A kind of cariprazine hydrochloride injection preparation and its preparation method and application
A technology for cariprazine hydrochloride and injection preparations, which are applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
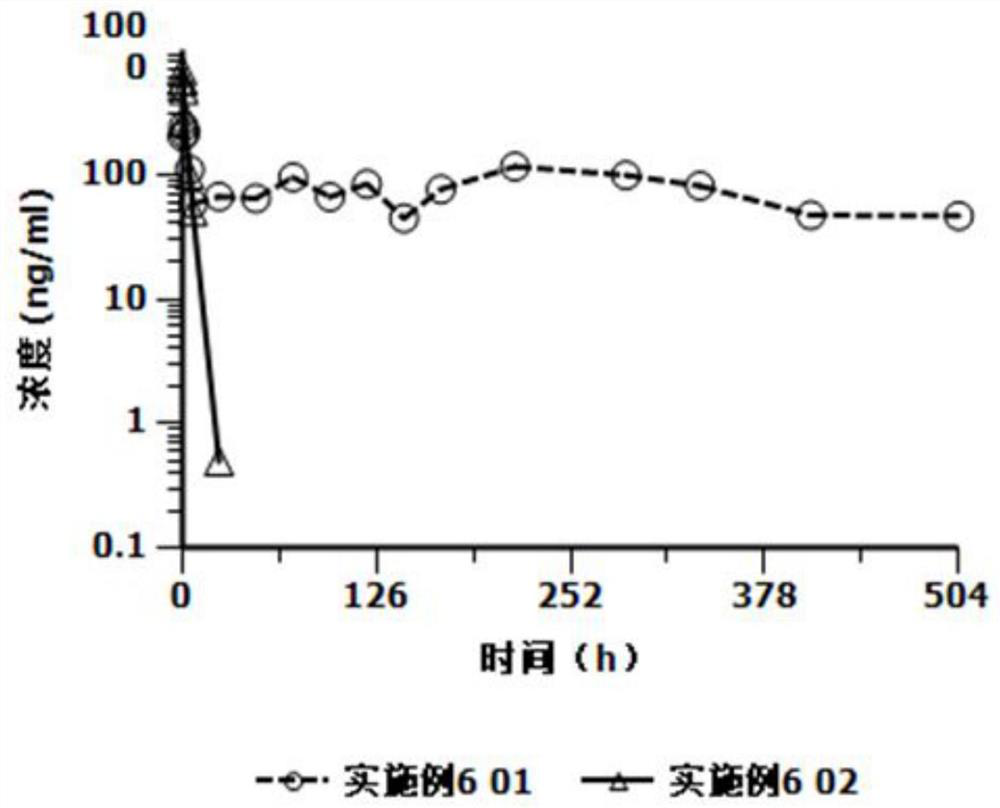
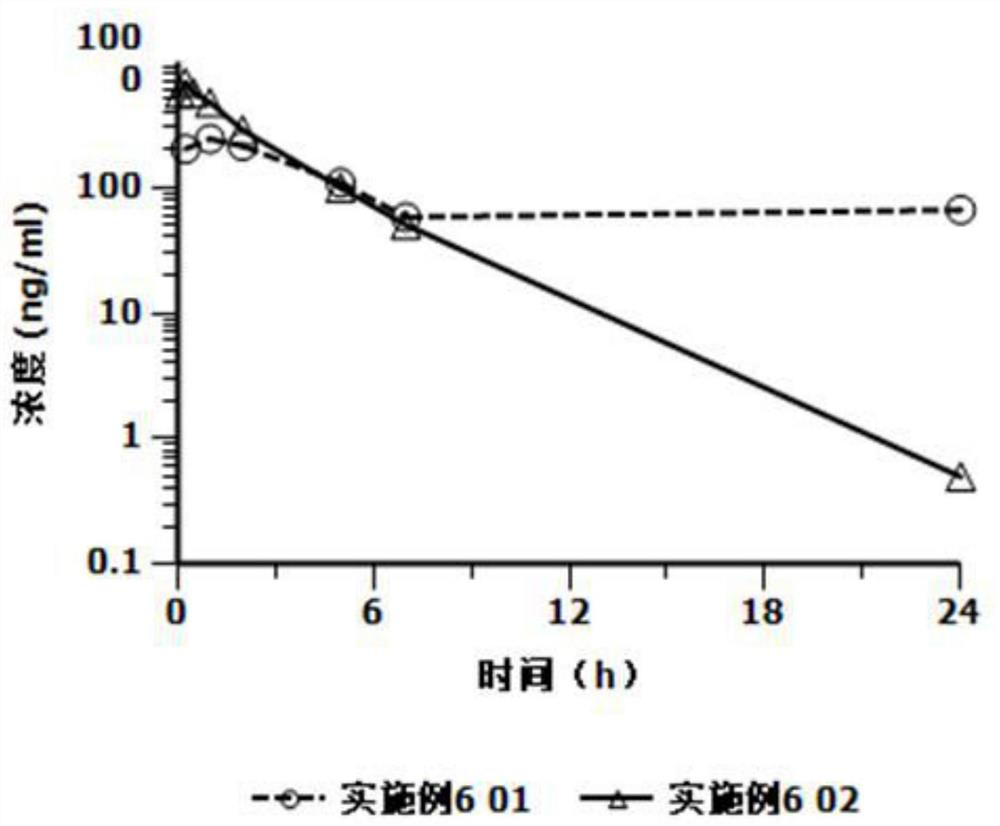
[0025] Based on the deficiencies in the prior art, the present invention has prepared an injection containing cariprazine hydrochloride through in-depth investigation and research. The preparation can be in the form of an aqueous suspension or in the form of a freeze-dried formulation. The former can be used directly. The latter is mixed with the supporting sterile water for injection and formulated into a suspension solution for use, and is used for intramuscular injection or subcutaneous injection. Compared with oral cariprazine hydrochloride capsules, the advantages of the invention include:
[0026] (1) Cariprazine hydrochloride exists as low-solubility granules in the suspension solution, which can continuously release the drug after injection, can significantly reduce the number of administrations, avoid peak-to-valley fluctuations, and thereby improve the patient's treatment compliance and safety;
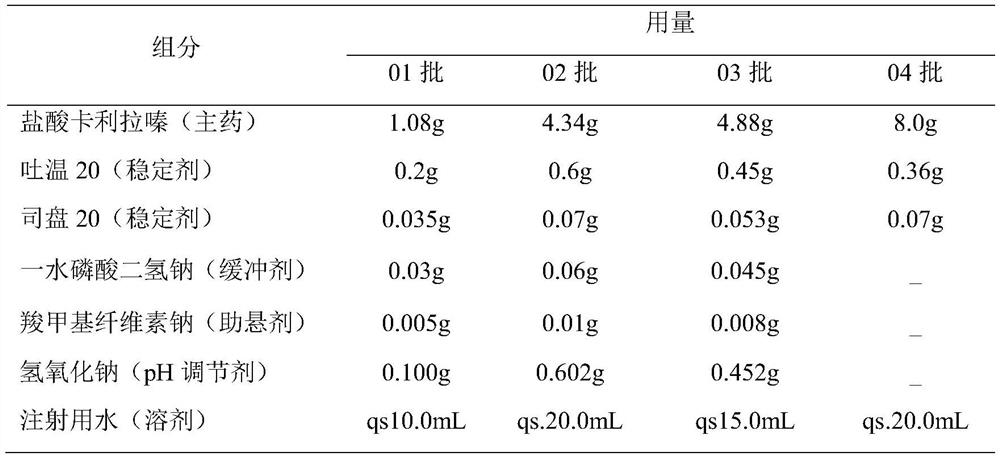
[0027] (2) The drug loading of this preparation is relatively high, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com