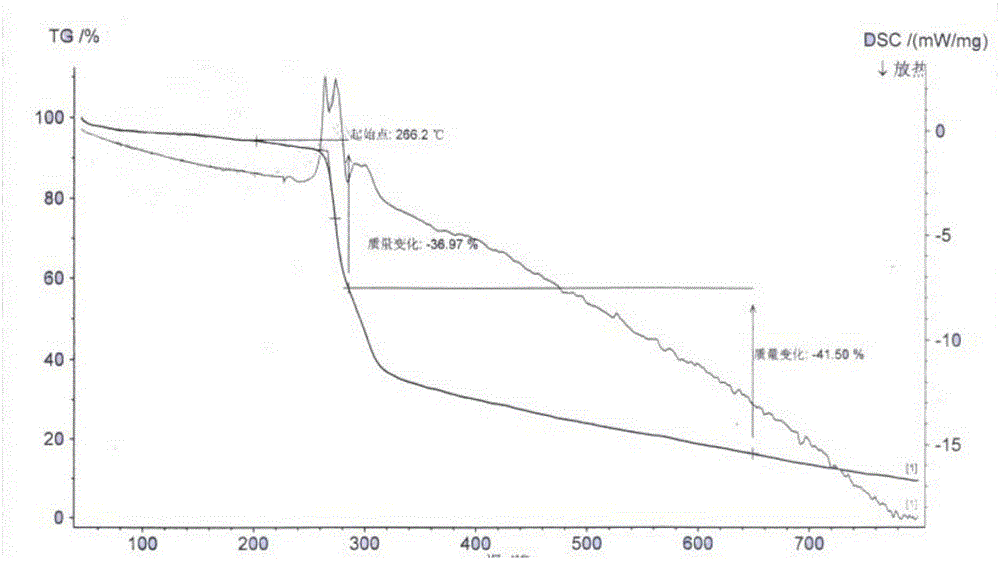
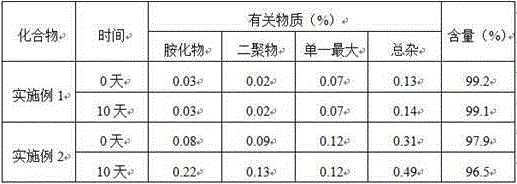
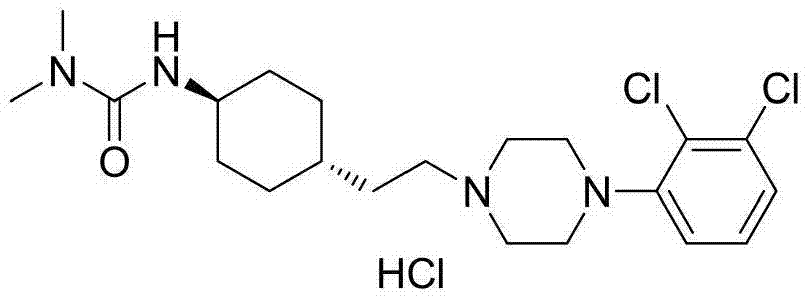
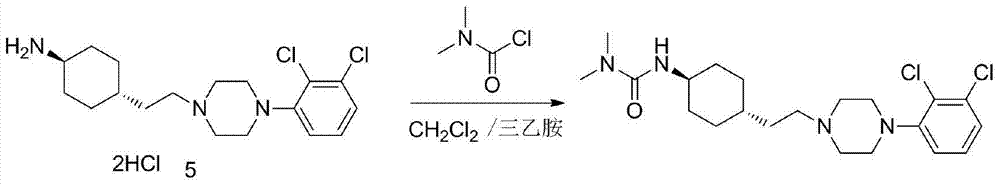
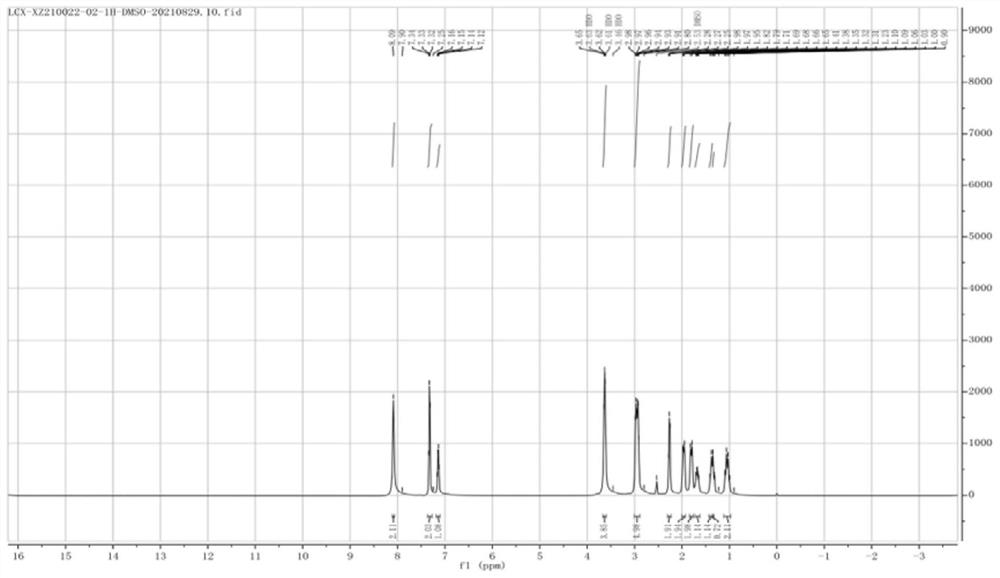
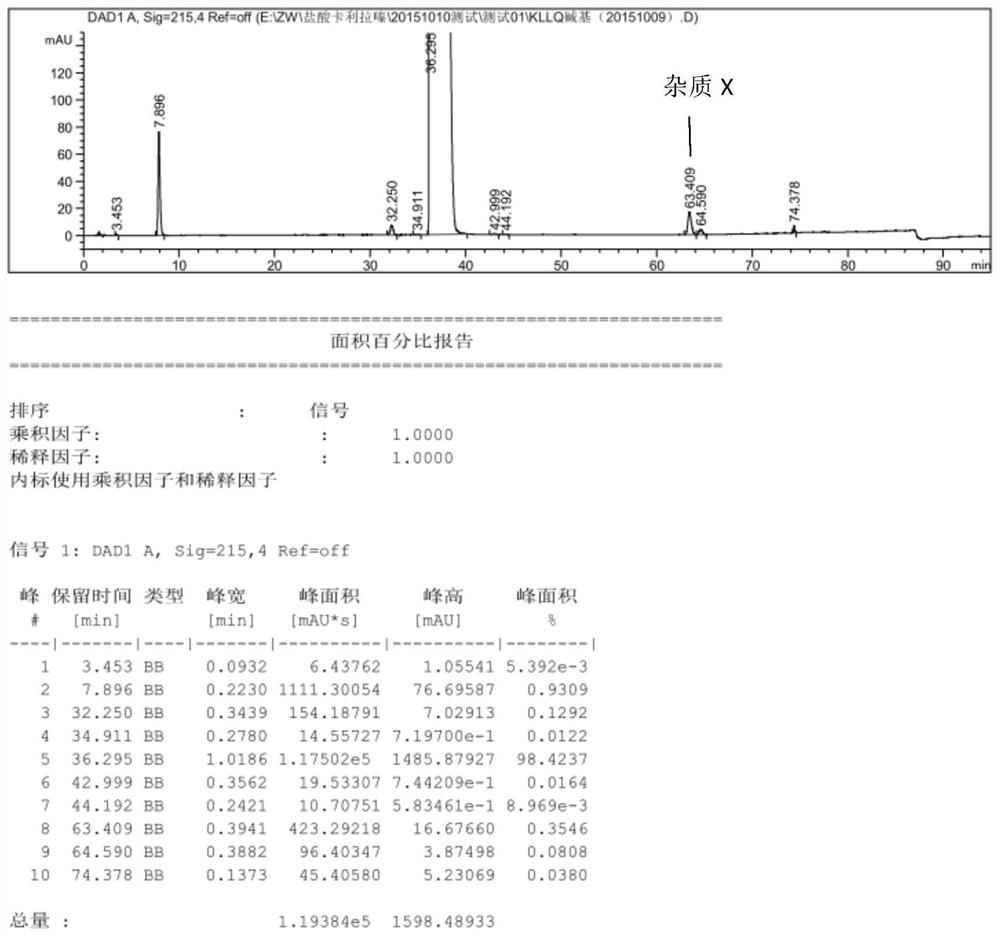
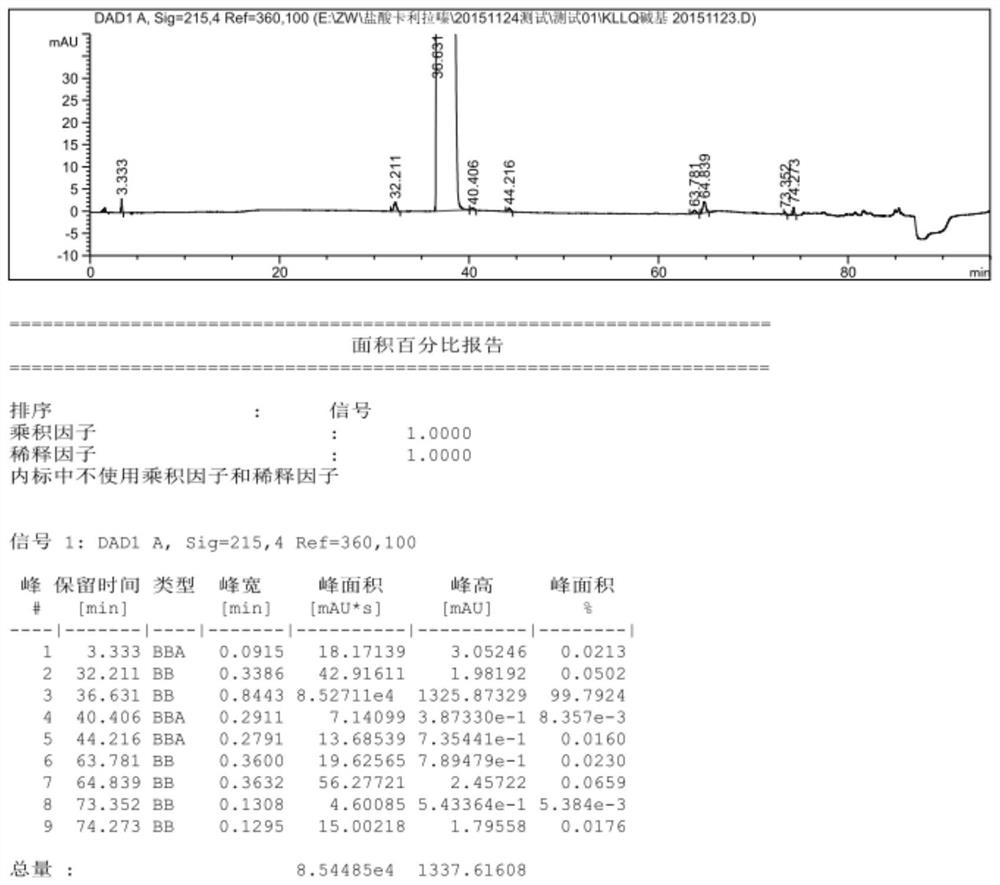
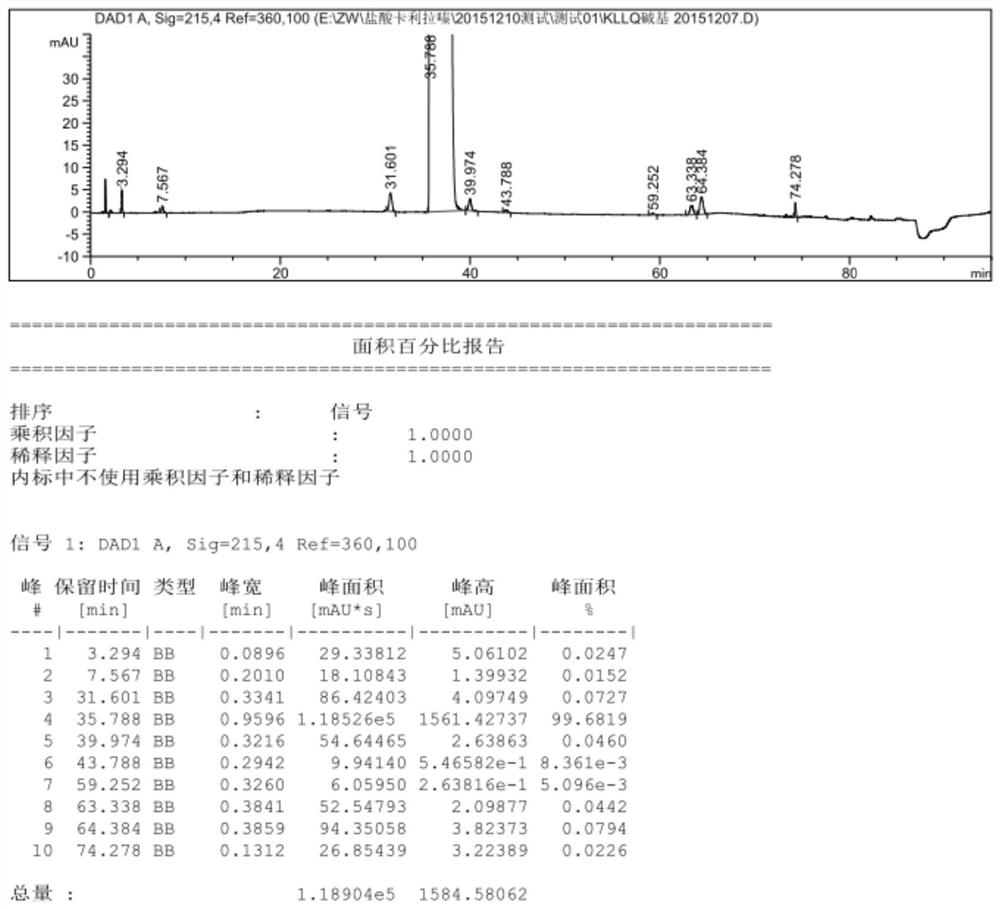
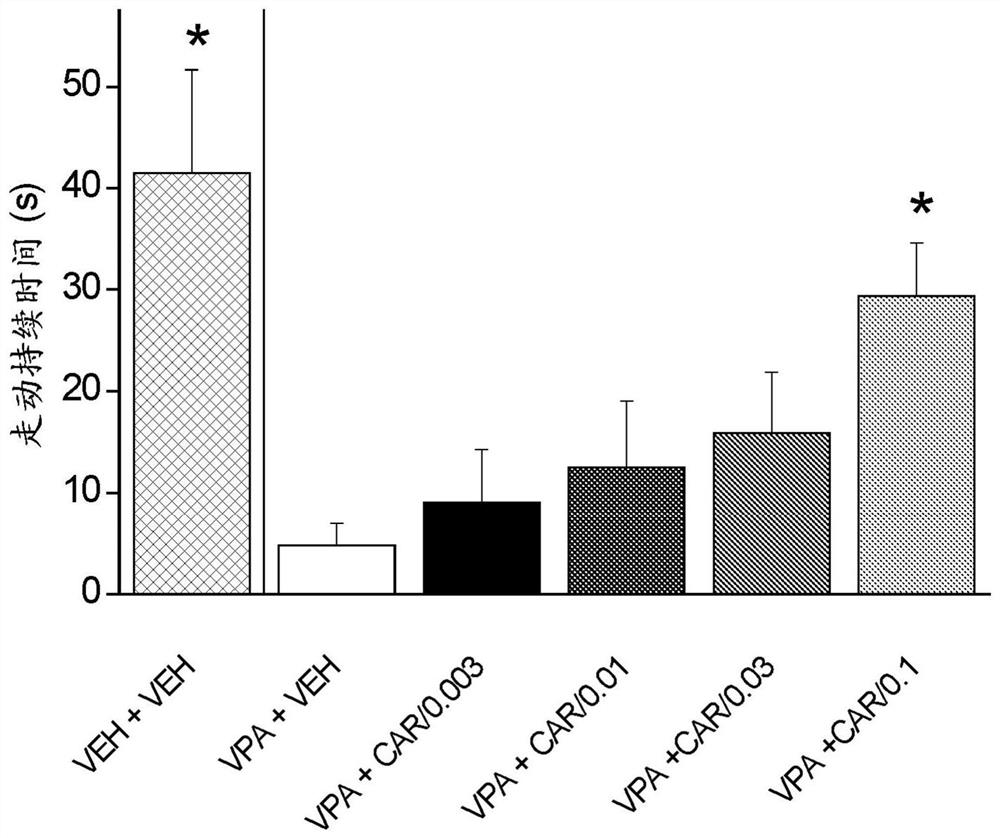
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Cariprazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
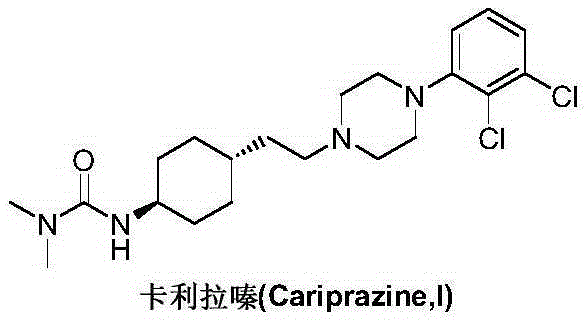
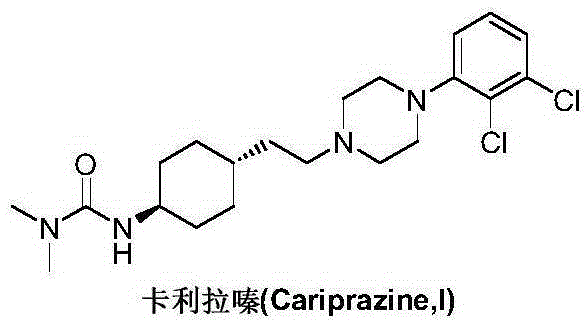
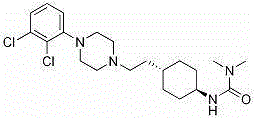
Cariprazine is used to treat certain mental/mood disorders (such as bipolar disorder, schizophrenia).
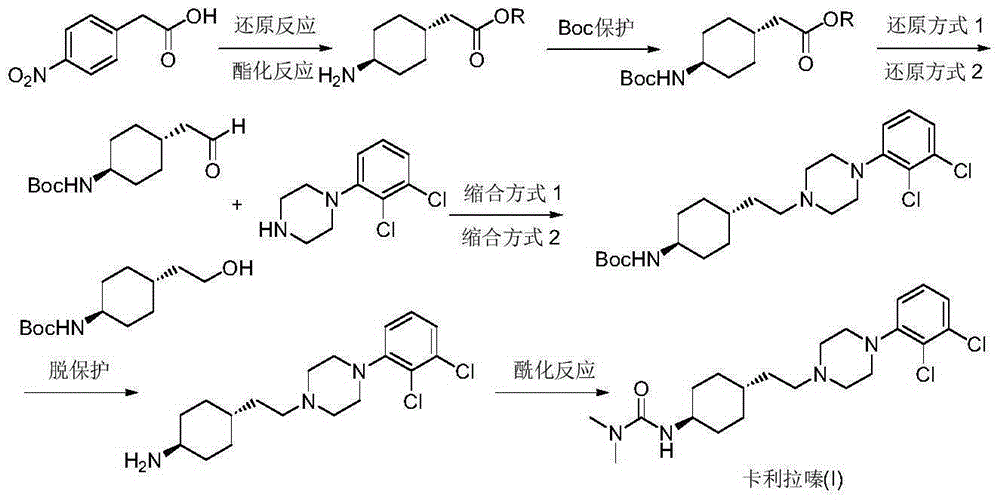
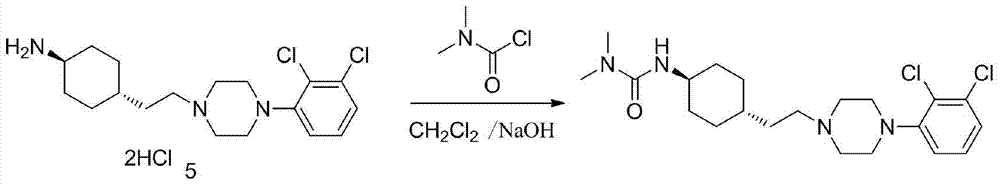
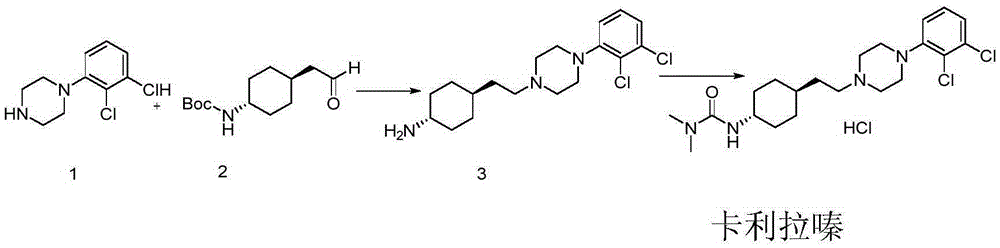
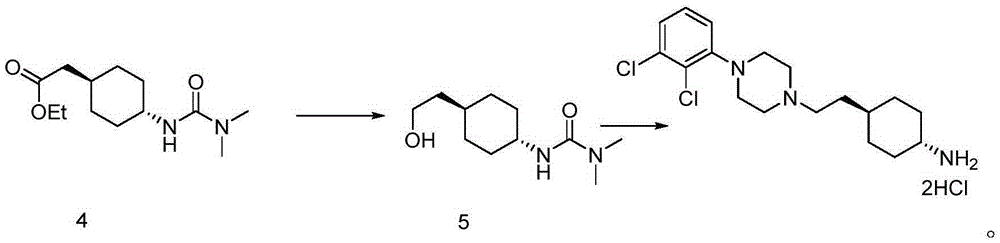
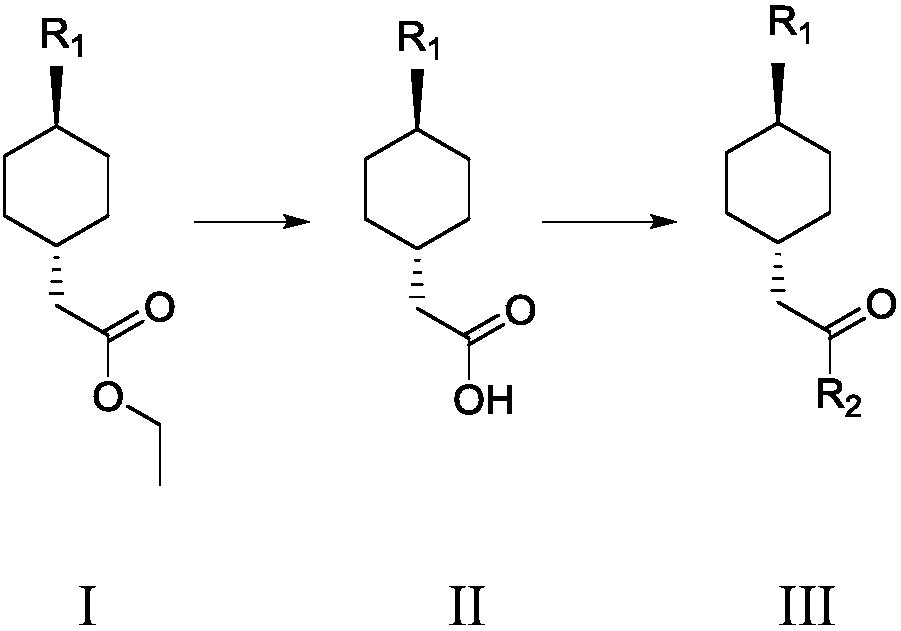
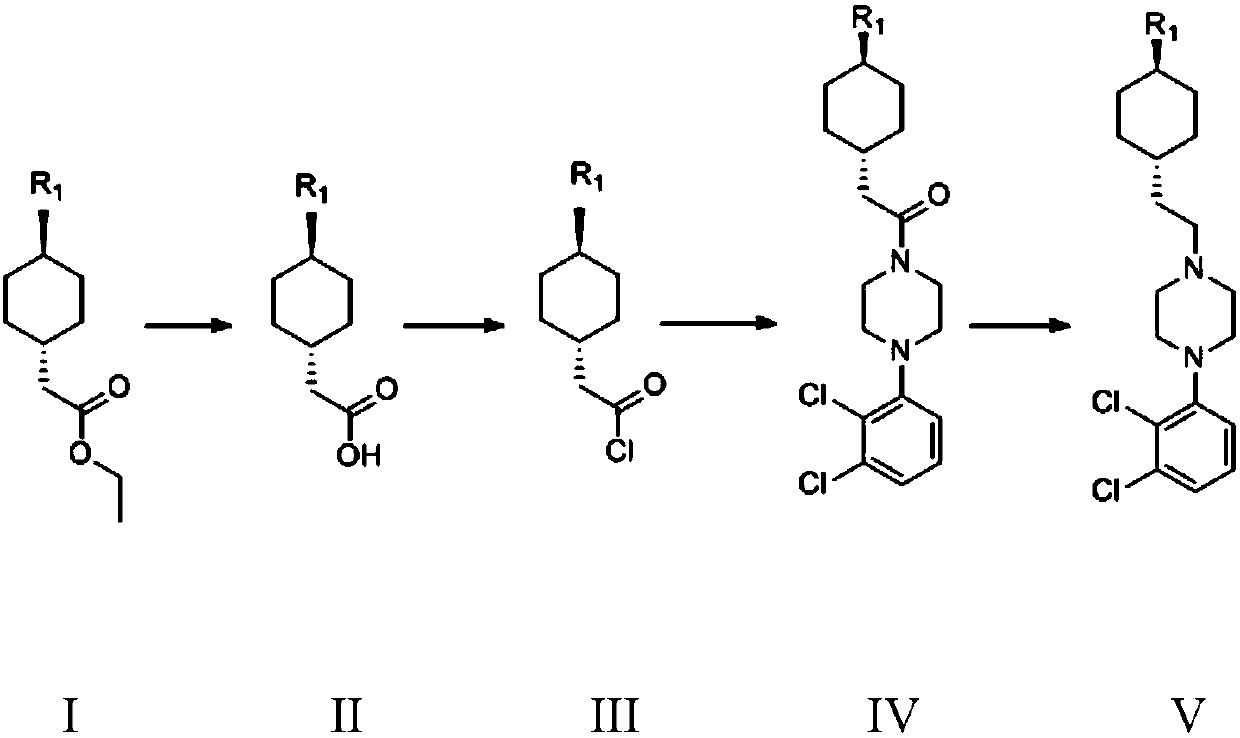
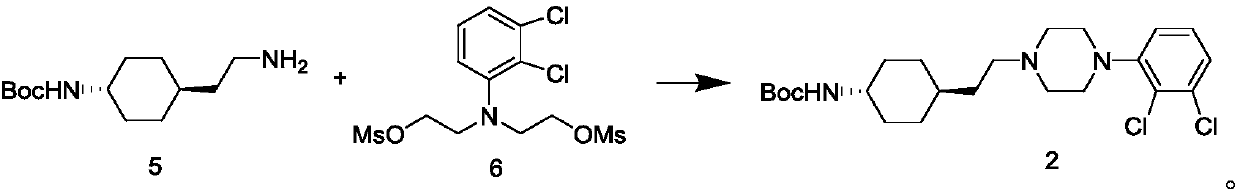
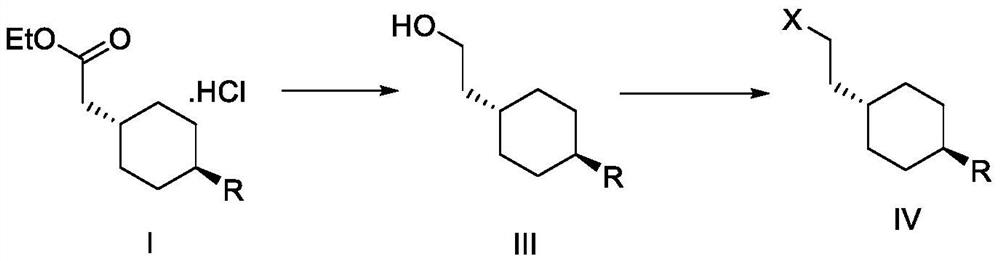
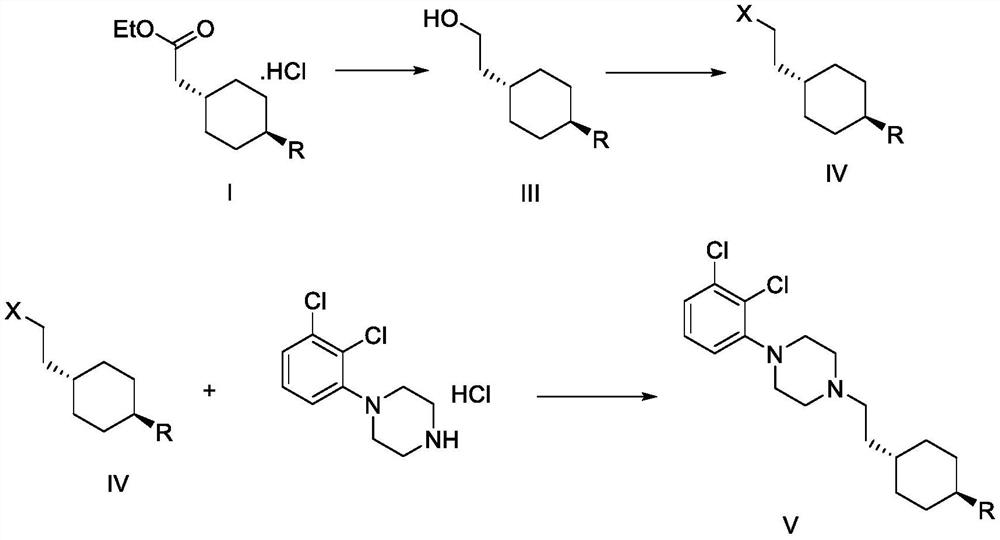
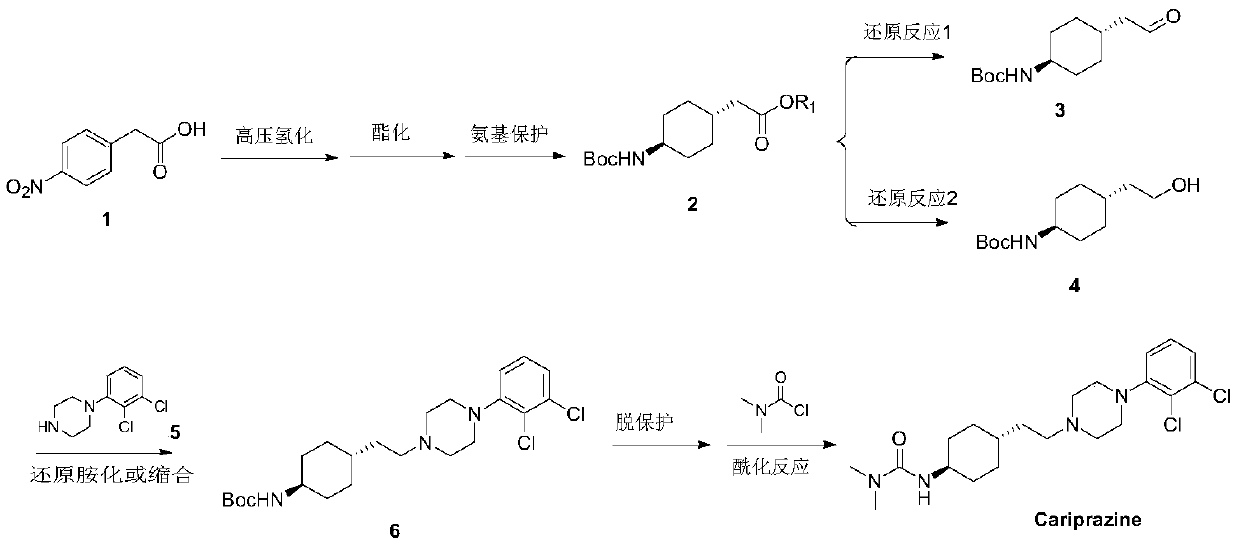
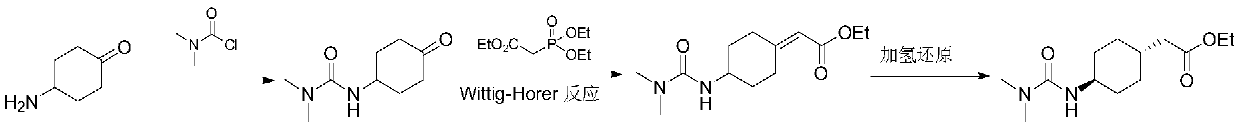
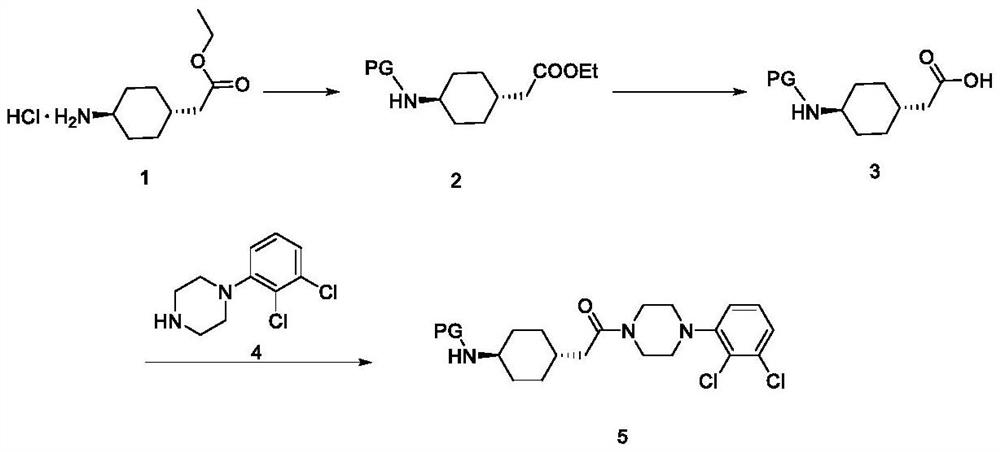
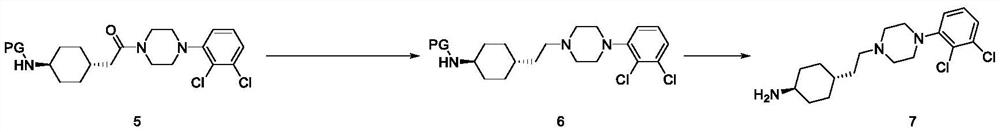
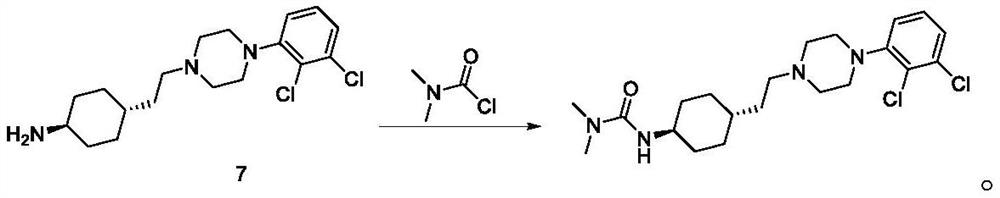
Preparation method of cariprazine
ActiveCN105330616AEase of industrial productionRaw materials are easy to getOrganic active ingredientsNervous disorderCariprazineCyclohexanone
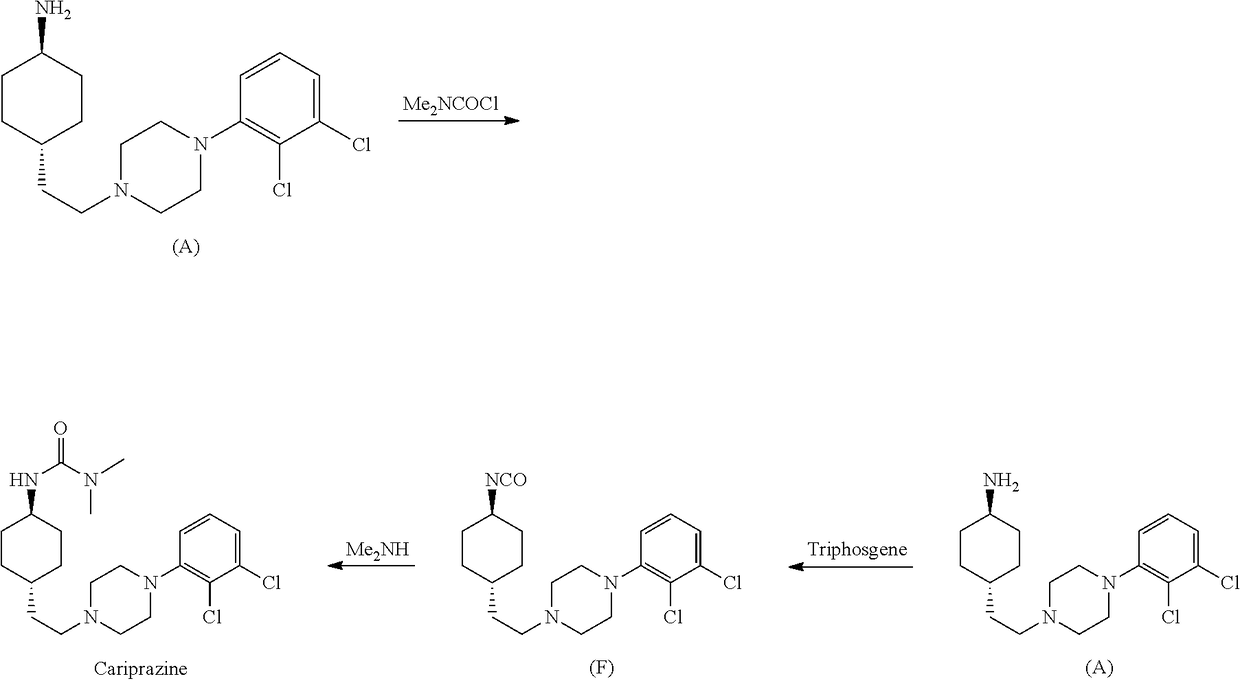
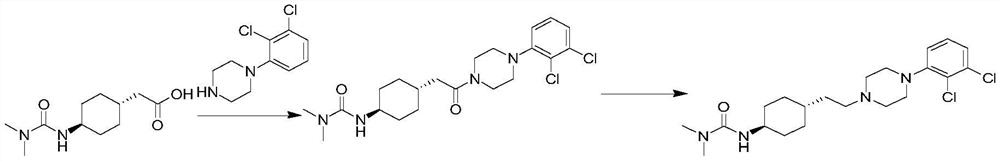
The invention discloses a preparation method of cariprazine (Cariprazine, RGH 188). The method includes the preparation steps of preparing 4-[2-[4-(2,3-dichlorophenyl)piperazine]-1-based]ethyl]cyclohexanone by making 4-(2-hydroxyethyl)cyclohexanone and 1-(2,3-dichlorophenyl)piperazine subjected to a condensation reaction, preparing trans-4-[[2-]4-(2,3-dichlorophenyl)piperazine]-1-based]ethyl]cyclohexylamine by making the obtained intermediate subjected to a reduction ammonolysis reaction, and preparing cariprazine by making the intermediate and N,N-dimethylcarbamyl chloride subjected to an acylation reaction. According to the preparation method, raw materials can be easily obtained, the process is simple, and the method is economical, environmentally friendly and suitable for industrialized production.
Owner:中科恩吉瑞特(烟台)科技发展有限公司
Cariprazine tartrate, preparation method therefor and medical use thereof
ActiveCN105218484ANot easy to absorb moistureImprove solubilityNervous disorderOrganic chemistry methodsCariprazinePharmaceutical drug
Owner:ANHUI HEALSTAR PHARM CO LTD
3-cyclohexyl-1,1-dimethylurea compound as well as preparation method and application thereof
ActiveCN104496854AAtom economy is highHigh purityUrea derivatives preparationOrganic compound preparationCariprazineDimethylurea
The invention discloses a 3-cyclohexyl-1,1-dimethylurea compound as well as a preparation method and application thereof. The 3-cyclohexyl-1,1-dimethylurea compound is used for preparing an antischizophrenic drug cariprazine. Compared with the prior art and report literatures, the preparation method of the 3-cyclohexyl-1,1-dimethylurea compound has the remarkable advantages of being free of removal of protecting groups such as Boc group, high in atom economy, low in cost and easy in getting of raw materials, mild in reaction condition, stable in yield, simple and convenient to operate, controllable in product quality, high in product purity, less in three waste pollution and easy to produce industrially. The structure formula of the 3-cyclohexyl-1,1-dimethylurea compound is as shown in (I) in the specification.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Compound for preparation of cariprazine and preparation method thereof
InactiveCN106543039AHigh purityLow cis contentCarbamic acid derivatives preparationOrganic compound preparationCariprazineState of art
The invention relates to a compound for preparation of cariprazine and a preparation method thereof. The method can overcome the defects in the prior art, the used raw materials and reagents are low toxic, cheap and easily available, the reaction conditions are mild, fewer three wastes are generated, at the same time, the operation is simple and safe, and the yield is good, therefore the method is suitable for industrialized production.
Owner:NHWA PHARMA CORPORATION
Preparation method for trans 4-amino-cyclohexyl acetate derivative
ActiveCN106565510AHigh purityGood effectOrganic compound preparationAmino-carboxyl compound preparationCariprazineCyclohexanone
The invention provides a preparation method for a trans 4-amino-cyclohexyl acetate derivative. The preparation method comprises the following steps that a formula (please see the description for the formula) is provided, wherein R is methyl or ethyl; a compound III is prepared from the raw materials of 4-amino cyclohexanone II and is subjected to hydrogenation reduction, a compound I crude product is obtained, acid is added, and salt is formed; and a compound I is prepared by adding alkali. According to the preparation method, 4-amino cyclohexanone not protected by amino is adopted as raw materials, witting reaction is conducted, and then through catalytic hydrogenation, the trans crude product of the preparation method is obtained. The crude product can be subjected to salifying crystallization so as to obtain the purer trans product, and the trans:syn ratio is 95-99.9:5-0.1. The product compound is high in purity and can be used for preparing cariprazine. Operation is simple, the raw materials are easy to obtain, industrial production is suitable, and large application value is achieved.
Owner:ZHEJIANG JINGXIN PHARMA +2
Novel method for synthesizing cariprazine
ActiveCN108586389AAvoid re-esterificationSteps to Avoid Reductive RehalogenationOrganic chemistryCariprazineAcetic acid
The invention belongs to the technical field of organic synthesis, and provides a novel method for synthesizing cariprazine. The novel method comprises the following steps: firstly, carrying out condensation reaction on trans-2-(4-(3,3-dimethyl ureido) cyclohexyl) acetic acid and 1-(2,3-dichlorophenyl) piperazine to obtain 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-2-oxo-ethyl}-cyclohexyl)-1,1-dimethylurea; and secondly, reducing 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-2-oxo-ethyl}-cyclohexyl)-1,1-dimethylurea by using borane to obtain the cariprazine. The method hasthe advantages that process steps are greatly shortened, the purity of a final product is ensured and the total yield is obviously increased.
Owner:成都福柯斯医药技术有限公司
Solid preparation of cariprazine for oral administration
The invention relates oral phamiaceuticai compositions for the modified release delivery of cariprazine (trans-N-{4-[2-[4-(2,3-dichlorophenyl)-piperazin-l-yl]-ethyl]-cyclohexyl}-N',N'- dimethylurea) or pharmaceutically acceptable salts thereof for less than daily dosing. The invention also relates to the use of said compositions in the treatment and / or prevention of pathological conditions which require the modulation of dopamine receptors. The invention also relates to the process for the preparation of said modified release pharmaceutical compositions.
Owner:RICHTER GEDEON NYRT
1,4-cyclohexylamine derivatives and processes for the preparation thereof
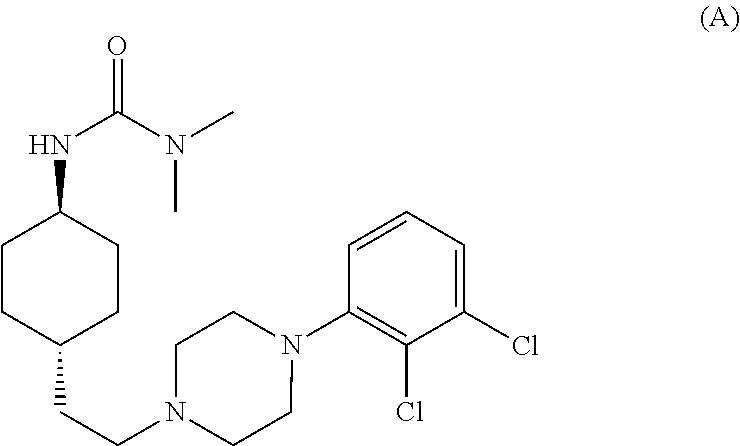
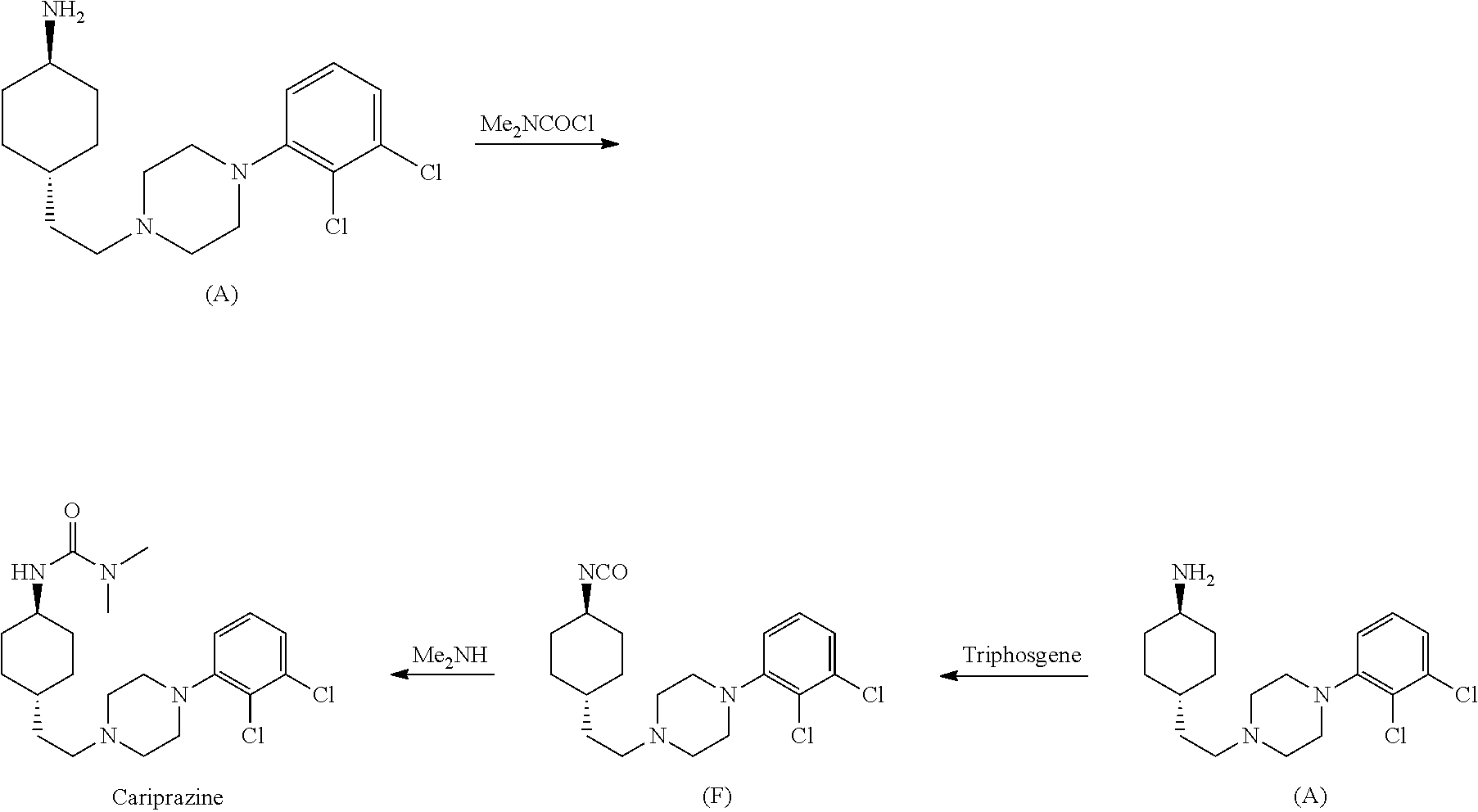
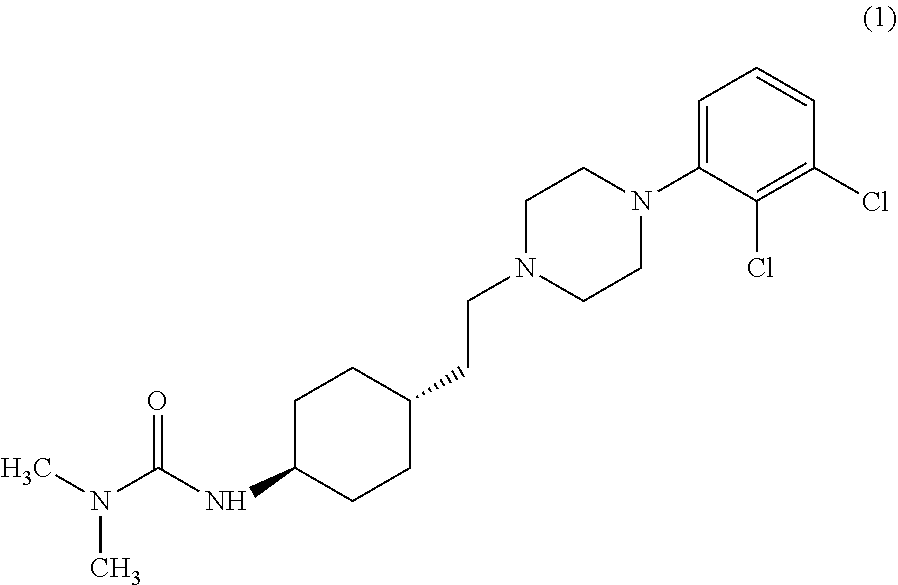
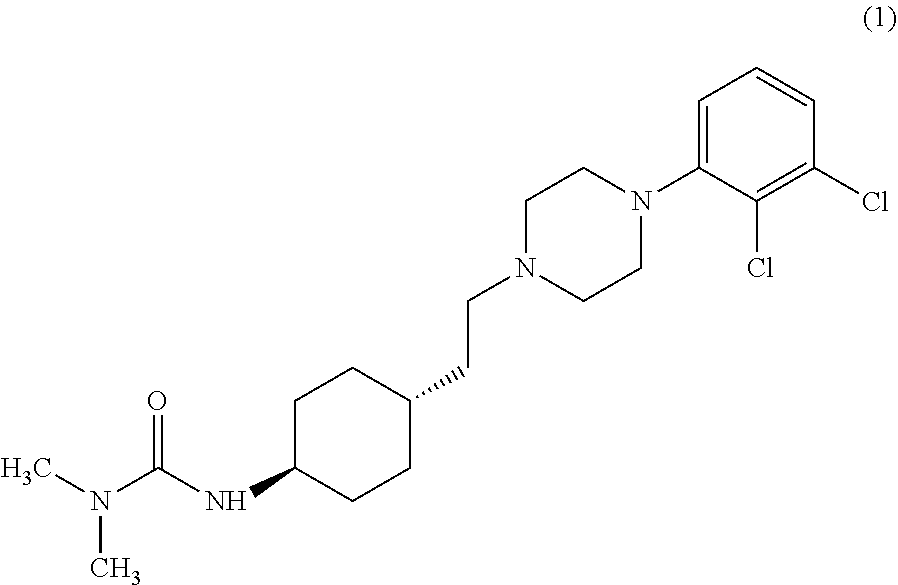
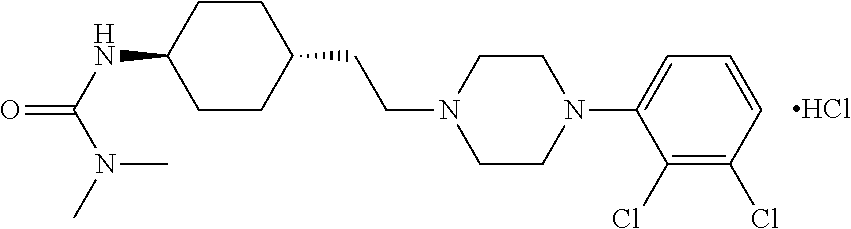
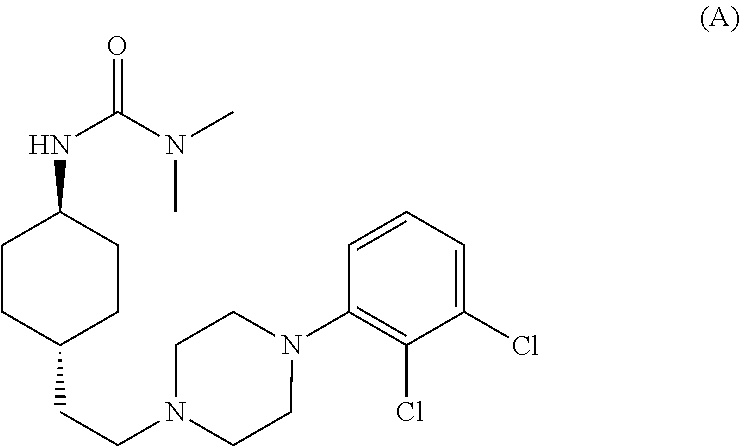
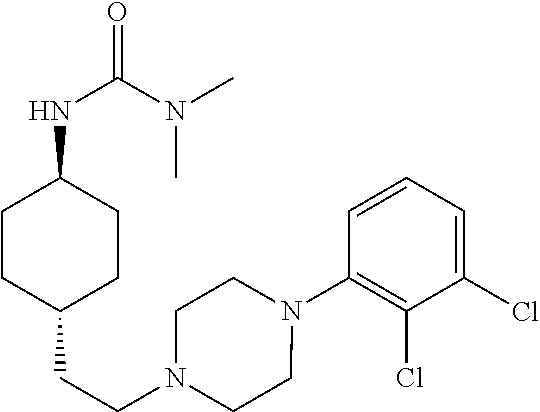
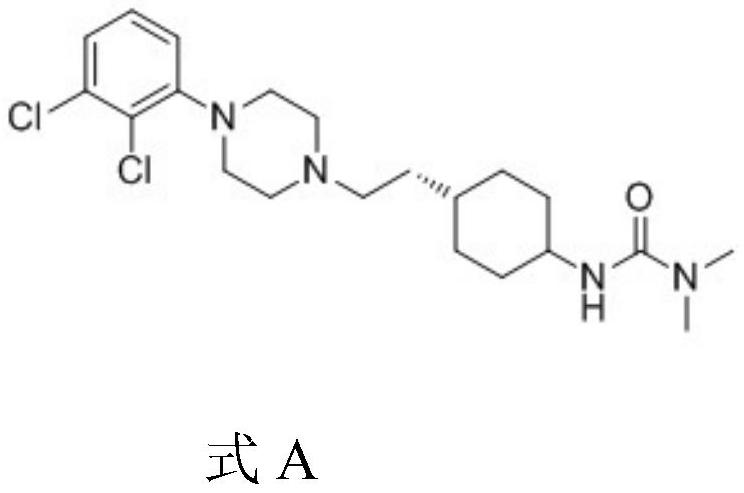
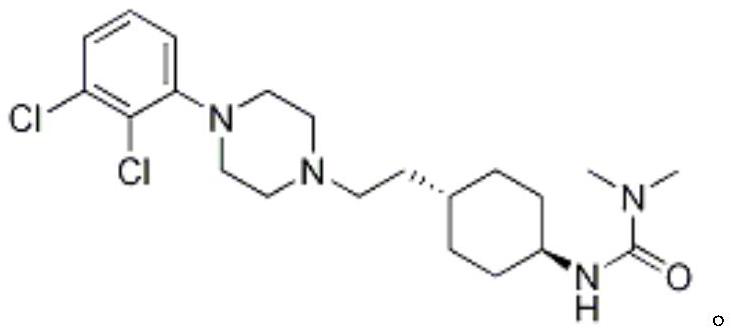
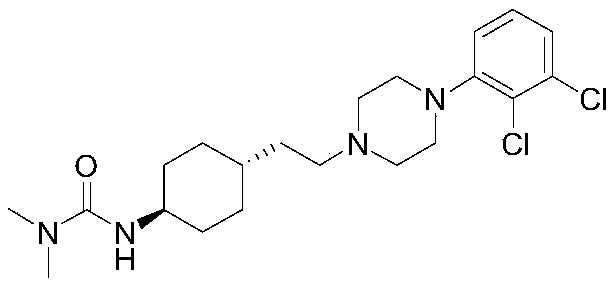
The invention relates to a process for the synthesis of Cariprazine, an antipsychotic compound useful in the treatment of positive and negative symptoms associated to schizophrenia, with the following structural formula: (A) The invention further relates to the synthesis of intermediates useful in the preparation of Cariprazine.
Owner:CHEMO RES
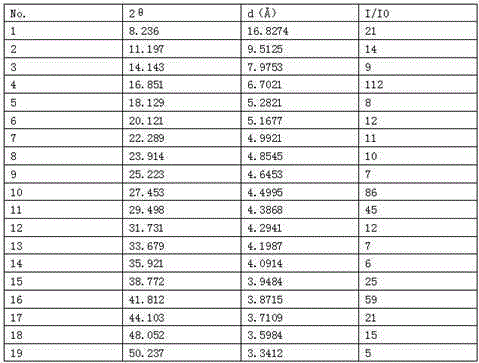
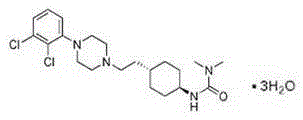
Cariprazine trihydrate compound
InactiveCN106699689AHigh purityImprove stabilityOrganic active ingredientsNervous disorderCariprazineHigh humidity
The invention belongs to the technical field of medicines and particularly relates to a cariprazine trihydrate compound. The obtained cariprazine trihydrate compound contains three crystallization waters and has the advantages as follows: the purity is high, the stability is good and moisture-absorption weight gain is not remarkable under the high-humidity condition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Industrial process for the preparation of cariprazine
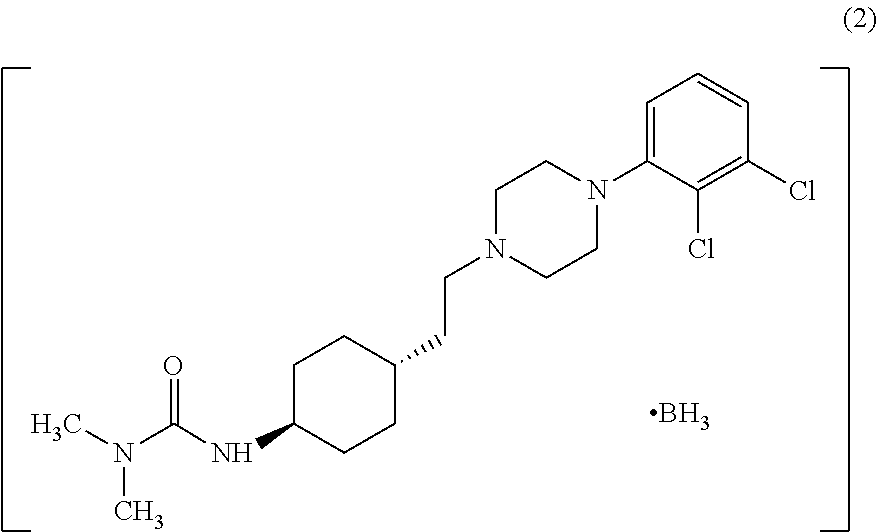
In the process of the present invention, cariprazine is prepared by converting (trans-4-amino-cyclohexyl)-acetic acid ethyl ester hydrochloride to trans-4-aminocyclohexyl) acetic acid or its hydrochloride by hydrolysis, from the obtained product with addition of dimethylcarbamoyl derivative as a suitable reagent (trans-4-{[(dimethylamino)carbonyl]amino}cyclohexyl) acetic acid is formed, then the obtained compound is linked to 1-{2,3-dichlorophenyl)˜piperazine in the presence of carboxylic acid activating coupling reagent, and so 1,1-dimethyl-3-[trans-4-(2-oxo-2-(4-(2,3-dichlorophenyl)piperazin-1-yl-ethyl)cyclohexyl] urea is formed, which is converted to cariprazine borane adduct of formula (2) in the presence of reducing agent, and finally the product itself is eliminated directly or is obtained from its salt by a known method. The invention also relates to a group of intermediate compounds that are formed and / or used in the process according to the present invention.
Owner:RICHTER GEDEON NYRT
Solid preparation of cariprazine for oral administration
InactiveUS20200222391A1Improved profileOrganic active ingredientsNervous disorderCariprazineOral medication
The invention relates oral pharmaceutical compositions for the modified release delivery of cariprazine (trans-N-{4[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-N′,N′-dimethylurea) or pharmaceutically acceptable salts thereof for less than daily dosing. The invention also relates to the use of said compositions in the treatment and / or prevention of pathological conditions which require the modulation of dopamine receptors. The invention also relates to the process for the preparation of said modified release pharmaceutical compositions.
Owner:RICHTER GEDEON NYRT
Carbamoyl cyclohexane derivatives for treating autism spectrum disorder
The present invention relates to trans-N-[4-[2-[4-(2,3-dichlorophenyl)piperazin-1-yl]ethyl]cyclohexyl]-N′,N′-dimethylurea (cariprazine), its salts, close analogs, derivatives, pharmaceutical compositions, metabolites and combinations for use in the treatment of symptoms of autism spectrum disorder in general, and preferably the object of the present invention is to treat one or more symptoms of autism.Furthermore, it was also found that cariprazine, its salts, close analogs, derivatives, pharmaceutical compositions, metabolites and combinations are suitable for treatment of conditions such as Asperger's syndrome, atypical autism (otherwise known as pervasive developmental disorder not otherwise specified; PDD-NOS), Rett syndrome, childhood disintegrative disorder, attention deficit hyperactivity disorder (ADHD) and sensory integration dysfunction.
Owner:RICHTER GEDEON NYRT
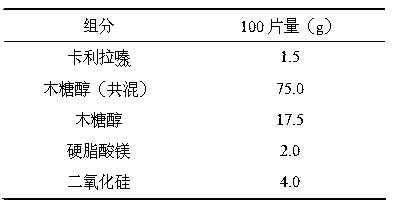
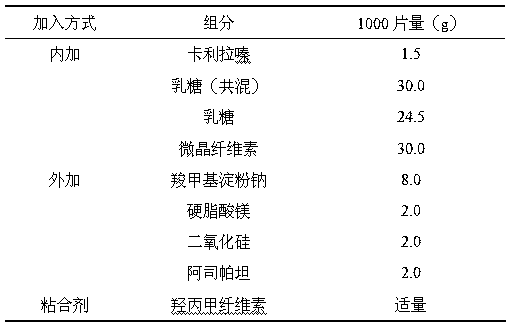
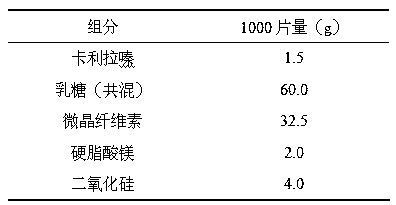
Orally disintegrating tablet of cariprazine and preparation method thereof
InactiveCN107970217ADisintegrates quicklyHigh hardnessOrganic active ingredientsNervous disorderCariprazineMedicine
The invention discloses an orally disintegrating tablet of cariprazine and a preparation method thereof. The orally disintegrating tablet of cariprazine comprises, by mass, 0.8 to 3.6% of cariprazinehydrochloride, 0 to 2.0% of a binder, 16 to 48% of a disintegrating agent, a diluent and a lubricant. The orally disintegrating tablet of cariprazine can be rapidly disintegrated and dissolved out inthe oral cavity, has good hardness and wear resistance, and can improve the compliance of patients, mitigate the burden of nursing people, effectively prevent psychopaths from hiding and spitting cariprazine and improve treatment effect. The preparation method for the orally disintegrating tablet of cariprazine is simple, can carry out production in conventional workshops for oral solid preparations, has low production cost and is capable of realizing large-scale industrial production.
Owner:ZHEJIANG JINGXIN PHARMA +1
Stable cariprazine formulations for oral use
ActiveUS20200155546A1Easy to fixGood bioavailablePowder deliveryOrganic active ingredientsCariprazinePharmaceutical medicine
The present invention relates to stabilized pharmaceutical formulations comprising therapeutically effective amount of Cariprazine premix / solid dispersion, or its pharmaceutically acceptable salts, esters, hydrates and solvates thereof, at least one diluent which is not having low water activity and optionally one or more excipients selected from lubricant, disintegrant and buffering agent or combinations thereof. The present invention further relates to process for preparation and method of using such stable formulations comprising Cariprazine.
Owner:AUROBINDO PHARMA LTD
Synthesis method for cariprazine
ActiveUS20210300883A1Short reaction timeSimple post-processingBulk chemical productionAmide group formation/introductionCariprazineCombinatorial chemistry
The present application relates to a synthesis method for cariprazine, comprising performing an acylation reaction between a compound represented by formula (I) and dimethylcarbamoyl chloride in a reaction solvent in the presence of an aqueous solution of an inorganic base, so as to obtain the cariprazine compound represented by formula (II). The synthesis method overcomes defects in the prior art such as a long reaction time, large size impurities and the difficulty of purification, and provides a new method suitable for commercial production wherein the reaction is fast, impurity sizes are small, the product is easily purified, the purity of the product can reach 99.0% or more, and the yield is high.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Preparation method of cariprazine
The invention provides a preparation method of cariprazine. The preparation method of the cariprazine includes that a trans-2-(trans-4-(3, 3-dimethylureido) cyclohexyl) derivative is enabled to reactwith 1-(2, 3-dichlorophenyl) piperazine or salt thereof in an acid-binding agent reaction condition, and then the cariprazine is generated in a reducing agent reaction condition. The preparation method of the cariprazine is few in a synthetic route, simple in technology and conformable to production requirements.
Owner:SHANGYU JINGXIN PHARMA +2
Preparation method of cariprazine
ActiveCN111269199AHigh yieldHigh purityOrganic chemistryCariprazineTert-Butyloxycarbonyl protecting group
The invention provides a preparation method of cariprazine, which comprises the following steps: reacting trans-N-tert-butyloxycarbonyl-4-(2-(4-(2,3-dichlorophenyl)-piperazine-1-yl)-ethyl)-cyclohexylamine with dimethylamine under the conditions of an organic solvent, an acid-binding agent and an additive to obtain the cariprazine. The preparation method of cariprazine provided by the invention hasthe advantages of short reaction time, high reaction yield, few byproducts, simple post-treatment and suitability for industrial production, and overcomes the problems of difficult solvent recovery,environmental pollution, complex operation and the like in the prior art.
Owner:ZHEJIANG JINGXIN PHARMA +2
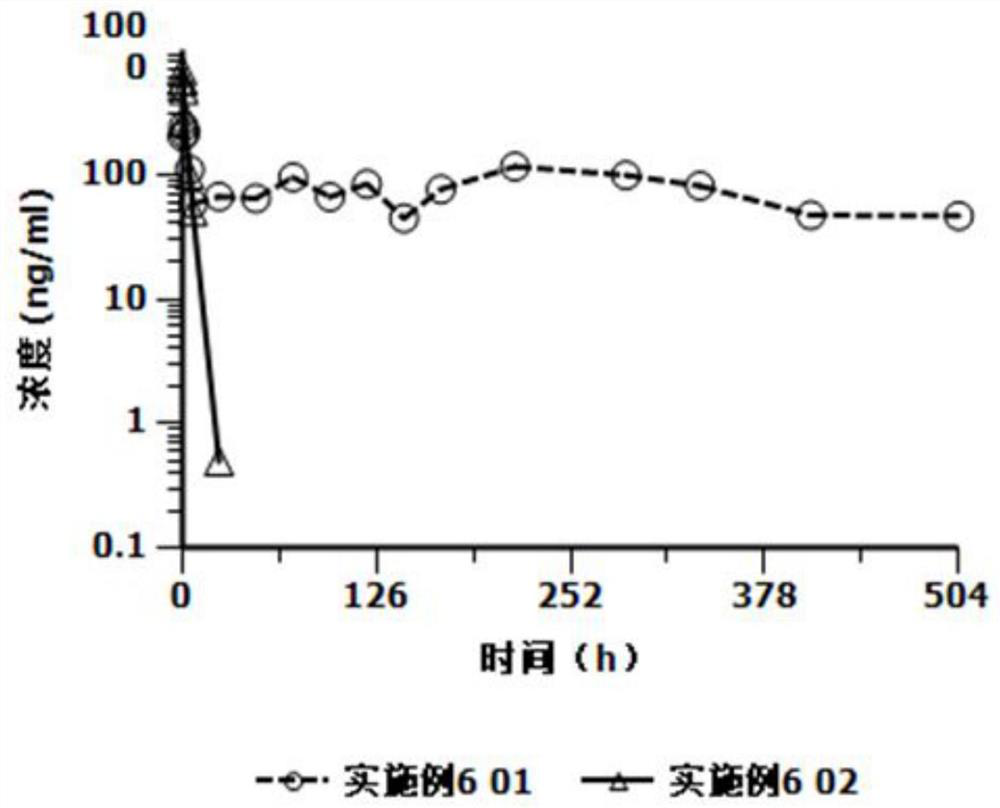
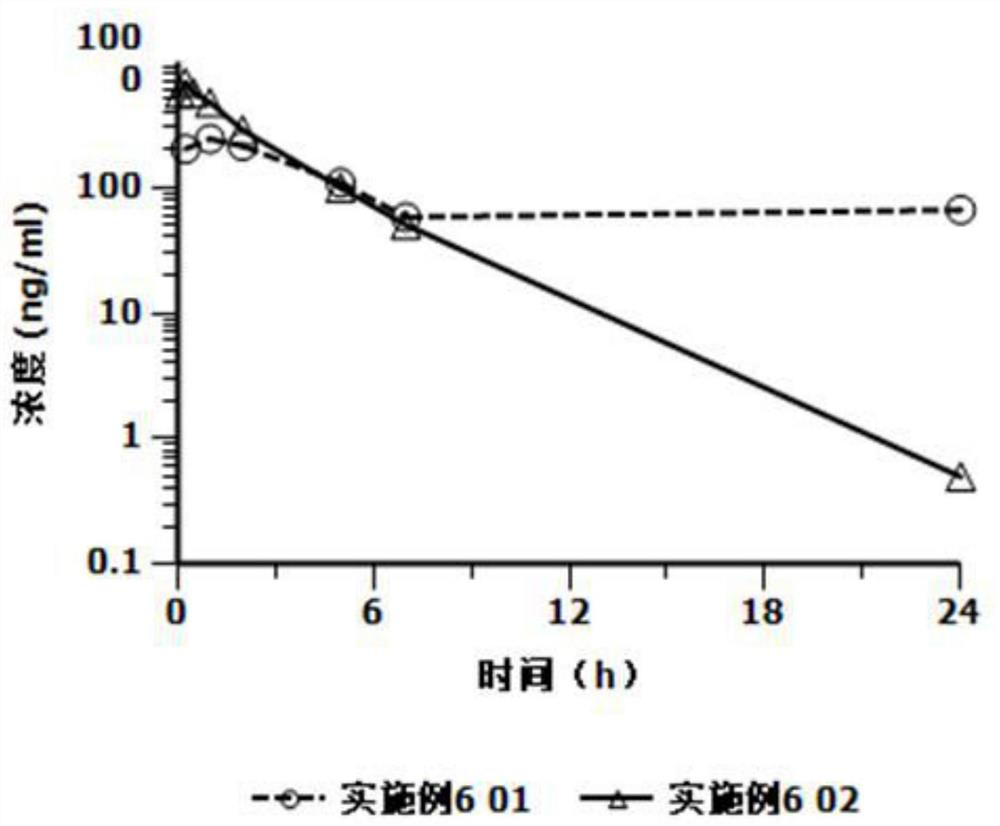
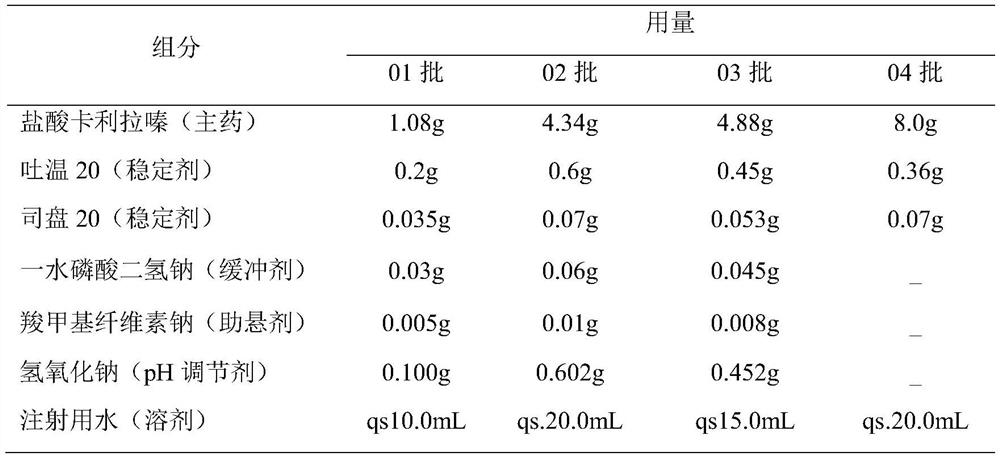
A kind of cariprazine hydrochloride injection preparation and its preparation method and application
ActiveCN108261394BImprove drug stabilitySmall batch-to-batch variancePowder deliveryOrganic active ingredientsCariprazineDosage adjustment
The invention provides a cariprazine hydrochloride injection preparation and its preparation method and application. When the injection preparation is used, it is a suspension aqueous solution, wherein the concentration of cariprazine hydrochloride is relatively high, and the stability is good, and a higher dosage can be obtained in a limited injection volume, and it can also be used according to the long-acting administration time. The dose can be flexibly adjusted, and by controlling the particle size distribution and the injection dose, a long-acting effect can be achieved. After injection, the preparation can release cariprazine continuously for at least 1 week, and can be administered once a week or more. Long dosing intervals to increase patient compliance. The invention also provides a preparation method of the cariprazine hydrochloride injection preparation, the injection preparation prepared by the method has good stability and high safety, the method is simple and easy, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of cariprazine-hydrophilic material blend solid preparation
The invention discloses a preparation method of a cariprazine-hydrophilic material blend solid preparation. The solid preparation comprises a cariprazine-hydrophilic material blend and other pharmaceutically acceptable ingredients, wherein the cariprazine-hydrophilic material blend effectively improves the dissolution speed of cariprazine in the solid preparation, is favorable for improving the bioavailability of a drug, and is used to treat a patient with schizophrenia and diphasic I type related psychlampsia or mixed episode, and the process is simple and convenient and is suitable for industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
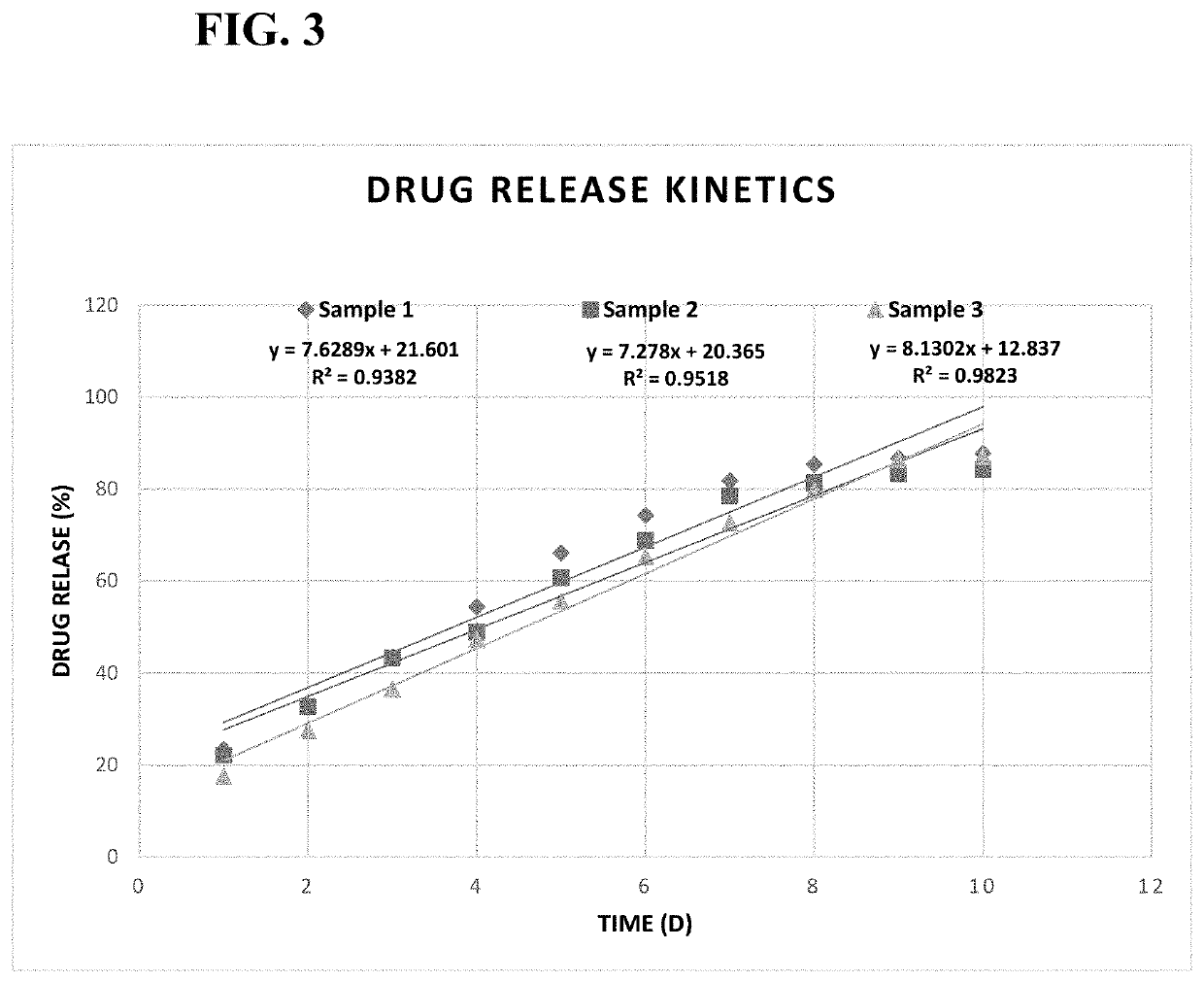
Cariprazine release formulations
ActiveUS20210177768A1Patient compliance is goodLong durationPowder deliveryOrganic active ingredientsCariprazineDisease
The patent discloses long-term injectable formulations and delivery systems of cariprazine and related salts and derivatives in the prevention and treatment of various psychotic diseases, such as schizophrenia, mania, and bipolar disorder. The dosage forms are either microsphere, microparticle, nanoparticle drug delivery systems in a pharmaceutically acceptable carrier, or devices that contain long-term injectable formulation of such cariprazine and related salts and derivatives.
Owner:HALO SCI LLC
Method for preparing cariprazine and intermediate thereof
PendingCN114262283AReaction raw materials are readily availableEasy to operateCarbamic acid derivatives preparationOrganic compound preparationCariprazineNucleophilic substitution
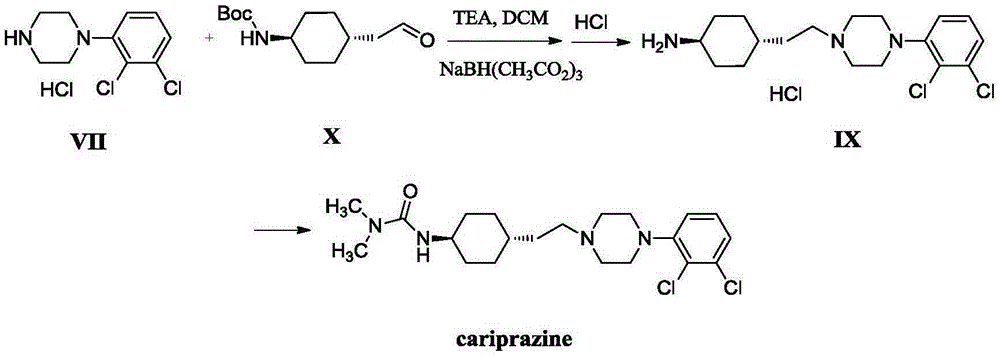
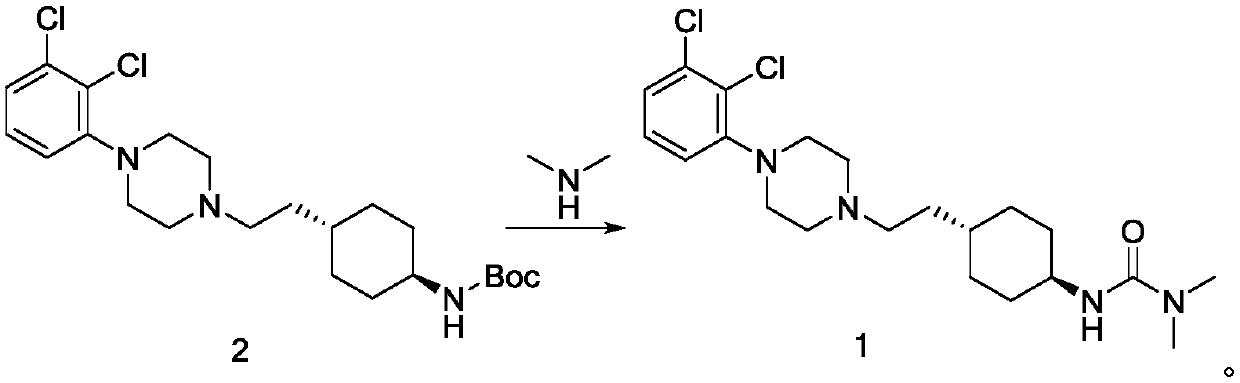
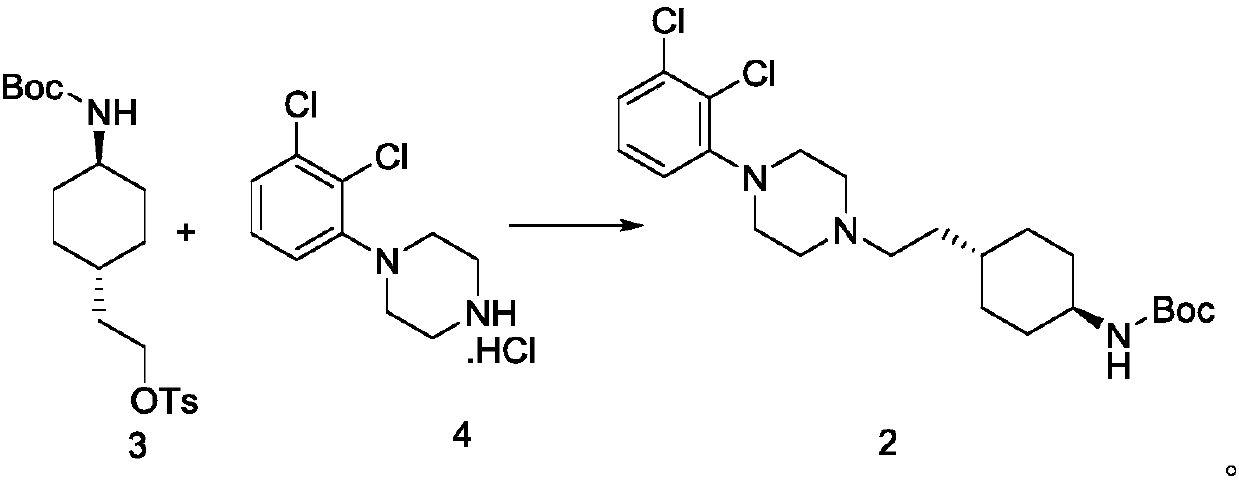
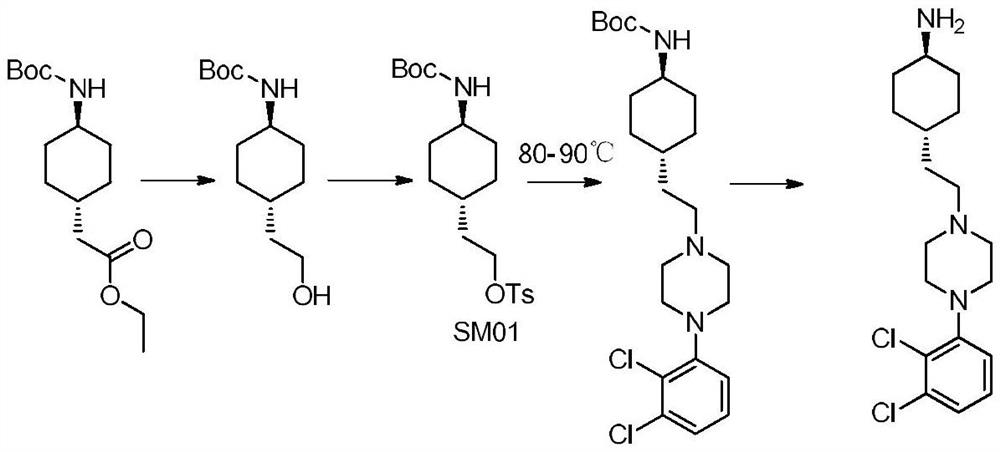
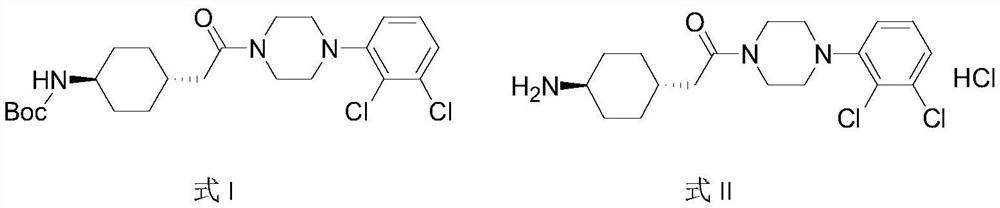
The invention relates to a method for preparing cariprazine and an intermediate thereof, and the method comprises the following steps: taking trans-4-aminocyclohexane acetate hydrochloride as a starting material, and carrying out Boc protection, reduction, halogenation, nucleophilic substitution, Boc protection removal and amidation reaction to prepare the cariprazine. The alcoholic hydroxyl group of the trans-2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl} ethanol is activated and converted into a group easy to leave, the alcoholic hydroxyl group is creatively converted into the halogen atom, the reaction condition is mild, the yield is high, the raw material medicine which is high in purity and low in impurity content and meets the medicinal requirement can be obtained, and the method is suitable for industrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
Cariprazine pharmaceutical salt and crystal form, preparation method and application thereof
ActiveCN114195740AReduce solubilityGood crystal stabilityOrganic active ingredientsPowder deliveryCariprazineOrganic acid
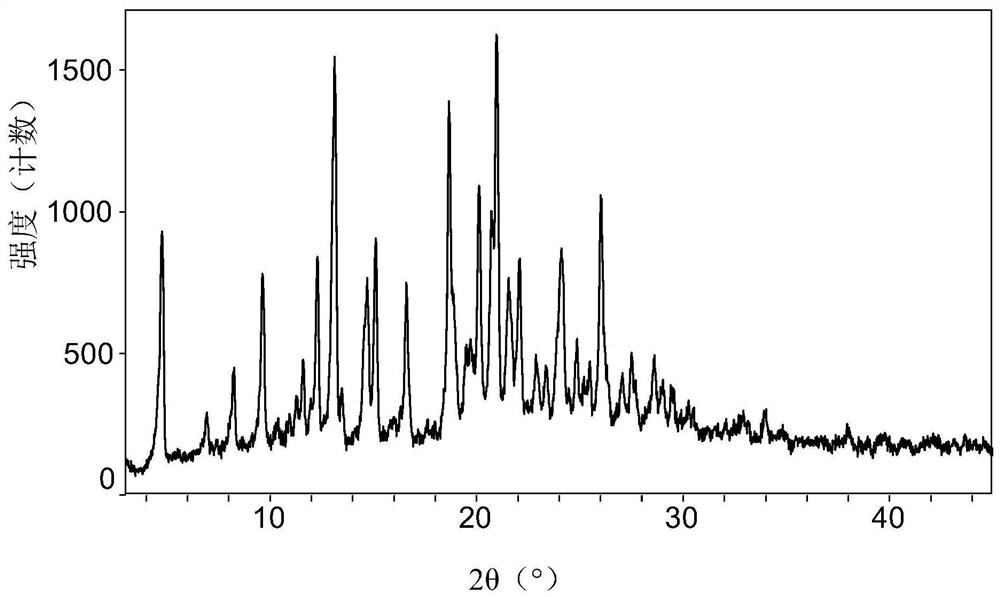
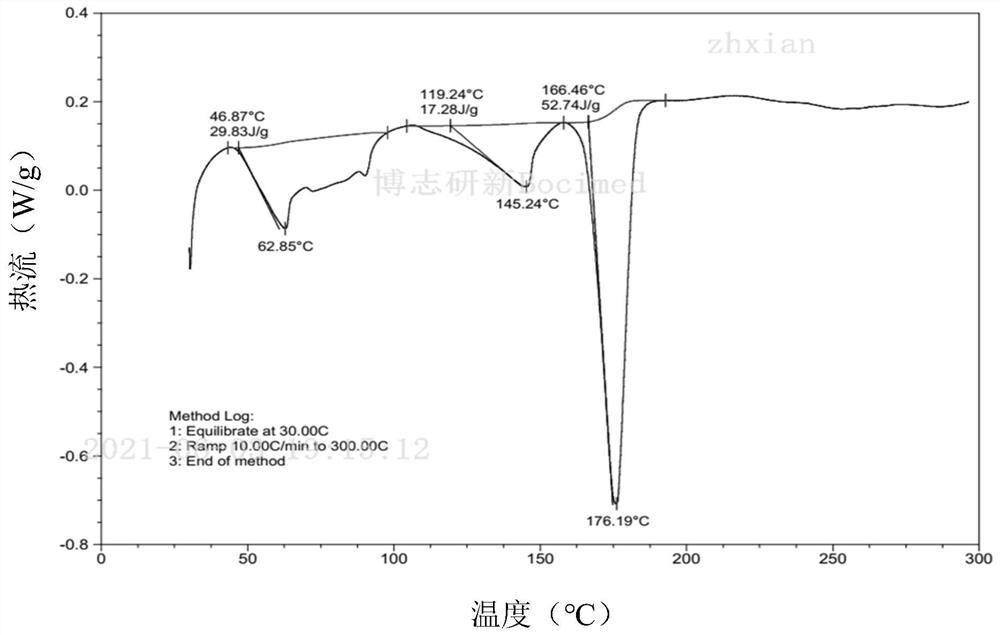
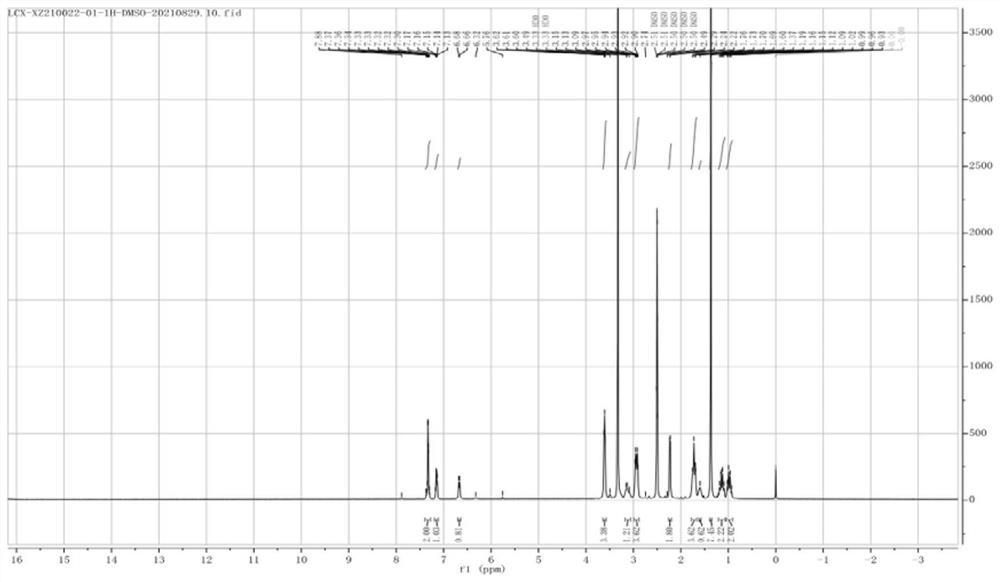
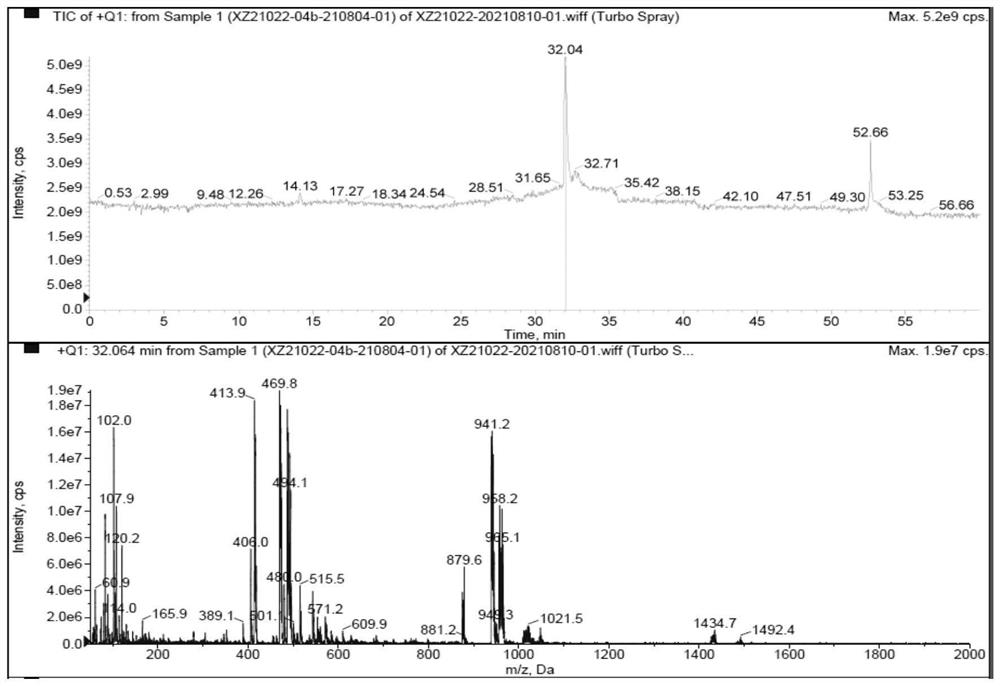
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Preparation method of cariprazine and intermediate compound
The invention provides a preparation method of cariprazine and an intermediate compound. The preparation method disclosed by the invention is simple in process, high-temperature and high-pressure conditions are not needed, a precious catalyst is not used, the cost is saved, and the prepared cariprazine is high in yield and purity.
Owner:四川奥邦古得药业有限公司
A kind of refining method of high-purity cariprazine
ActiveCN111320593BSolution is not easy to removeEfficient removalOrganic chemistry methodsCariprazineAlcohol
Owner:NHWA PHARMA CORPORATION
1,4-cyclohexylamine derivatives and processes for the preparation thereof
The invention relates to a process for the synthesis of Cariprazine, an antipsychotic compound useful in the treatment of positive and negative symptoms associated to schizophrenia, with the following structural formula: (A) The invention further relates to the synthesis of intermediates useful in the preparation of Cariprazine.
Owner:CHEMO RES
Carbamoyl cyclohexane derivatives for treating autism spectrum disorder
The present invention relates to trans-N-[4-[2-[4-(2,3-dichlorophenyl)piperazin-1-yl]ethyl]cyclohexyl]-N',N'-dimethylurea (cariprazine), its salts, close analogs, derivatives, pharmaceutical compositions, metabolites and combinations for use in the treatment of symptoms of autism spectrum disorder in general, and preferably the object of the present invention is to treat one or more symptoms of autism. Furthermore, it was also found that cariprazine, its salts, close analogs, derivatives, pharmaceutical compositions, metabolites and combinations are suitable for treatment of conditions such as Asperger's syndrome, atypical autism (otherwise known as pervasive developmental disorder not otherwise specified; PDD-NOS), Rett syndrome, childhood disintegrative disorder, attention deficit hyperactivity disorder (ADHD) and sensory integration dysfunction.
Owner:RICHTER GEDEON NYRT
A kind of preparation method of cariprazine
The invention provides a kind of preparation method of cariprazine, it comprises the steps: trans-2-(trans-4-(3,3-dimethylureido) cyclohexyl) derivative reacts in acid-binding agent Reaction with 1‑(2,3‑dichlorophenyl)piperazine or its salts under conditions, followed by reaction with reducing agent to generate cariprazine. The synthesis route of cariprazine of the present invention has few steps, simple process and meets production requirements.
Owner:SHANGYU JINGXIN PHARMA +2
Preparation method of cariprazine
PendingCN113527227AShort reaction timeHigh purityOrganic chemistryCariprazineBiochemical engineering
The invention relates to a preparation method of cariprazine. The invention provides a novel intermediate of cariprazine and a method for preparing cariprazine from the intermediate. According to the method, use of a genotoxic impurity dimethyl carbamoyl chloride is avoided, so that the safety is good, the product yield and purity are ideal, and the method is suitable for industrial production.
Owner:四川弘远药业有限公司
A kind of method of synthesizing cariprazine
ActiveCN108586389BAvoid re-esterificationSteps to Avoid Reductive RehalogenationOrganic chemistryCariprazineAcetic acid
The invention belongs to the technical field of organic synthesis, and provides a novel method for synthesizing cariprazine. The novel method comprises the following steps: firstly, carrying out condensation reaction on trans-2-(4-(3,3-dimethyl ureido) cyclohexyl) acetic acid and 1-(2,3-dichlorophenyl) piperazine to obtain 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-2-oxo-ethyl}-cyclohexyl)-1,1-dimethylurea; and secondly, reducing 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazine-1-yl]-2-oxo-ethyl}-cyclohexyl)-1,1-dimethylurea by using borane to obtain the cariprazine. The method hasthe advantages that process steps are greatly shortened, the purity of a final product is ensured and the total yield is obviously increased.
Owner:成都福柯斯医药技术有限公司
Preparation method of cariprazine and intermediate thereof
PendingCN114539185AEasy to purifyEffective quality controlOrganic chemistryBulk chemical productionCariprazineOrganic solvent
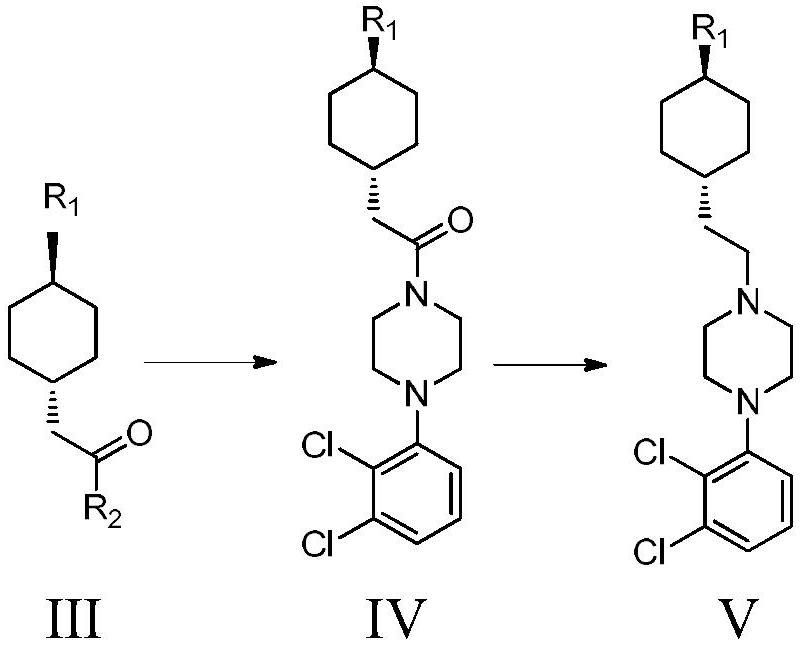
The invention provides a preparation method of cariprazine and an intermediate thereof. The invention discloses a preparation method of a cariprazine intermediate 5, which comprises the following steps: a) carrying out condensation reaction on 2-[1-(4-amino]-cyclohexyl)-ethyl acetate hydrochloride 1 and an amino protection reagent in a solvent in the presence of alkali to obtain a cariprazine intermediate 2; b) in a solvent, hydrolyzing the cariprazine intermediate 2 under an alkaline condition, and then acidifying to obtain a cariprazine intermediate 3; and c) carrying out condensation reaction on the cariprazine intermediate 3 and 1-(2, 3-dichlorophenyl)-piperazine 4 in an organic solvent in the presence of a catalyst and alkali to obtain the cariprazine intermediate 5. The preparation method has the advantages of easily available raw materials, simple operation and high reaction yield, and is suitable for industrial production.
Owner:上海新礼泰药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com