embodiment 3
[0070] At room temperature (20-25°C), mix 137g of L-proline methyl ester hydrochloride in 130ml of MeOH, control the temperature not to exceed 25°C, add a solution of 44.7g of sodium methoxide and 132ml of MeOH dropwise, and the addition is complete Then add 4800ml of MTBE and 225g of compound 1, stir for half an hour and evaporate about 2680ml of MTBE / MeOH mixed solvent under normal pressure. The distillation temperature is 50-55℃. °C, the solids in the system were removed by filtration under reduced pressure. Evaporate the filtrate to about 900ml under reduced pressure (a water pump can be used to distill under reduced pressure, the temperature is generally -5 ~ 5°C), add 1000ml of water, adjust the pH of the system to acidic with concentrated hydrochloric acid (the pH value can be controlled from 1 to 4), and let stand to separate. The water layer was removed, the aqueous phase was back-extracted with 400ml MTBE, the MTBE was combined, dried over sodium sulfate, filtered, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com