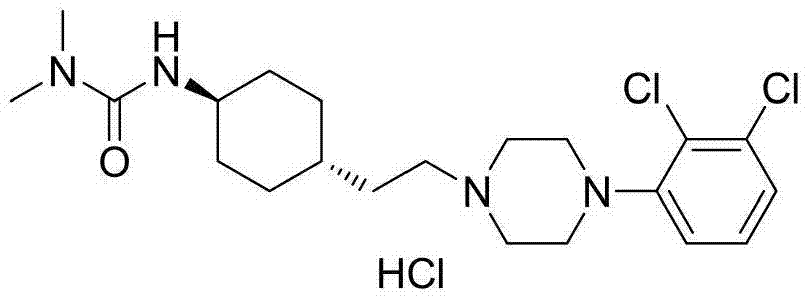
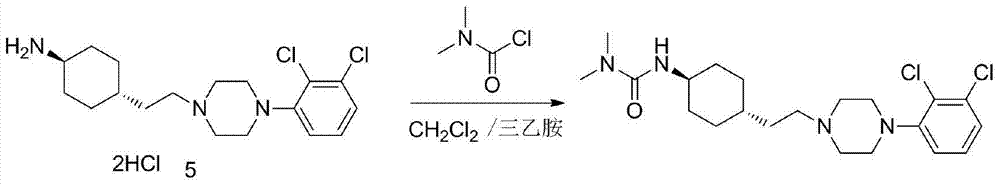
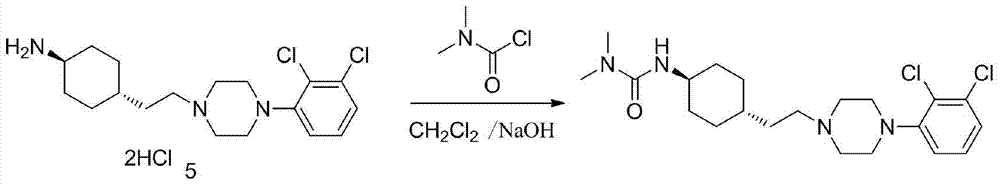
3-cyclohexyl-1,1-dimethylurea compound as well as preparation method and application thereof
A technology of dimethyl urea and compound, which is applied in the field of 3-cyclohexyl-1,1-dimethyl urea compounds, can solve the problem of difficult synthesis of 4-aminocyclohexanoic acid, unfavorable industrial production, severe reaction conditions, etc. problems, to achieve the effect of controllable product quality, stable yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Preparation of 1,1-dimethyl-3-(trans-4-(2-oxoethyl)cyclohexyl)urea (I-1);
[0091] (1) Synthesis of 1,1-Dimethyl-3-(4-oxocyclohexyl)urea (1)
[0092] Combine 4-aminocyclohexanone (113.16g, 1.0mol), dichloromethane (566mL), tetra-n-butylammonium bromide (0.6g, 1.9mmol), DMAP (0.6g, 4.9mmol), 40% NaOH aqueous solution (NaOH:60g) was added to a 1000mL four-necked flask, cooled to 0℃ in an ice bath, slowly added dimethylcarbamoyl chloride (129g, 1.2mol) dropwise, the temperature did not exceed 10℃, after the addition, stirred at room temperature for 2h, reflux React for 6h, cool to room temperature, add H 2 O (200mL) was stirred and separated, the organic layer was washed with 10% HCl (20mL×2), saturated brine (200mL×1), anhydrous MgSO 4 Evaporate to dryness, filter, and evaporate to dryness to obtain 159.16 g of white solid with a yield of 86.5%, which is directly used in the next reaction.
[0093] (2) Synthesis of 2-(4-(3,3-dimethylureido)cyclohexylalkenyl) ethyl acetate (2)
...
Embodiment 2
[0102] Preparation of Cariprazine (Cariprazine)
[0103] 1,1-Dimethyl-3-(trans-4-(2-oxoethyl)cyclohexyl)urea (I-1) (9.0g, 42.39mmol), 1-(2,3-di Chlorophenyl) piperazine (9.8g, 42.39mmol), sodium triacetoxyborohydride (13.1g, 61.8mmol), 1,2-dichloroethane (300mL) were added to a 500mL single-necked flask and stirred at room temperature for 18h , Add potassium carbonate aqueous solution (250mL), separate the layers, extract the aqueous layer with 1,2-dichloroethane (150mL×1), combine the organic layers, wash with saturated brine (150mL×1), and dry with anhydrous sodium sulfate , Filter, concentrate, remove most of the solvent, filter, wash the filter cake with ethyl acetate (50 mL×3), combine the filtrate, and concentrate to obtain 16.2 g of white solid with a yield of 89.5%. The obtained white solid, ethanol (160 mL), and 10% HCl (39.84 mmol) were added to a 250 mL single-neck flask, refluxed for 1 h, cooled to room temperature, and filtered to obtain 16.7 g of cariprazine (white...
Embodiment 3
[0107] Preparation of 3-(trans-4-(2-hydroxyethyl)cyclohexyl)-1,1-dimethylurea (II-1)
[0108] The trans-2-(4-(3,3-dimethylureido) cyclohexyl) ethyl acetate (3) (51.27g, 0.2mol), NaBH 4 (37.8g, 1mol), THF (190mL) were added to a 500mL single-necked flask, refluxed for 1h, cooled to 50℃, methanol (80mL) was added in batches, after the addition, refluxed for 7h, the reaction solution was cooled to room temperature and concentrated Adjust the reaction solution to pH 1-2 with hydrochloric acid, stir for 1 hour, adjust the reaction solution to pH 7-8 with 20% NaOH aqueous solution, stir for 1 hour, extract with dichloromethane (200mL×3), and wash with saturated brine (100×1) , Anhydrous MgSO 4 After drying, filtering, and concentrating, a colorless oil was obtained, which was allowed to stand and solidified to obtain 37.7 g of white solid with a yield of 88%.
[0109] 1 H NMR(DMSO-d 6 ,δ:ppm):0.89-0.95(m,2H,A-H),1.13-1.19(m,5H,A-H),1.67-1.75(m,4H,A-H),2.75(s,6H,CH 3 ), 3.35-3.44(m,3H,A-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com