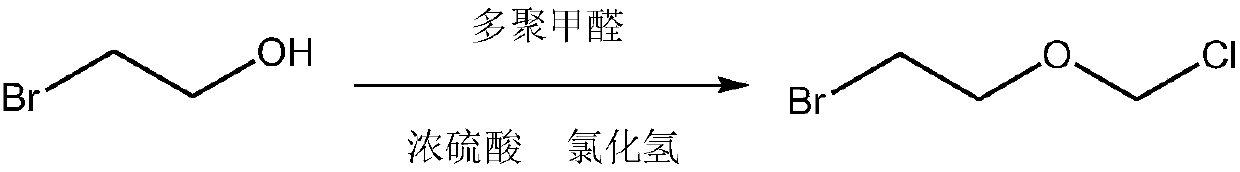
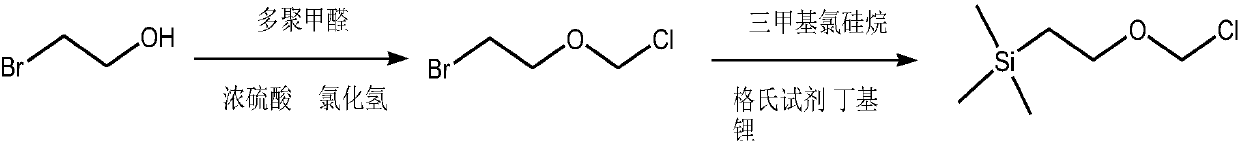
A simple synthetic method of SEM-CI, an important intermediate of anti-myelofibrosis ruxolitinib
A technology of myelofibrosis and synthesis method, which is applied in the field of organic synthesis, can solve the problems of severe reaction, low purity and difficult preservation, and achieves the effects of improving reaction efficiency, simple and convenient operation and stable quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Take 500g of 2-bromoethanol, 500ml of petroleum ether, 130g of paraformaldehyde, and 80g of concentrated sulfuric acid, put them into a 2L reaction bottle, and start stirring. The system was cooled to -5-0°C, and HCL gas was introduced. Introduce slowly at the beginning, after about 10 minutes, increase the aeration rate, control the internal temperature at 5-8°C, and slow down the aeration rate again when the reaction solution becomes clear. The gas phase is monitored until the raw material is less than 5%, and the aeration is stopped (about 240 g of shared hydrogen chloride). Add 1 g of dimethylethylamine to the reaction liquid, and stir for 5 min. The layers were separated, and the lower layer was extracted once with 500ml petroleum ether, and the organic phases were combined. Concentrate under reduced pressure at 50°C (recovery of petroleum ether) until there is no bubble and no reflux to obtain the crude product. 60-90 ° C, water pump rectification, to obtain th...
Embodiment 2
[0040] Take 5000g of 2-bromoethanol, 5000ml of petroleum ether, 1300g of paraformaldehyde, and 800g of concentrated sulfuric acid, put them into a 20L reaction bottle, and start stirring. The system was cooled to -5-0°C, and HCL gas was introduced. Introduce slowly at the beginning, after about 10 minutes, increase the aeration rate, control the internal temperature at 5-8°C, and slow down the aeration rate again when the reaction solution becomes clear. The gas phase is monitored until the raw material is less than 5%, and the aeration is stopped (about 2500 g of shared hydrogen chloride). Add 10 g of triethylamine to the reaction liquid, and stir for 5 min. The layers were separated, and the lower layer was extracted once with 500ml petroleum ether, and the organic phases were combined. Concentrate under reduced pressure at 50°C (recovery of petroleum ether) until there is no bubble and no reflux to obtain the crude product. 60-90°C, water pump rectification, 4.96kg of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com