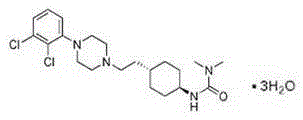
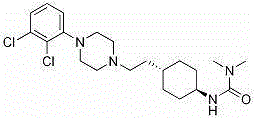
Cariprazine trihydrate compound
A technology of cariprazine trihydrate and trihydrate, applied in the field of medicine, can solve the problems of high impurity content, moisture absorption and weight gain, difficulty in purifying cariprazine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
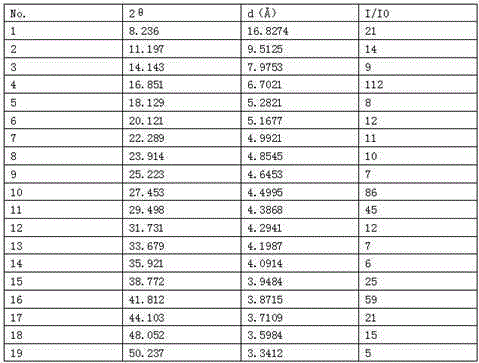
[0033] In a 100L reactor, add 6 kg of cariprazine trihydrate to a mixture of 10 times (weight-volume ratio) tetrahydrofuran-ethyl acetate-water=4.3:0.8:5, heat to 70°C and keep warm 30 minutes, strain while hot. The filtrate was kept at 47°C for 1 hour; then naturally cooled to 35°C and held for 10 hours, the precipitated crystals were filtered and dried naturally at room temperature to obtain 6.02 kg of white crystals with a purity of 99.92%, and the solvent residue test met the requirements. The moisture measured by the Karl Fischer method is 0.89%.
[0034] Microparticles or microspheres are prepared by combining the compounds of the invention with a pharmaceutically acceptable solid or liquid carrier, and optionally with pharmaceutically acceptable adjuvants and vehicles, using standard and conventional techniques. The composition is used for preparing oral preparations and injections. It is given by way of example only and in no way is it intended to limit the scope of ...
Embodiment 2
[0036] Tablets containing cariprazine trihydrate: 25% by weight of cariprazine trihydrate, appropriate amount of lactose, 30% microcrystalline cellulose, 18% pregelatinized starch, and 8% low-substituted hydroxypropyl cellulose , 1% sodium lauryl sulfate, 0.8% magnesium stearate, appropriate amount of polyvinyl pyrrolidone.
[0037] Process: Preparation of tablet cores: mix the main ingredients and auxiliary materials evenly according to the determined prescription, granulate, ventilate and dry the granules below 40°C, sieve the granules with l6 mesh, add magnesium stearate and remaining starch, and press into tablets. Instantly.
[0038] Isolation layer coating: Add talcum powder to 5% polyvinylpyrrolidone (PVP) absolute ethanol solution, stir evenly, and prepare a 20% suspension as the isolation layer coating solution. Coating is carried out in a fluidized bed, and the process conditions are as follows: spray pressure 0.3 MPa, liquid inlet speed 5mL / min, inlet temperature 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com