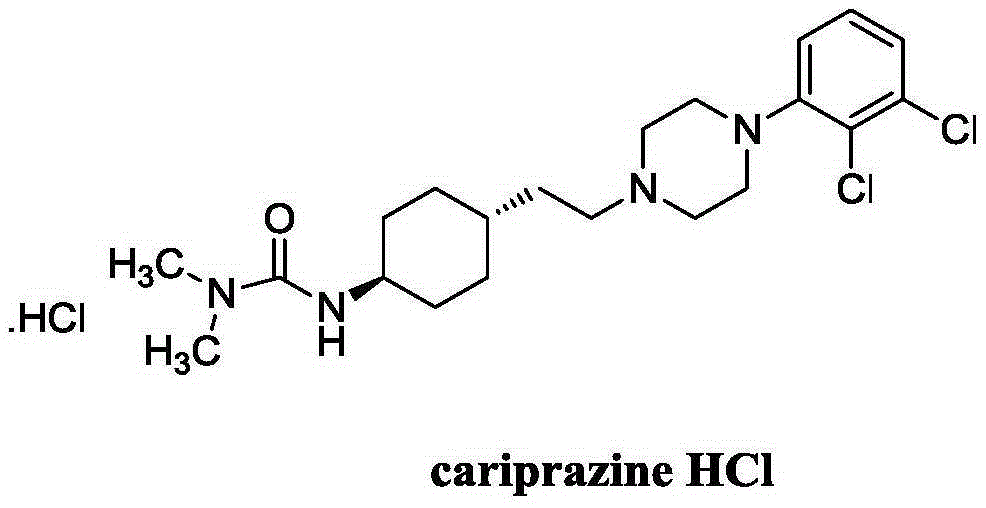
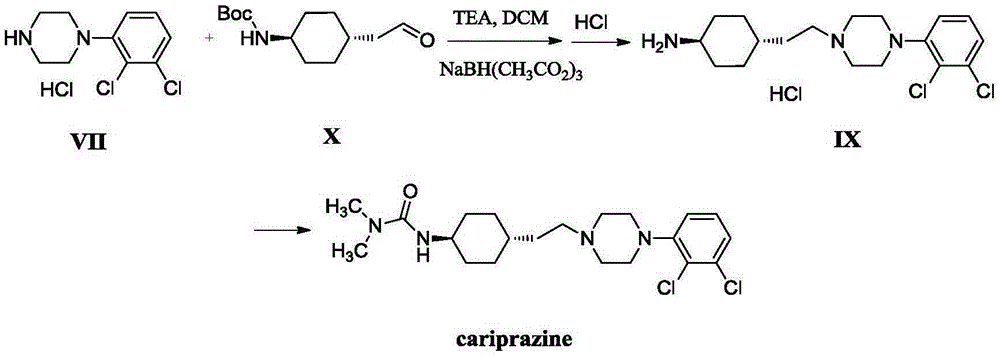
Compound for preparation of cariprazine and preparation method thereof
A compound and a technology for sodium hydride, applied in the field of compounds for the preparation of cariprazine, can solve problems such as rare raw materials, unsuitable for industrial production, environmental pollution, etc., and achieve the effects of good yield, short reaction time, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0053] Preparation of ethyl 2-{4-[(N-tert-butoxycarbonyl)amino]cyclohexylylidene}acetate
[0054]
[0055] Add sodium hydrogen (60 g) and 200 ml of dry tetrahydrofuran to a 2 l four-necked flask. Cool down to 5°C, add dropwise a mixture of triethyl phosphonoacetate (69g, 0.31mol) and THF (400ml) under stirring; dropwise, react at the same temperature for 30 minutes; dropwise add tert-butyl (4-oxocyclohexyl ) carbamate (60 g, 0.28 mol) and dry THF (500 ml) mixture. After dropping, slowly warm up to room temperature and react for 3 hours.
[0056] After the reaction was completed, 430ml of water was added dropwise, followed by ethyl acetate (500mlX2) for extraction; the organic phases were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate. After filtering and concentrating until most solids are precipitated, add 150ml of n-hexane and stir at room temperature for 1 hour. After filtration and vacuum drying at 40°C to constant weight, 72.2 g of...
example 2
[0058] Preparation of ethyl 2-{4-[(N-tert-butoxycarbonyl)amino]cyclohexylylidene}acetate
[0059] Sodium hydrogen (50 g) and 170 ml of dry tetrahydrofuran were added to a 2 l four-necked flask. Cool down to 5°C, add dropwise a mixture of triethyl phosphonoacetate (79g, 0.35mol) and THF (450ml) under stirring; dropwise, react at the same temperature for 30 minutes; dropwise add tert-butyl (4-oxocyclohexyl ) carbamate (50 g, 0.23 mol) and dry THF (400 ml). After dropping, slowly warm up to room temperature and react for 3 hours.
[0060] After the reaction was completed, 430ml of water was added dropwise, followed by ethyl acetate (500mlX2) for extraction; the organic phases were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate. Filter and concentrate until most solids are precipitated, add 125ml of n-hexane, and stir at room temperature for 1 hour. After filtering and vacuum drying at 40°C to constant weight, 60 g of white solid was obtained ...
example 3
[0062] Preparation of methyl 2-{4-[(N-tert-butoxycarbonyl)amino]cyclohexylylidene}acetate
[0063]
[0064] Add sodium hydrogen (30 g) and 100 ml of dry tetrahydrofuran to a 1 l four-necked flask. Cool down to 5°C, add dropwise a mixture of trimethyl phosphonoacetate (51.2g, 0.28mol) and THF (300ml) under stirring; Hexyl) carbamate (30 g, 0.14 mol) and dry THF (260 ml). After dropping, slowly warm up to room temperature and react for 3 hours.
[0065] After the reaction was completed, 220ml of water was added dropwise, followed by ethyl acetate (260mlX2) for extraction; the organic phases were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate. Filter and concentrate until most solids are precipitated, add 100ml of n-hexane, and stir at room temperature for 1 hour. After filtration and vacuum drying at 40° C. to constant weight, 34.9 g of white solid was obtained, yield 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com