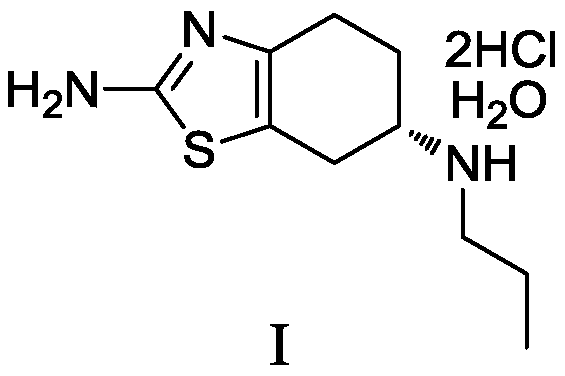
Preparation method of pramipexole hydrochloride and its intermediate
A pramipexole and intermediate technology, which is applied in the field of preparation of pramipexole hydrochloride and its intermediates, can solve the problems of unsuitability for industrial production, poor product purity, low total molar yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the preparation of pramipexole intermediate III
[0070]
[0071]Under the protection of nitrogen, add 200g of 1,4-cyclohexanedione to 1.2L of methanol, stir to dissolve and cool to 5°C, add 286g of liquid bromine dropwise within 15 minutes and stir for 15 minutes, add 163g of thiourea, and then ℃~25℃ and stirred for 16 hours. Add 200 mL of concentrated hydrochloric acid (12 mol / L) and 1 L of water, and stir at 65°C to 70°C for 1 hour. After cooling, concentrate in vacuo to remove most of the methanol (temperature 35°C ~ 45°C, vacuum pressure -0.085MPa ~ -0.1MPa), after cooling, extract twice with 500mL of toluene, and use 20% sodium hydroxide aqueous solution for the water phase (the said mass percentage refers to the percentage of the quality of sodium hydroxide in the total mass of sodium hydroxide aqueous solution) adjust the pH value to 12~14, extract three times with dichloromethane 1L, combine the organic phase with a mass percentage of 10% sod...
Embodiment 2
[0072] Embodiment 2: the preparation of pramipexole II
[0073]
[0074] In a 500mL hydrogenation kettle, add methanol 245mL and pramipexole intermediate III 24.5g (HPLC purity 98.20%), then add propylamine 7.79g, (+)-1,2-bis(2S,5S)-diethylcyclo 87 mg of trifluoromethanesulfonate rhodium trifluoromethanesulfonate was hydrogenated at 50° C. and 5-8 atmospheres for 6 hours. Filtrate, concentrate in vacuo (45°C~55°C, -0.085MPa~-0.1MPa) to remove the solvent, add 100mL of dichloromethane, and use a mass percentage of 20% sodium hydroxide aqueous solution (the mass percentage refers to The quality of sodium hydroxide accounts for the percentage of the total mass of sodium hydroxide aqueous solution) to adjust the pH value to 12~14, leave to stand for stratification, the aqueous phase is extracted twice with dichloromethane 100mL, and the organic phase is combined with 10% by mass percentage of carbonic acid Sodium bicarbonate aqueous solution (the described mass percentage comp...
Embodiment 3
[0076] Embodiment 3: the preparation of pramipexole II
[0077] In a 500mL hydrogenation kettle, add methanol 320mL and pramipexole intermediate III 24.5g (HPLC purity 98.20%), then add propylamine 10.5g, (+)-1,2-bis(2S,5S)-diethylcyclo 174 mg of rhodium trifluoromethanesulfonate was hydrogenated at 40° C. and 7 to 10 atmospheres for 10 hours. Filtrate, concentrate in vacuo (45°C~55°C, -0.085MPa~-0.1MPa) to remove the solvent, add 100mL of dichloromethane, and use a mass percentage of 20% sodium hydroxide aqueous solution (the mass percentage refers to The quality of sodium hydroxide accounts for the percentage of the total mass of sodium hydroxide aqueous solution) to adjust the pH value to 12~14, leave to stand for stratification, the aqueous phase is extracted twice with dichloromethane 100mL, and the organic phase is combined with 10% by mass percentage of carbonic acid Sodium bicarbonate aqueous solution (the described mass percentage composition refers to the quality pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com