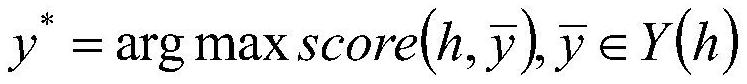Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "Negative symptom" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A negative symptom is absence of a function or feeling normally present in an average person. [citation needed] For example, in describing mental disorders, especially schizophrenia, positive and negative symptoms are as follows.
Combination of serotonin reuptake inhibitors and norephinephrine reuptake inhibitors
InactiveUS20050014848A1Prevent relapseIncreasing and improving neuronal processBiocideAmine active ingredientsStress inducedNorepinephrine reuptake inhibitor
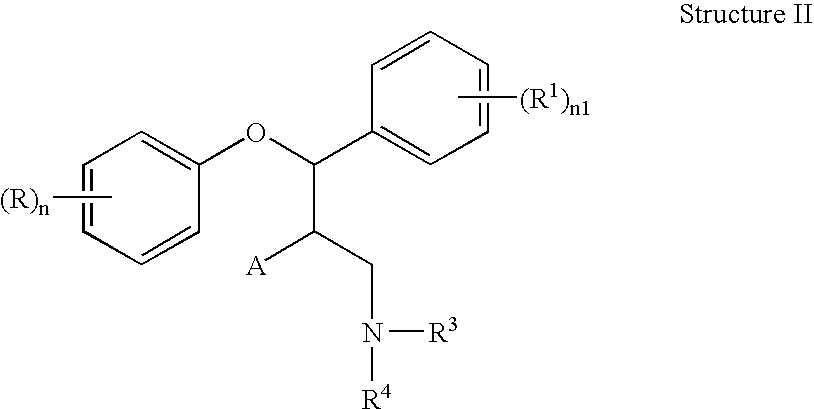
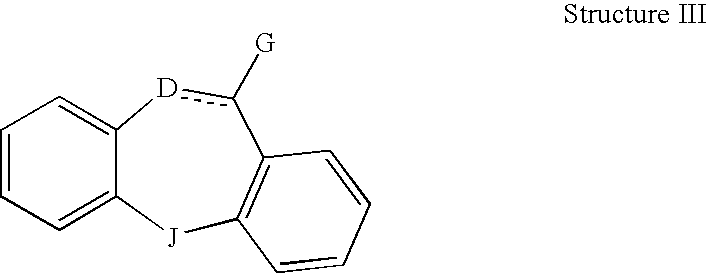
This invention is directed to pharmaceutical compositions and methods for treating a disorder or condition selected from the group consisting of depression, anxiety disorders, phobias, avoidant personality disorder, eating disorders, chemical dependencies, Parkinson's diseases, obsessive-compulsive disorder, negative symptoms of schizophrenia, cognitive dysfunction related to schizophrenia, premenstrual syndrome, stress-induced incontinence, headache, neuropathic pain, chronic pain, urinary incontinence, post-traumatic stress disorder, chronic stress, acute stress, fibromyalgia, depression comorbid with fibromyalgia, obesity, migraine and a combination thereof in a mammal. The methods in one embodiment comprise administering to a mammal in need of treatment for the disorder or condition: (i) at least one serotonin reuptake inhibitor or pharmaceutically acceptable salt thereof; (ii) at least one norepinephrine reuptake inhibitor or pharmaceutically acceptable salt thereof, wherein the norepinephrine reuptake inhibitor is selected from the group consisting of Structure II, Structure III, and Structure IV as defined in the specification; and (iii) a pharmaceutically acceptable carrier. The pharmaceutical compositions and methods of the invention are also useful for preventing a relapse associated with one of the foregoing disorders or conditions, and for treating a symptom associated with one of the foregoing disorders or conditions, wherein the symptom is selected from the group consisting of cognitive dysfunctions and somatic complaints.
Owner:PFIZER INC
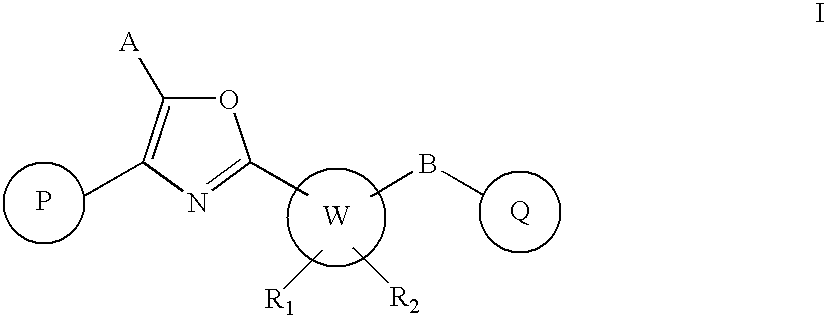
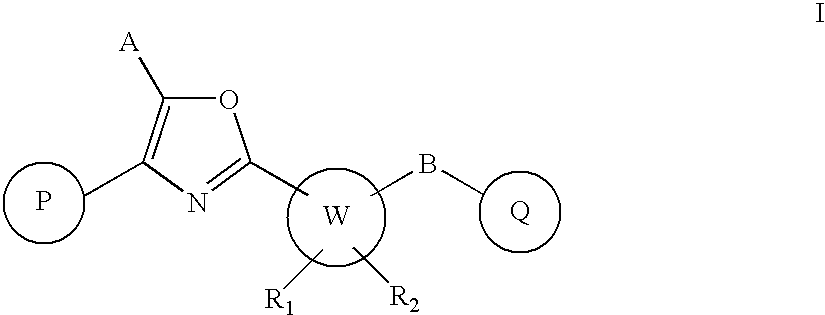
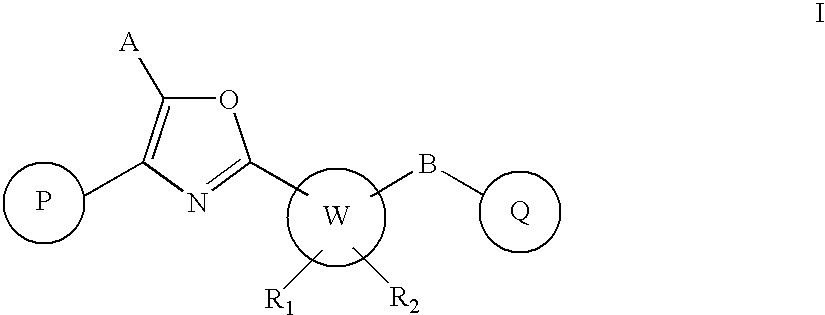
Oxazole derivatives as positive allosteric modulators of metabotropic glutamate receptors
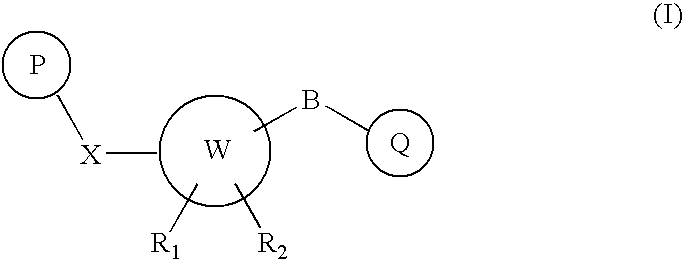
The present invention provides new compounds of formula I, wherein P, A, W, B, Q, R1 and R2 are defined as in formula I; invention compounds are positive allosteric modulators of metabotropic receptors—subtype 5 (“mGluR5”) which are useful for the treatment or prevention of central nervous system disorders such as for example: cognitive decline, both positive and negative symptoms in schizophrenia as well as other disorders in which the mGluR5 subtype of glutamate metabotropic receptor is involved.
Owner:ADDEX PHARM SA
Combination of atypical antipsychotics and 5HT-1B receptor antagonists
InactiveUS20050256112A1Reduce morbidityDifferent recognizableNervous disorderMetabolism disorderDiseaseHeadaches
The present invention relates to a pharmaceutical composition for treating, for example, a disorder or condition selected from the group consisting of hypertension, depression, generalized anxiety disorder, phobias, posttraumatic stress disorder, avoidant personality disorder, sexual dysfunction, eating disorders, obesity, chemical dependencies, cluster headache, migraine, pain, Alzheimer's disease, obsessive-compulsive disorder, panic disorder, memory disorders, Parkinson's diseases, endocrine disorders, cerebellar ataxia, gastrointestinal tract disorders, negative symptoms of schizophrenia, premenstrual syndrome, Fibromyalgia Syndrome, stress incontinence, Tourette syndrome, trichotillomania, kleptomania, male impotence, cancer, chronic paroxysmal hemicrania and headache in a mammal, preferably a human, comprising (i) an atypical antipsychotic or a pharmaceutically acceptable salt thereof, (ii) a 5-HT1B receptor antagonist or a pharmaceutically acceptable salt thereof, wherein the 5-HT1B receptor antagonist is selected from the group consisting of (A) a compound of the formula I as described in the specification and (B) a compound of the formula II as described in the specification, and optionally (iii) a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Dual NK1/NK3 receptor antagonists
The present invention provides a method for the treatment of schizophrenia which comprises administering a compound of formulawherein the substituents are as described herein or a pharmaceutically active acid-addition salt thereof. In particular, the invention provides methods for treating both positive and negative symptoms of schizophrenia through dual inhibition of NK1 and NK3 receptors. The invention also provides novel compounds with formula I and methods for preparing compounds of the invention.
Owner:F HOFFMANN LA ROCHE INC
Combination of glyt1 compound with antipsychotics
InactiveUS20120035156A1Affecting/increasing side-effect profileBiocideNervous disorderNegative symptomAtypical antipsychotic
The present invention relates to a pharmaceutical combination of a glycine transporter inhibitor (GlyT1) and an atypical antipsychotic drug which may be used for the treatment of positive and negative symptoms of schizophrenia.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for identifying antipsychotic drug candidates
The present invention provides a method for identifying a compound or a combination of compounds having a pharmacological behavior that qualifies it as a candidate for clinical development of a drug for treatment of a psychiatric disease or disorder, preferably schizophrenia. According to this method, a candidate drug is assessed for its ability to produce a biochemical profile, in either or both in vitro and in vivo test systems, which is similar to a unique reference biochemical profile obtained following treatments with drugs or drug combinations effective against both positive and negative symptoms of psychiatric diseases or disorders.
Owner:TECHNION RES & DEV FOUND LTD
Traumatic injury plaster
InactiveCN102302752AConvenient treatmentWith swelling and pain reliefAntibacterial agentsHydroxy compound active ingredientsCentipedeOleoresin
The invention discloses a traumatic injury plaster. The plaster comprises Chinese herbal medicines of: 6 to 9g of radix notoginseng, 6 to 9g of dried ginger, 6 to 9g of incised notopterygium rhizome and root, 6 to 9g of stiff silkworm, 6 to 9g of saposhnikovia divaricata, 10 to 14g of raw radix aconite, 10 to 14g of raw aconitum kusnezoffii, 2 to 4g of centipede, 6 to 9g of trigonobalanus, 6 to 9g of zedoary, 6 to 9g of safflower, 6 to 9g of peach seed, 6 to 9g of galangal oleoresin, 6 to 9g of drynariae baronii, 6 to 9g of red peony root, 6 to 9g of ricinus communis, 6 to 8g of curcuma, 10 to 14g of raw nux vomica liquid medicine, 6 to 9g of pangolin scale, 6 to 9g of clove fruit, 6 to 9g of cinnamomum cassia, 6 to 9g of borneol, 6 to 9g of frankincense, and 1 to 3g of forest musk. With the traumatic injury plaster provided by the invention, traumatic injuries can be well treated; and arthralgia, fractures, dislocation, beriberoid pyretic arthralgia, tuberculosis, tortoise back and chest, and nameless swellings with negative symptoms can be treated.
Owner:周荣萍
Dual NK1/NK3 receptor antagonists
The present invention provides a method for the treatment of schizophrenia which comprises administering a compound of formula wherein the substituents are as described herein or a pharmaceutically active acid-addition salt thereof. In particular, the invention provides methods for treating both positive and negative symptoms of schizophrenia through dual inhibition of NK1 and NK3 receptors. The invention also provides novel compounds with formula I and methods for preparing compounds of the invention.
Owner:F HOFFMANN LA ROCHE & CO AG
Methods for evaluation prognosis and follow-up of drug treatment of psychiatric diseases or disorders
The present invention provides methods for evaluating the pharmacological efficacy of drugs or drug candidates in treatment of psychiatric diseases or disorders, particularly schizophrenia, and for predicting the efficacy of drugs or drug combinations indicated for treatment of both positive and negative symptoms of psychiatric diseases or disorders in an individual having such a disease or disorder. In both methods, the drugs or drug candidates evaluated are assessed for their ability to produce certain changes in the expression of specific genes in peripheral mononuclear cells in blood of psychiatric patients, which are similar to the changes obtained following treatments with reference drugs or drug combinations effective against both positive and negative symptoms of psychiatric diseases or disorders.
Owner:TECHNION RES & DEV FOUND LTD
Remedy for integration dysfunction syndrome
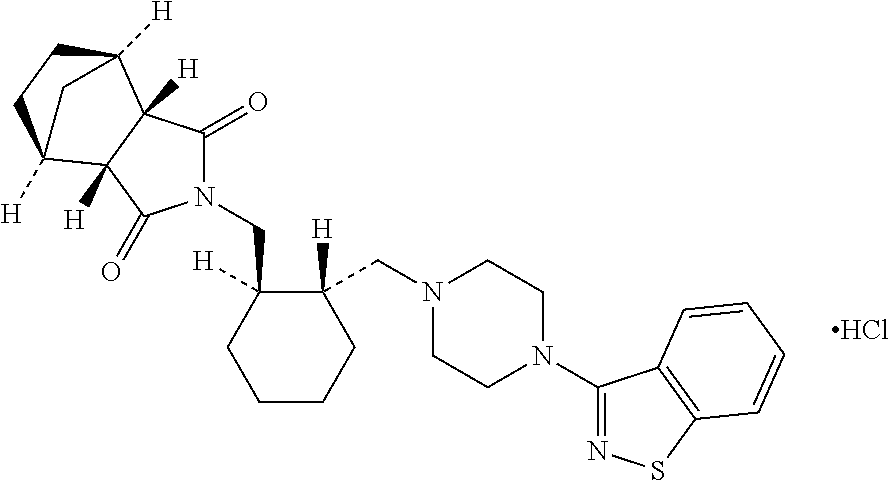
ActiveUS20060025422A1Good effectSafety managementOrganic active ingredientsNervous disorderNegative symptomSchizophrenia
The present invention provides a novel method for treatment of schizophrenia which can improve wide-ranging symptoms of schizophrenia, especially positive symptoms and negative symptoms without being accompanied by extrapyramidal symptoms, which comprises orally administering as an active compound (1R,2S,3R,4S)—N-[(1R,2R)-2-[4-(1,2-benzoisothiazol-3-yl)-1-piperazinylmethyl]-1-cyclohexylmethyl]-2,3-bicyclo[2.2.1]heptanedicarboxyimide or a pharmaceutically acceptable salt thereof (e.g., hydrochloride) at a daily dose of 5 mg to 120 mg once a day to a patient with schizophrenia, and a therapeutic agent to be used in said method.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
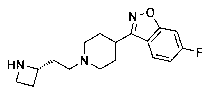
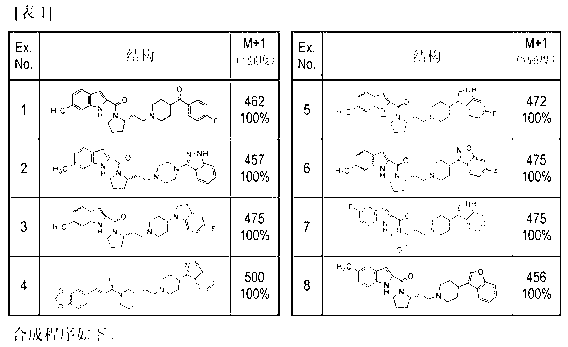
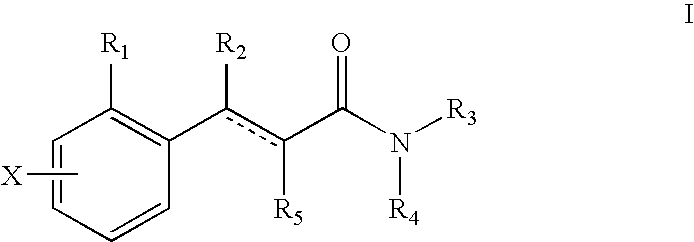
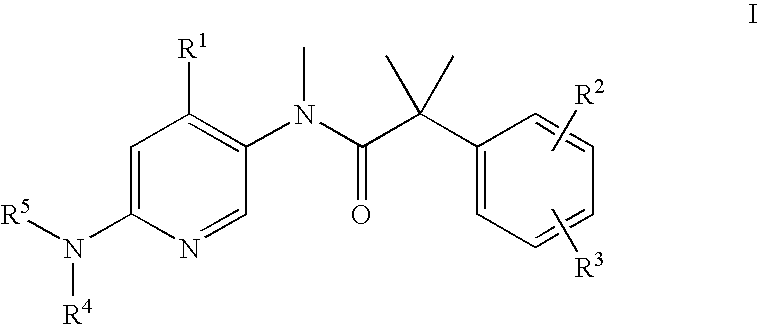
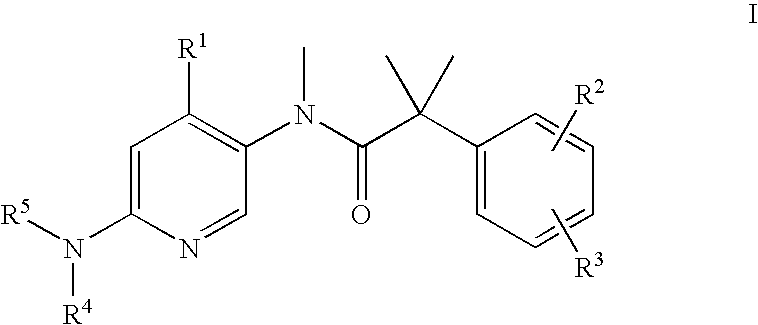
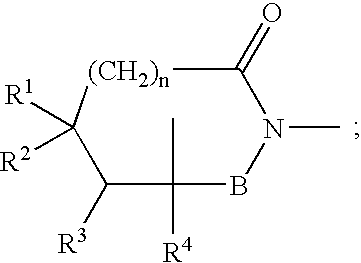
Bicyclic [3.1.0.] heteroaryl amides as type 1 glycine transport inhibitors
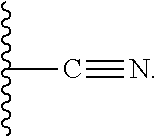
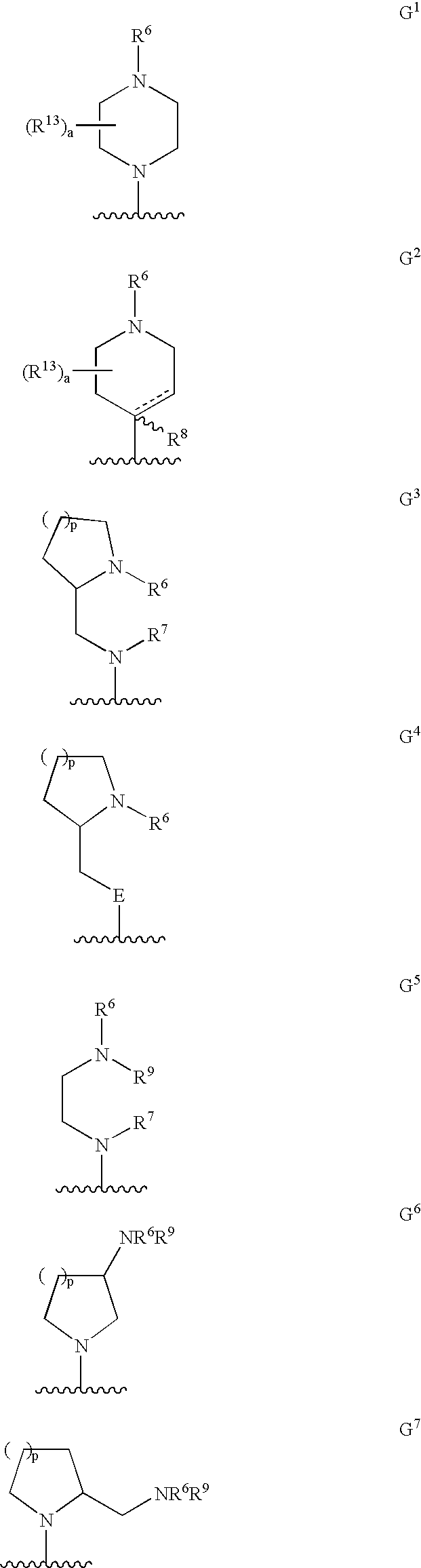
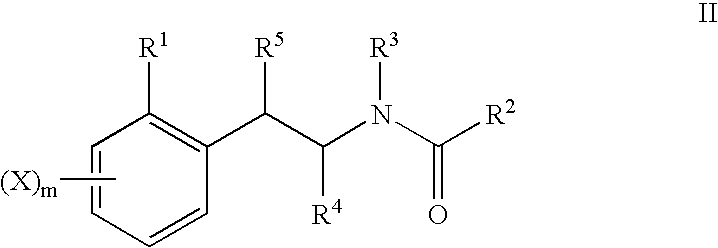
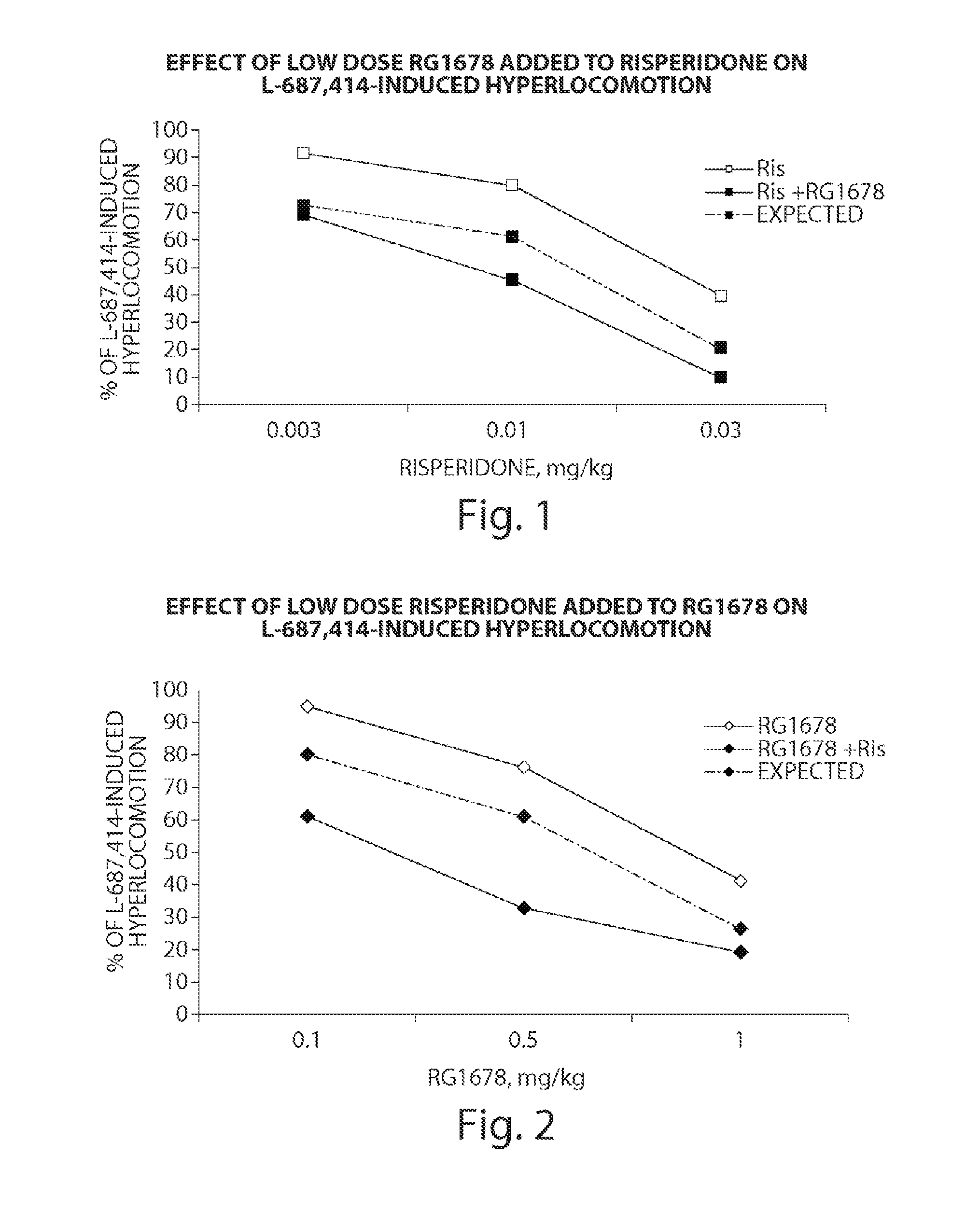
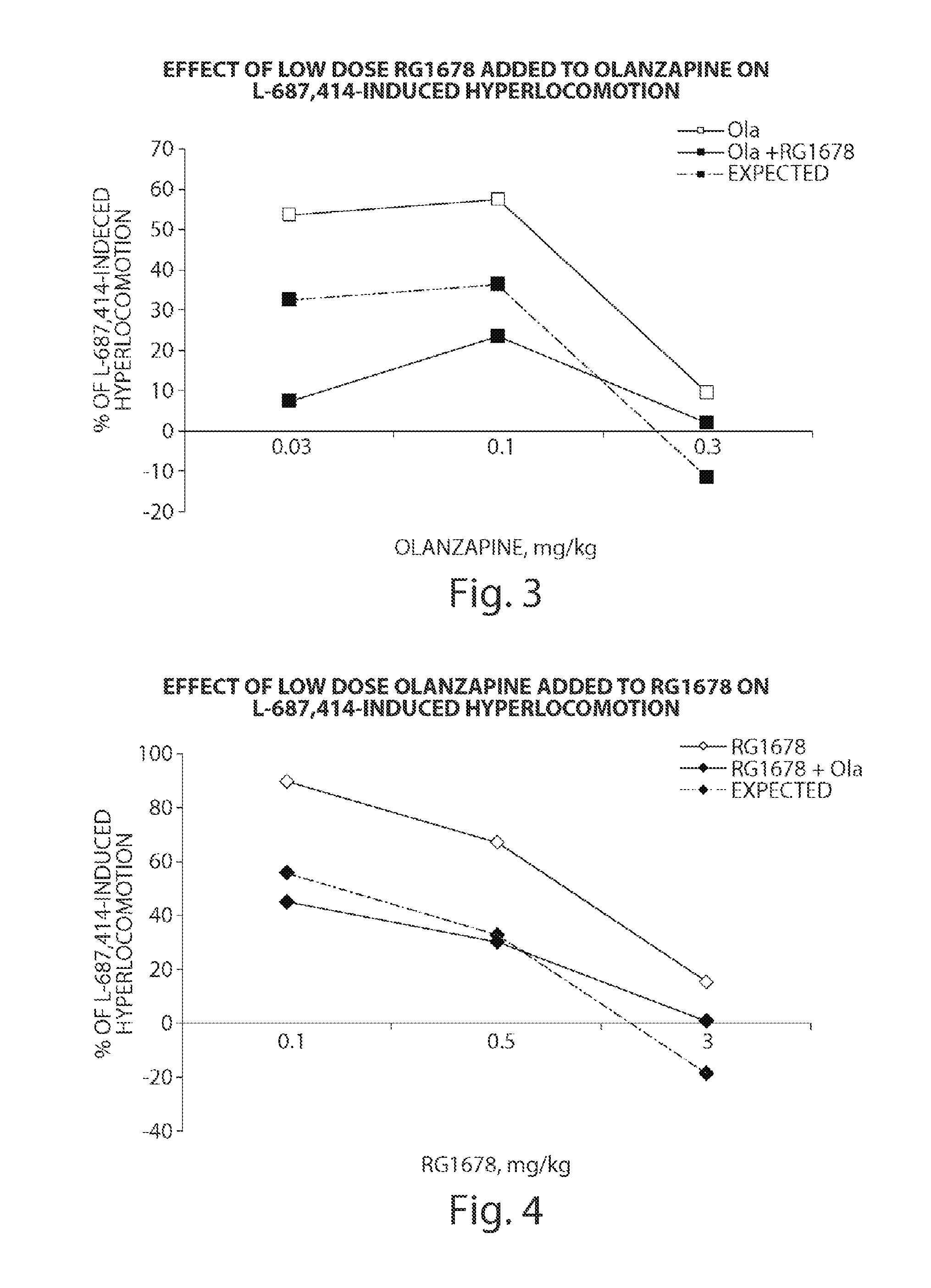
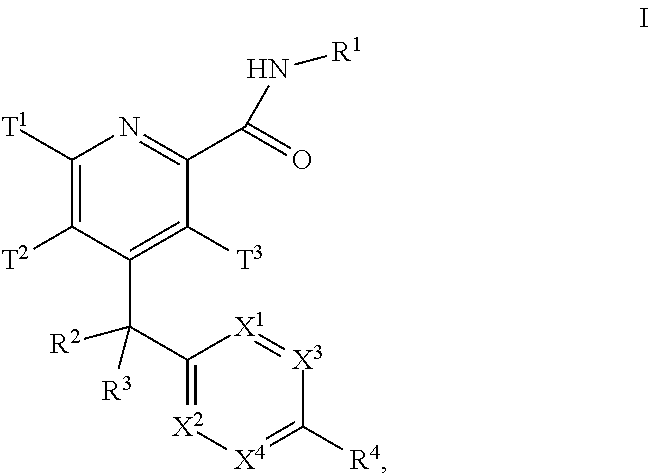
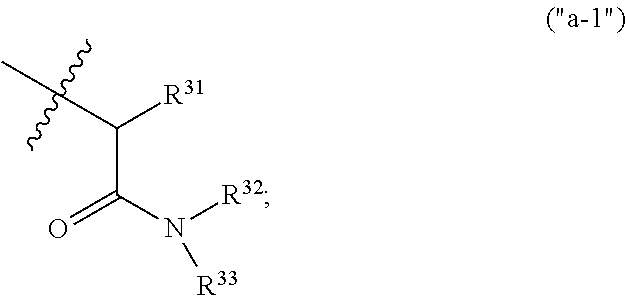
The present invention relates to a series of substituted bicyclic[3.1.0]heteroaryl amides of the Formula I, wherein A, Q, X, Y, Z and R1-R5 groups are defined as in the specification, that exhibit activity as glycine transport inhibitors, their pharmaceutically acceptable salts, pharmaceutical compositions containing them, and their use for the enhancement of cognition and the treatment of the positive and negative symptoms of schizophrenia and other psychoses in mammals, including humans.
Owner:PFIZER INC
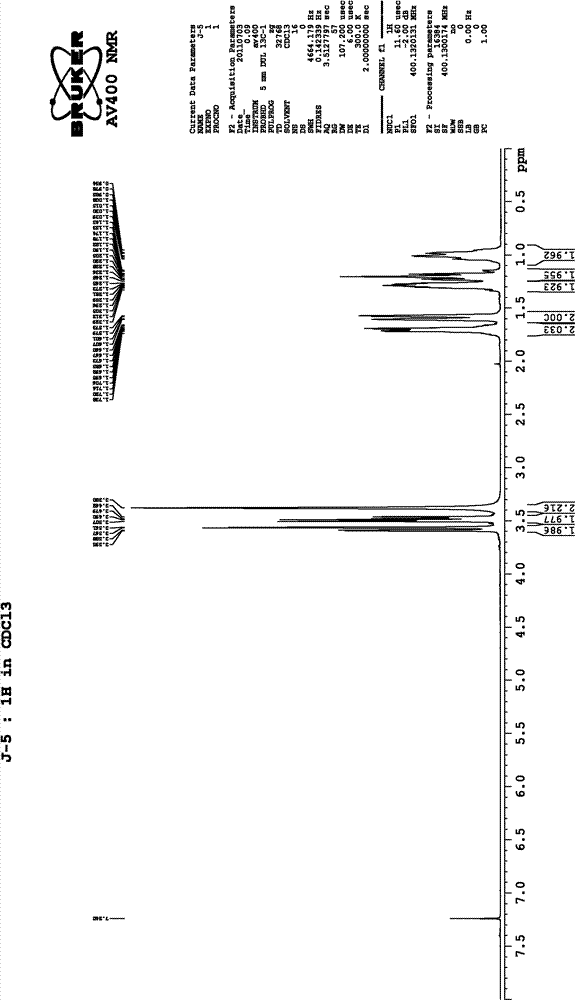
Preparation method of chiral intermediate cyclohexane dimethanol
InactiveCN102952001ALow priceStable in natureOrganic compound preparationHydroxy compound preparationNegative symptomLurasidone
The invention belongs to the technical field of medicine, and specifically relates to a preparation method of a chiral intermediate 1R,2R-cyclohexane dimethanol. The chiral intermediate 1R,2R-cyclohexane dimethanol is an important intermediate of an antischizophrinic medicine lurasidone. Lurasidone has substantially treatment effects against both positive and negative symptoms of mental patients. A reduction agent adopted by the invention has low cost and stable property. The preparation method is suitable for industrialized productions.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Methods and compositions for treating schizophrenia
InactiveUS20140206667A1Prevent and slow progressionLong and improved therapeutic effectBiocideNervous disorderNegative symptomSynaptic vesicle
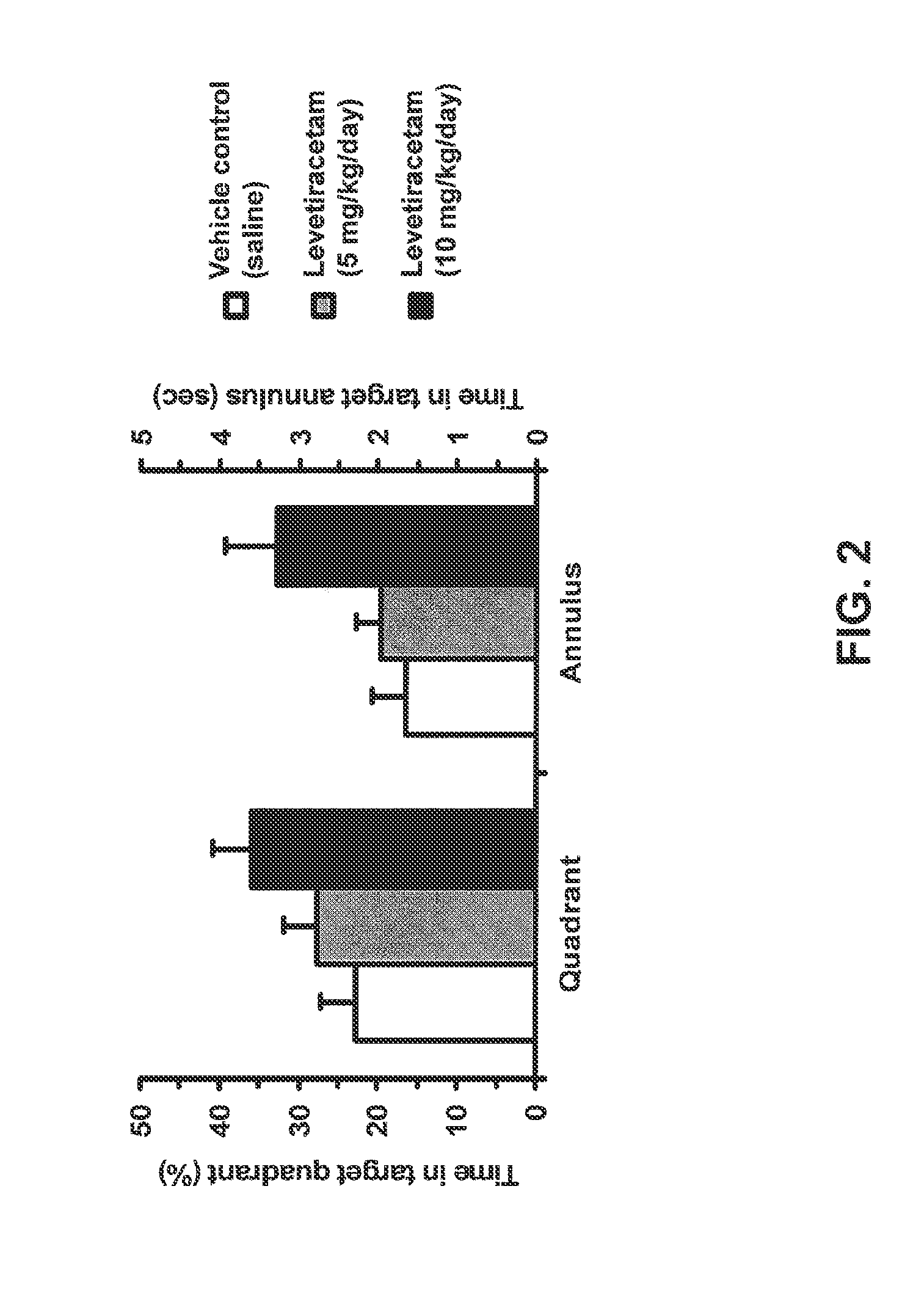
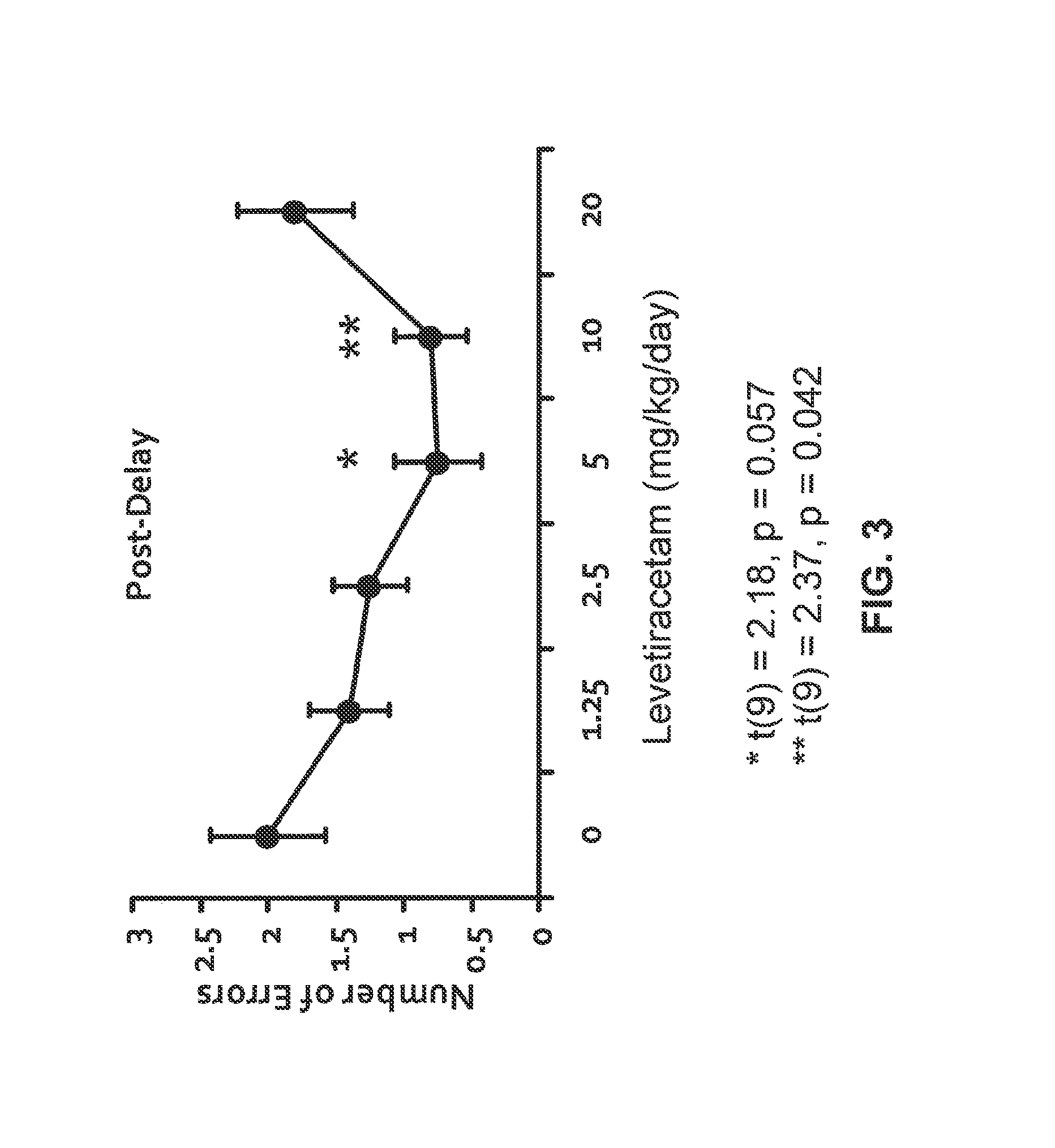
The invention relates to methods and compositions for treating schizophrenia or bipolar disorder (in particular, mania) by using a combination of a synaptic vesicle protein 2A (SV2A) inhibitor and an antipsychotic or their pharmaceutically acceptable salts, hydrates, solvates, polymorphs thereof. In some embodiments, the methods and the compositions are for treating one or more positive and / or negative symptoms, as well as cognitive impairment, associated with schizophrenia or bipolar disorder (in particular, mania).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Use of a CB1 Antagonist for Treating Side Effects and Negative Symptoms of Schizophrenia
InactiveUS20080221078A1Useful in treatmentSymptoms improvedOrganic active ingredientsBiocideDiseaseNegative symptom
The present invention discloses and claims a method of treating cognition deficits in a patient suffering from schizophrenia by administering to said patient a therapeutically effective amount of a CB1 receptor antagonist as described herein. In another aspect, this invention also discloses and claims a combination of one or more CB1 receptor antagonists and of one or more antipsychotic agents useful in the treatment of psychiatric disorders. The combination of this invention provides synergistic results in that the combination improves positive and negative symptoms of schizophrenia, weight gain and catalepsy.
Owner:AVENTIS PHARMA INC
Methods of treating schizophrenia
InactiveUS7115256B1Improve cognitive functionImproving cognitive deficitsBiocideNervous disorderSide effectNeurotrophic factors
The invention provides methods for the treatment of abnormal psychiatric states, particularly the negative symptoms of schizophrenia and extrapyramidal side effects (EPS) of antipsychotic drugs. The inventive methods relate to the administration of therapeutic cells (which produce dopamine or dopamine precursors) adhered to support matrices to subjects suffering from the negative symptoms of schizophrenia and / or EPS. The therapeutic cells may be coadministered with cells which protect the therapeutic cells from immune rejection and / or cells which produce neurotrophic factors which improve the viability of the therapeutic cells.
Owner:TITAN PHARMA
Pharmaceutical compositions of Lurasidone and Process for preparation thereof
Pharmaceutical compositions comprising an atypical antipsychotic as an active agent, process of preparation thereof and method of using the same are provided. Particularly the present invention relates to pharmaceutical compositions comprising lurasidone, process of preparation thereof and method to treat various psychotic disorders such as schizophrenia, positive and negative symptoms of schizophrenia, memory or learning dysfunctions caused by schizophrenia, senile dementia, attention deficit / hyperactivity disorder (ADHD), central nervous system (CNS) disorder responsive to modulation of glutamate levels, major depressive episodes associated with bipolar I disorder and other associated CNS disorders.
Owner:AUROBINDO PHARMA LTD
Methods and compositions for treating schizophrenia
ActiveUS20150313876A1Prevent and slow progressionLong and improved therapeutic effectBiocideNervous disorderNegative symptomSynaptic vesicle
The invention relates to methods and compositions for treating schizophrenia or bipolar disorder (in particular, mania) by using a combination of a synaptic vesicle protein 2A (SV2A) inhibitor and an antipsychotic or their pharmaceutically acceptable salts, hydrates, solvates, polymorphs, and prodrugs thereof. The methods and the compositions can be used for treating one or more positive and / or negative symptoms, as well as cognitive impairment, associated with schizophrenia or bipolar disorder (in particular, mania).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Pyridine derivatives as muscarinic m1 receptor positive allosteric modulators
InactiveUS20160016907A1Improve sleep qualityImproving sleep maintenanceBiocideNervous disorderNegative symptomAllosteric modulator
The present invention provides, in part, compounds of Formula I:N-oxides thereof, and pharmaceutically acceptable salts of the compounds or N-oxides; processes for the preparation of; intermediates used in the preparation of; and compositions containing such compounds, N-oxides, or salts, and their uses for treating M1-mediated (or M1-associated) disorders including, e.g., Alzheimer's disease, schizophrenia (e.g., its cognitive and negative symptoms), pain, addiction, and a sleep disorder.
Owner:PFIZER INC
Carbamate derivatives as positive allosteric modulators of metabotropic glutamate receptors
The present invention relates to new compounds which are Carbamate derivatives of formula I wherein X, B, P, Q5W, R1 and R2 are defined in the description. Invention compounds are useful for treating CNS or PNS disorders which are affected by the neuromodulatory effect of mGluR5 positive allosteric modulators such as cognitive decline and also to treat both positive and negative symptoms in schizophrenia
Owner:ADDEX PHARM SA
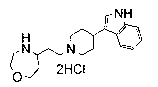
Bicyclic [3.1.0] derivatives as glycine transporter inhibitors
ActiveUS7473787B2Effective treatmentEase of detectabilityBiocideNervous disorderMedicinal chemistryGlycine transporter
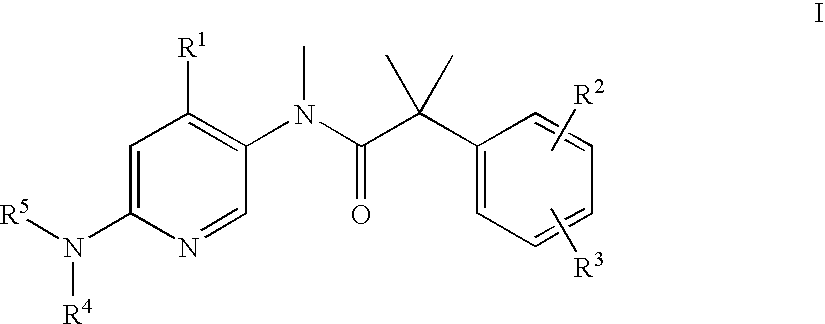
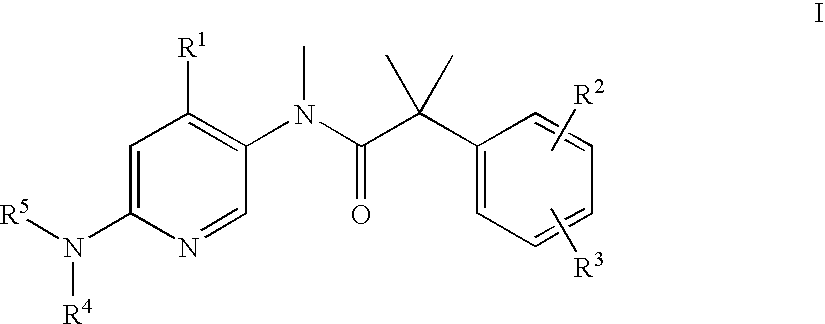
The present invention relates to a series of substituted bicyclic[3.1.0]amines of the Formula I:wherein A, B, D, Q, V, W, X, Y, Z, R2, R3, R4, R5, R14, R15, R30. o, p, s,t and q are as defined in the specification, their pharmaceutically acceptable salts, pharmaceutical compositions thereof, and their use for the enhancement of cognition and the treatment of the positive and negative symptoms of schizophrenia and other psychoses in mammals, including humans.
Owner:PFIZER INC +1
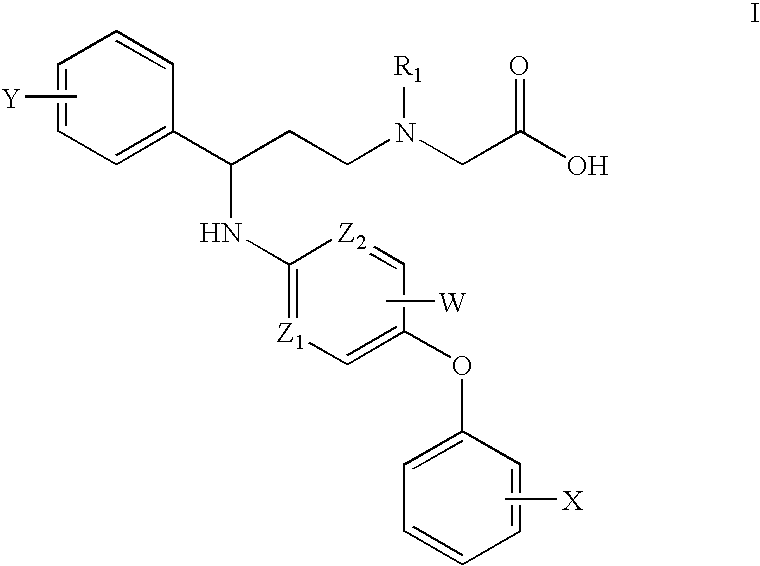
Pyridylamino compounds and methods of use thereof
InactiveUS20050080100A1Ease of detectabilityEasy to prepareBiocideGroup 5/15 element organic compoundsNegative symptomPyridine
This invention relates to a series of pyridylamino compounds of the formula I wherein Z1, Z2, W, X, Y and R1 are defined as in the specification, that exhibit activity as glycine transport inhibitors, their pharmaceutically acceptable salts, pharmaceutical compositions containing them, and their use for the enhancement of cognition and the treatment of the positive and negative symptoms of schizophrenia and other psychoses in mammals, including humans.
Owner:PFIZER INC
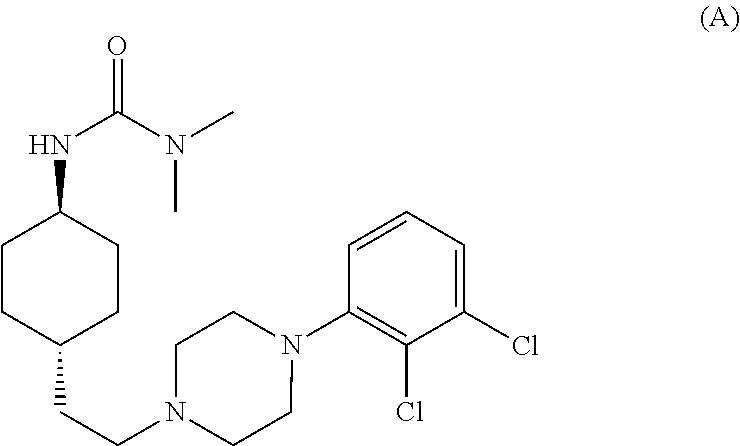
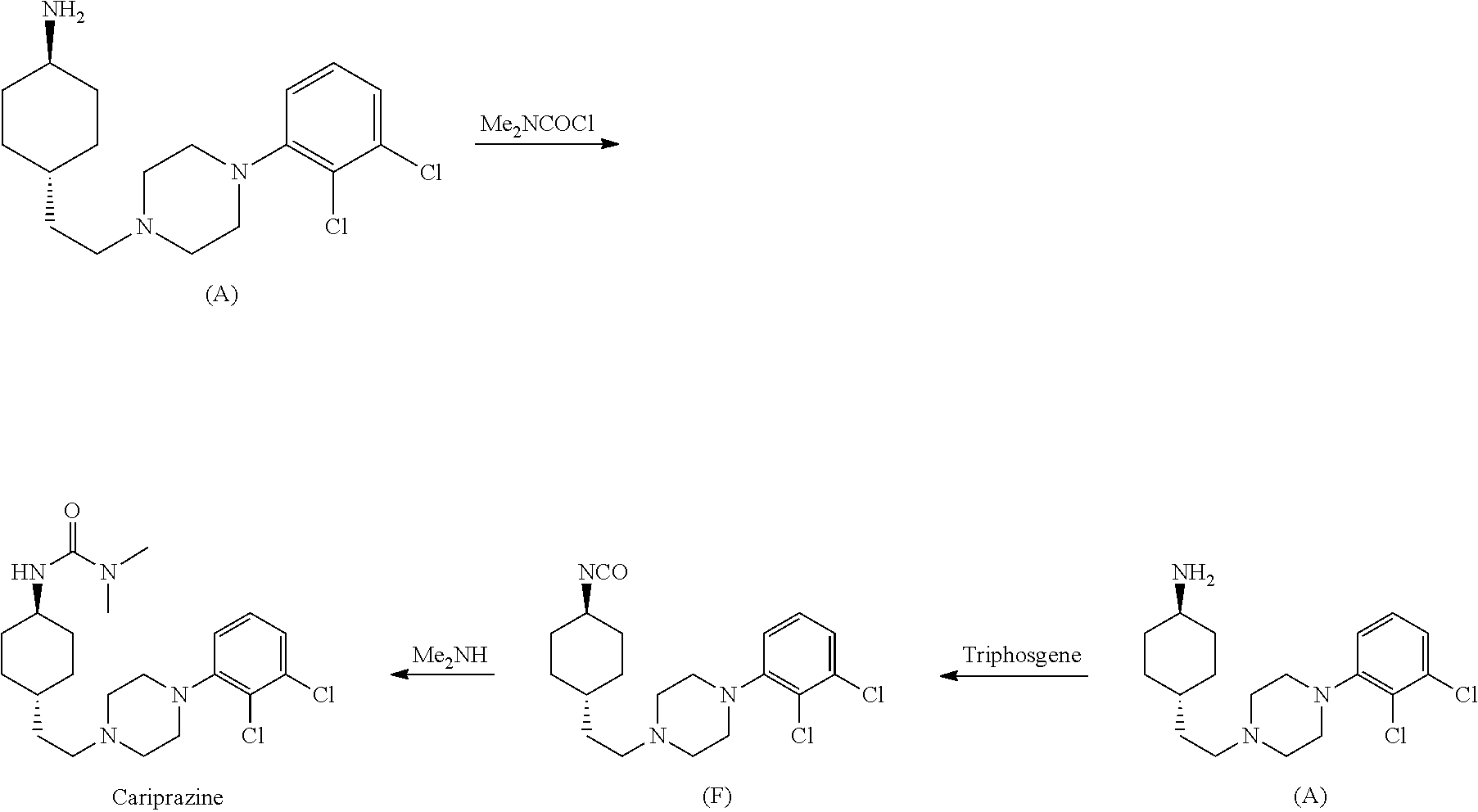
1,4-cyclohexylamine derivatives and processes for the preparation thereof
The invention relates to a process for the synthesis of Cariprazine, an antipsychotic compound useful in the treatment of positive and negative symptoms associated to schizophrenia, with the following structural formula: (A) The invention further relates to the synthesis of intermediates useful in the preparation of Cariprazine.
Owner:CHEMO RES
Methods of treating schizophrenia
InactiveUS20060292128A1Improve cognitive functionImproving cognitive deficitsBiocideNervous disorderNegative symptomSide effect
Owner:TITAN PHARMA
2-Substituted-oxy-5-methylsulfonyl aryl piperazine acidamide analogue and preparation method and application thereof
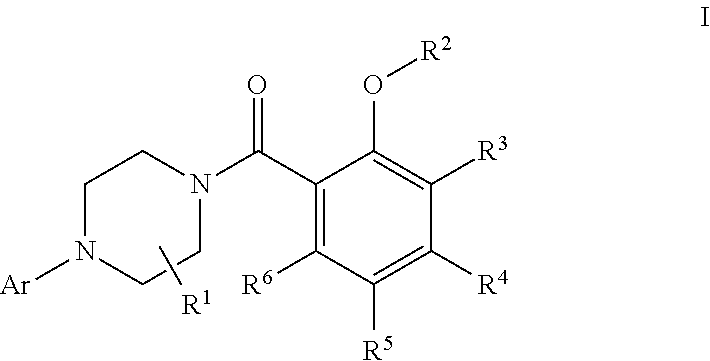
The invention discloses 2-substituted-oxy-5-methylsulfonyl aryl piperazine acidamide analogue and a preparation method and application thereof, and particularly, relates to 2-substituted-oxy-5-methylsulfonyl aryl piperazine acidamide analogue with a formula (I) compound and a preparation method and application thereof, wherein substitutions in the formula (I) compound are defined as in the description. This serial compound can inhibit the activity of glycine transport protein-1 (GlyT1), is useful in treating related diseases in central nerve and psychological fields, for example, schizophrenia (including positive symptoms, negative symptoms and cognitive symptoms), senile dementia, Parkinson's disease and other related psychological diseases, is widely applicable to the drugs for preventing and treating central nerve and psychological diseases, and is expected to be developed into new-generation GlyT1 inhibitors.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Fault positioning method, device and equipment for power communication network
InactiveCN110336590AShort timeImprove fault location efficiencyPower distribution line transmissionData switching networksNegative symptomFault propagation
The invention discloses a fault positioning method, device and equipment for a power communication network and a readable storage medium, and the method can build a fault propagation model based on the Bayesian theory according to the historical operation and maintenance data of the power communication network; in the fault positioning process, the fault propagation model is firstly segmented intoa plurality of independent sub-models based on the D-segmentation theory, and then fault positioning is realized according to the detected negative symptom set and each sub-model, so that the time consumption of the fault positioning process is remarkably reduced, and the fault positioning efficiency is improved.
Owner:GUANGDONG POWER GRID CO LTD +1
Chinese clinical phenotype fine-grained named entity recognition method and system
PendingCN114564959APrecisely structured dataCharacter and pattern recognitionNatural language data processingConditional random fieldMedical record
The invention provides a Chinese clinical phenotype-based fine-grained named entity recognition method and system, and belongs to the technical field of clinical medical record information processing, and the method comprises the steps: carrying out the character-level embedded feature extraction of a clinical text through a natural language pre-training model BERT; integrating the character-level embedded features and the sequence features of the clinical text by using a bidirectional long and short word memory model BiLSTM, and carrying out feature coding to obtain tags; and carrying out decoding prediction on the tag by using a conditional random field (CRF) to obtain a named entity recognition result. According to the method, a clinical fine-grained phenotype entity standard data set for a fine-grained named entity experiment is established, negative symptoms and positive symptoms are distinguished, and more accurate structured data is provided for clinical analysis.
Owner:BEIJING JIAOTONG UNIV
Predicting negative symptom change during drug treatment
A method for predicting a negative symptom change in a subject during drug therapy is provided. The method comprises isolating genomic DNA from a sample of the subject, and genotyping a T5988C marker of GRIN2B gene. A GRIN2B 5988 T / T genotype is predictive of a negative symptom improvement in the subject in response to drug therapy. The drug therapy may be clozapine therapy. Also provided is a method of identifying a polymorphism in a nucleotide sequence of interest that is predictive of response to drug therapy comprising the steps of assessing negative symptom improvement in a plurality of subjects during the course of drug therapy, isolating a sample comprising DNA from each subject, genotyping one or more nucleotide sequences of interest in the DNA of each subject to identify one or more polymorphisms that exist in the one or more nucleotide sequences of interest, wherein correlation of a significant improvement of negative symptoms with one or more polymorphisms is predictive of response to drug therapy.
Owner:CENT FOR ADDICTION & MENTAL HEALTH
N-acyl cyclic amine derivative or pharmaceutically acceptable salt thereof
InactiveCN102884059AImprove efficiencyReduce the risk of side effectsNervous disorderOrganic chemistryDiseaseSide effect
The present invention provides compounds which show high effectiveness against positive symptoms, negative symptoms and cognitive dysfunction in schizophrenia and reduce conventional side-effect risks as well as have remarkable effects for central neurological diseases associated with cognitive dysfunction other than schizophrenia. N-Acyl cyclic amine derivatives of formula (1): wherein Ar1 and Ar2 are aryl or heteroaryl; V is nitrogen, or CR3; W<1> is a single bond, -C(O)-, etc.; W<2> is C1- alkylene; W3 is a single bond, methylene, -NH-, -CR<4>=CR<5>-, etc.; Ring Q is a group of formula (a) in which n is 0 or 1; m is 0 to 2; k is 1 to 3; Z is a single bond, methylene, oxygen, etc.; R<1a>, R<1b> and R<1c> are each, same or different, hydrogen, hydroxyl, halogen, cyano, C1-6 alkyl, etc.
Owner:SUMITOMO PHARMA CO LTD
5,7-Dihydro-Pyrrolo-Pyridine Derivatives
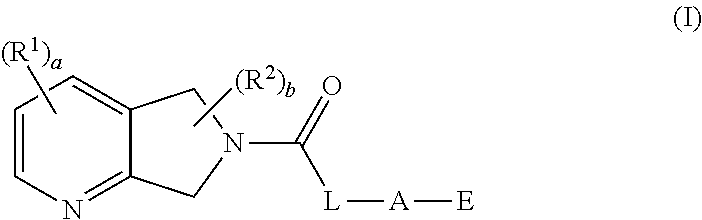
The present invention provides, in part, compounds of Formula I:or an N-oxide thereof, or a pharmaceutically acceptable salt of the compound or the N-oxide, wherein: R1, R2, L, A, and E are as described herein; processes for the preparation of; intermediates used in the preparation of; and compositions containing such compounds, N-oxides, or salts, and their uses for treating M4-mediated (or M4-associated) disorders including, e.g., Alzheimer's Disease, schizophrenia (e.g., its cognitive and negative symptoms), pain, addiction, and a sleep disorder.
Owner:PFIZER INC
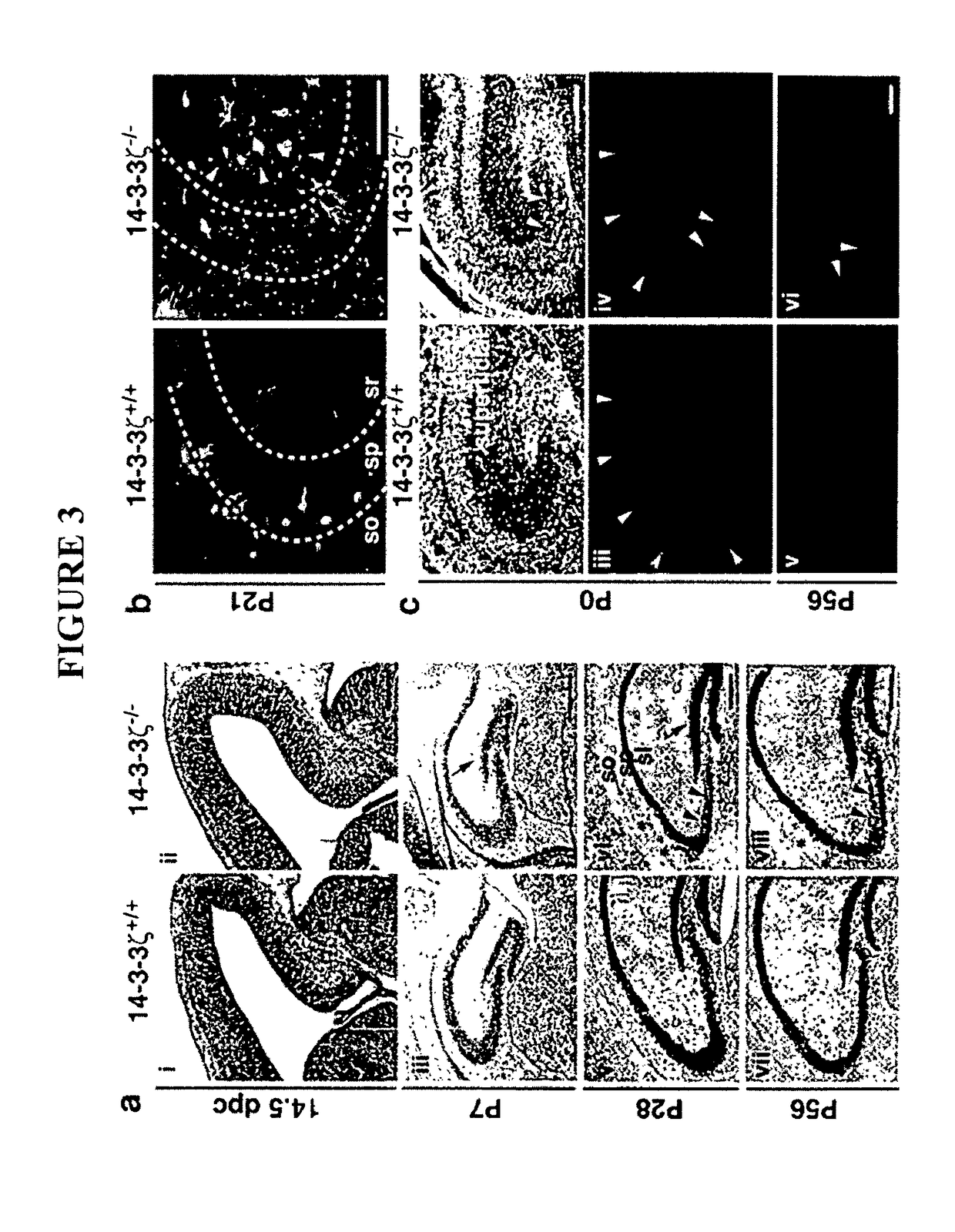
Screening method
The present invention provides a method of screening a mammal for the onset or predisposition to the onset of a neuropsychiatric disorder. More particularly, the present invention provides a method of screening a mammal for the onset or predisposition to the onset of schizophrenia by screening for a decrease in the functional level of protein 14-3-3ζ. In a related aspect, the present invention also provides a means of monitoring a patient diagnosed with a neuropsychiatric disorder, such as schizophrenia, by screening for changes to functional levels of protein 14-3-3ζ. This may be useful, for example, in the context of evaluating the effectiveness of a prophylactic or therapeutic treatment regime or otherwise monitoring the impact of physiological or metabolic changes which may occur in a patient. The method of the present invention is useful in a wide range of applications including, inter alia, providing a means of identifying mammals susceptible to the onset of a neuropsychiatric condition, such as a condition characterized by one or more symptoms of schizophrenia, thereby enabling the implementation of prophylactic or early therapeutic intervention in an effort to either minimize or prevent the onset of the condition. It also provides a means of confirming diagnoses which would otherwise be based solely on an assessment of positive and negative symptoms.
Owner:PRECISION MEDICINE HLDG PTY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com









![Bicyclic [3.1.0.] heteroaryl amides as type 1 glycine transport inhibitors Bicyclic [3.1.0.] heteroaryl amides as type 1 glycine transport inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b3093b82-9c8c-4ca2-8805-17c0a5e15b29/US20060229455A1-20061012-C00001.png)
![Bicyclic [3.1.0.] heteroaryl amides as type 1 glycine transport inhibitors Bicyclic [3.1.0.] heteroaryl amides as type 1 glycine transport inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b3093b82-9c8c-4ca2-8805-17c0a5e15b29/US20060229455A1-20061012-C00002.png)
![Bicyclic [3.1.0.] heteroaryl amides as type 1 glycine transport inhibitors Bicyclic [3.1.0.] heteroaryl amides as type 1 glycine transport inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b3093b82-9c8c-4ca2-8805-17c0a5e15b29/US20060229455A1-20061012-C00003.png)










![Bicyclic [3.1.0] derivatives as glycine transporter inhibitors Bicyclic [3.1.0] derivatives as glycine transporter inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0df82db1-84b0-4789-9f0a-8cd30b6dfdf9/US07473787-20090106-C00001.png)
![Bicyclic [3.1.0] derivatives as glycine transporter inhibitors Bicyclic [3.1.0] derivatives as glycine transporter inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0df82db1-84b0-4789-9f0a-8cd30b6dfdf9/US07473787-20090106-C00002.png)
![Bicyclic [3.1.0] derivatives as glycine transporter inhibitors Bicyclic [3.1.0] derivatives as glycine transporter inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0df82db1-84b0-4789-9f0a-8cd30b6dfdf9/US07473787-20090106-C00003.png)