Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Bipolar I disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bipolar I disorder (BD-I; pronounced "type one bipolar disorder") is a type of bipolar spectrum disorder characterized by the occurrence of at least one manic episode, with or without mixed or psychotic features. Most patients also, at other times, have one or more depressive episodes, and all experience a hypomanic stage before progressing to full mania.
Organic compounds
ActiveUS20120053190A1Useful in treatmentInhibitory activityBiocideOrganic active ingredientsPhosphodiesteraseBipolar mood disorder
The present invention relates to a new use of phosphodiesterase 1 (PDE1) inhibitors for the treatment of psychosis, schizophrenia, schizoaffective disorder, schizophreniform disorder, psychotic disorder, delusional disorder, mania, or bipolar disorder.
Owner:INTRA CELLULAR THERAPIES INC
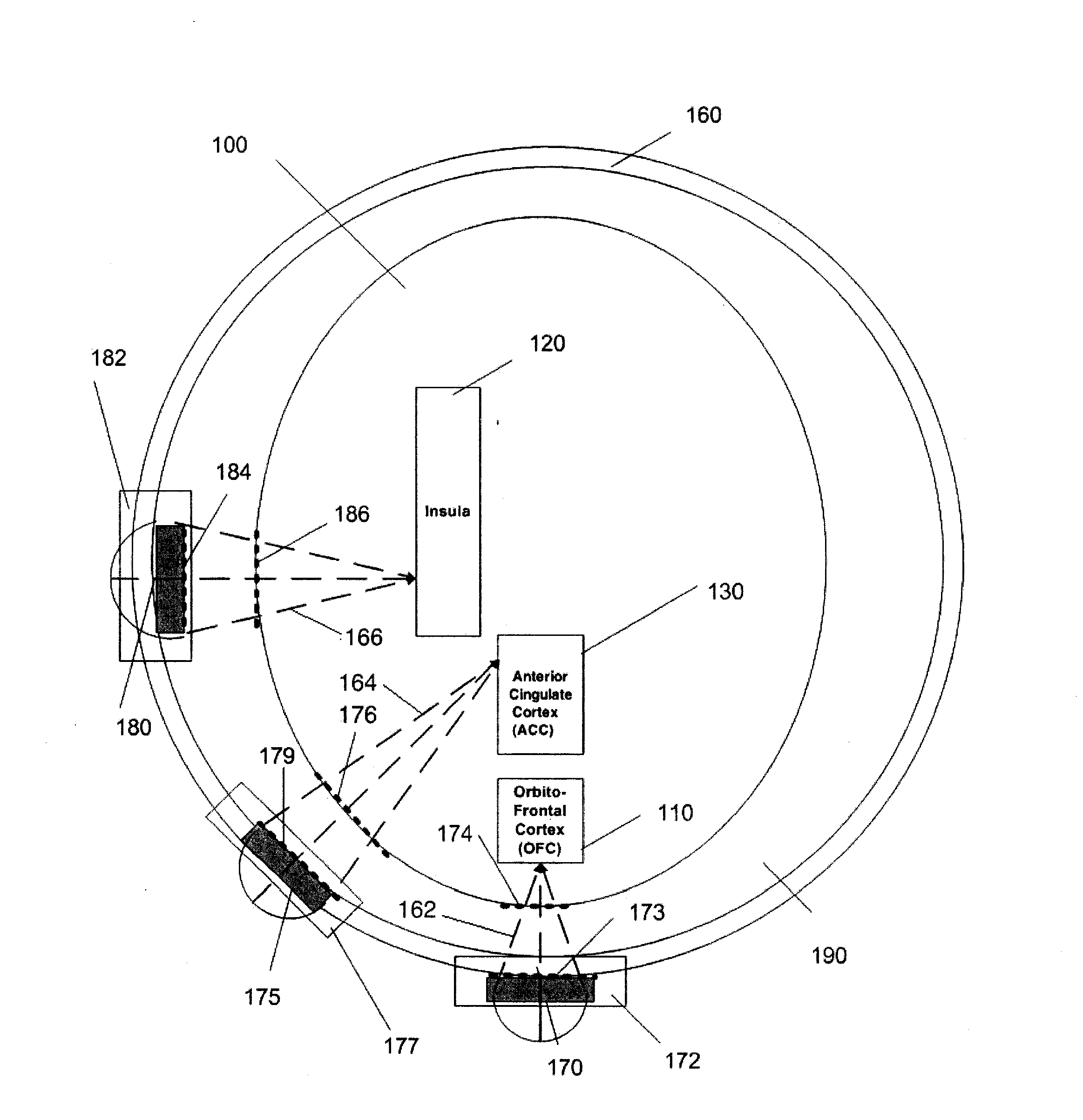
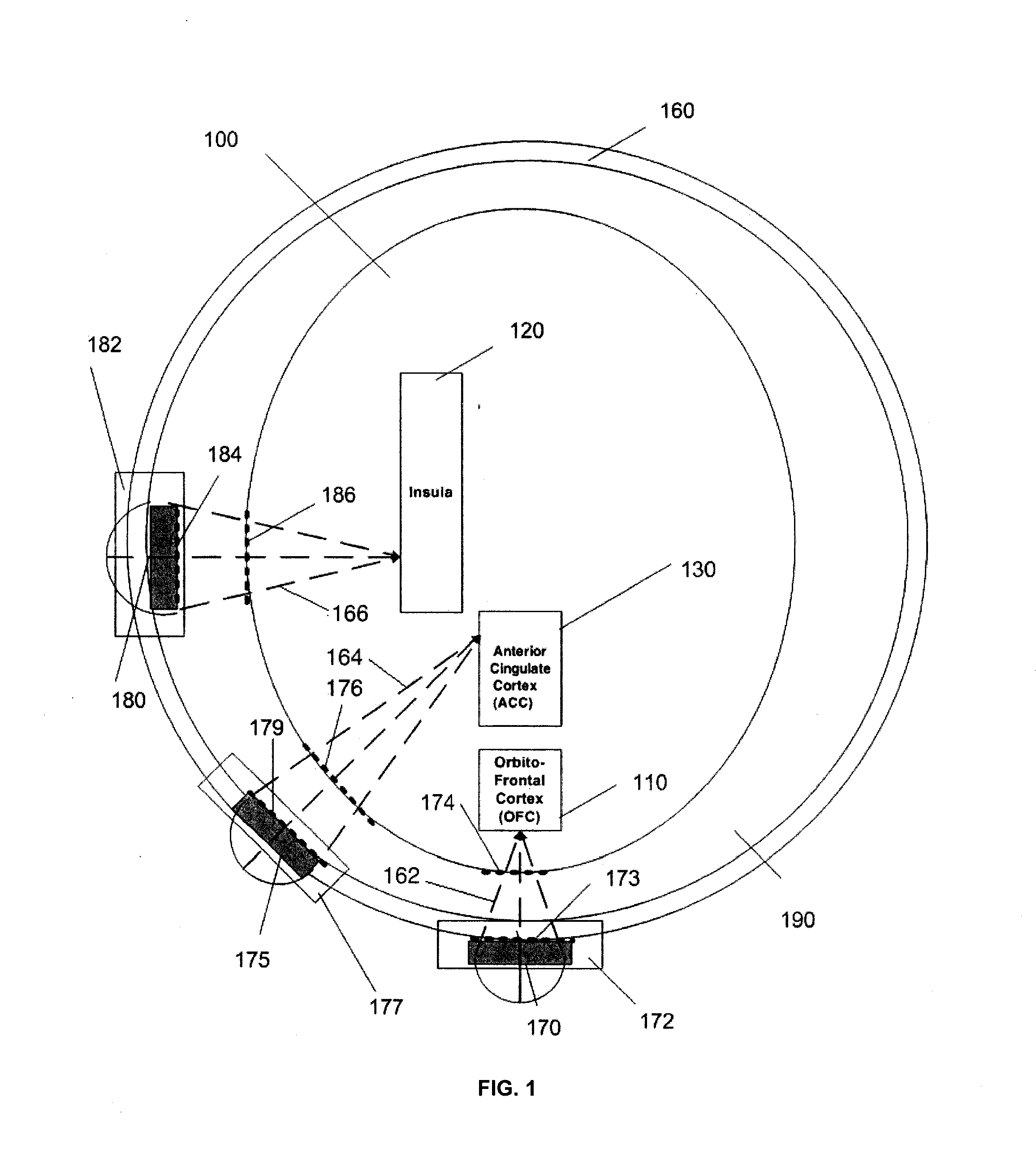
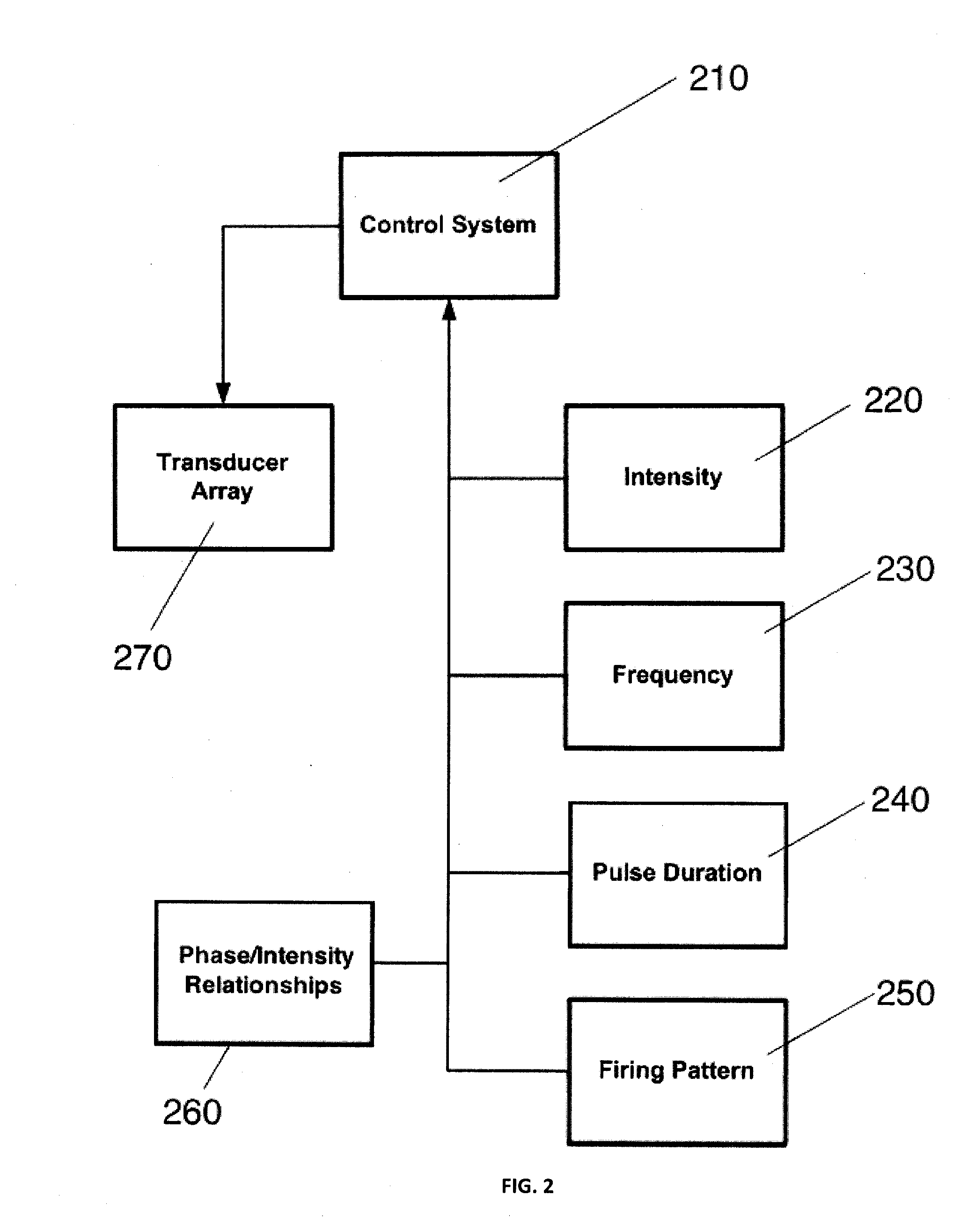
Ultrasound neuromodulation treatment of depression and bipolar disorder
InactiveUS20120283502A1Treatment for depressionAffect stateUltrasound therapyDiagnosticsBipolar mood disorderBipolar I disorder
Owner:NEUROTREK
Biomarkers for diagnosing schizophrenia and bipolar disorder
InactiveUS20050208519A1Monitoring therapeutic efficacyMonitor efficacySugar derivativesMicrobiological testing/measurementBipolar mood disorderBipolar I disorder
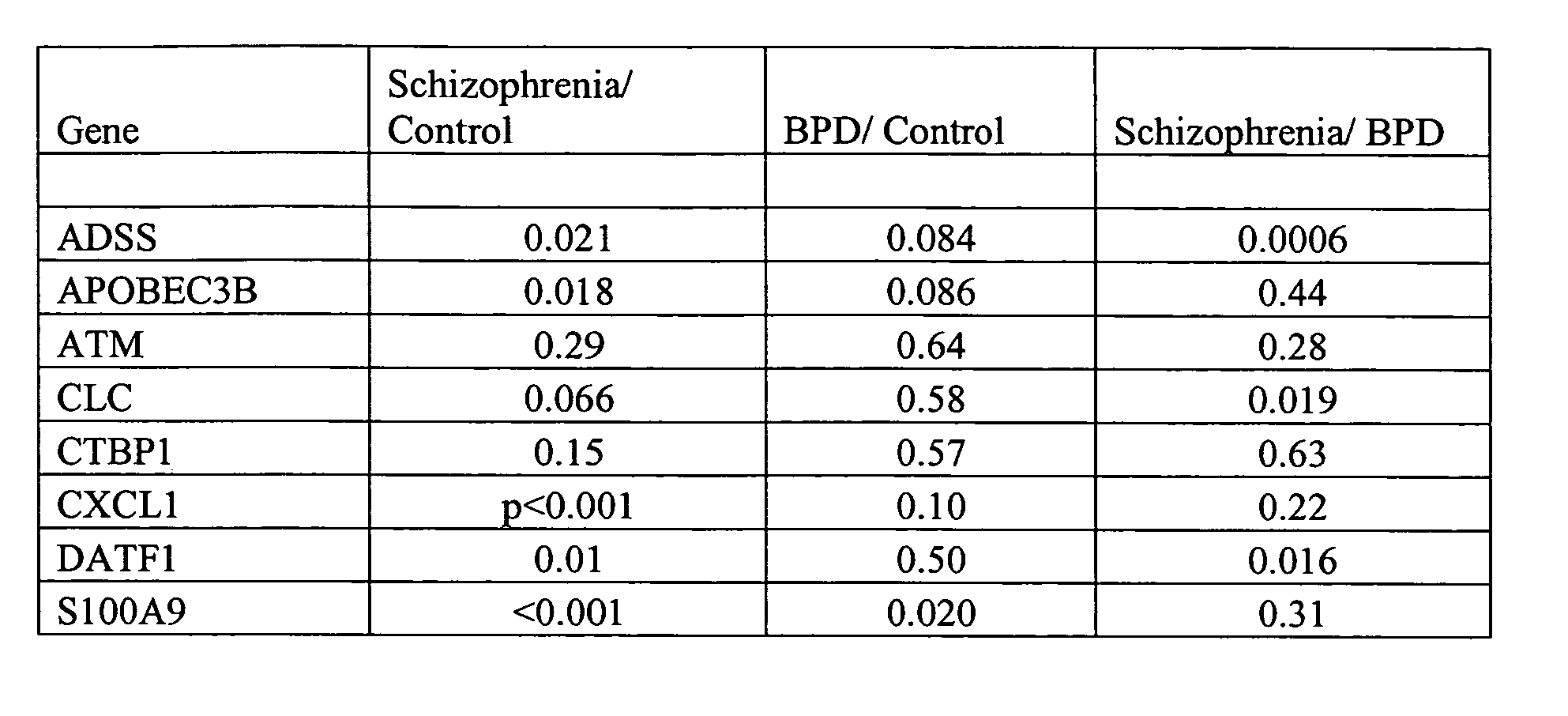
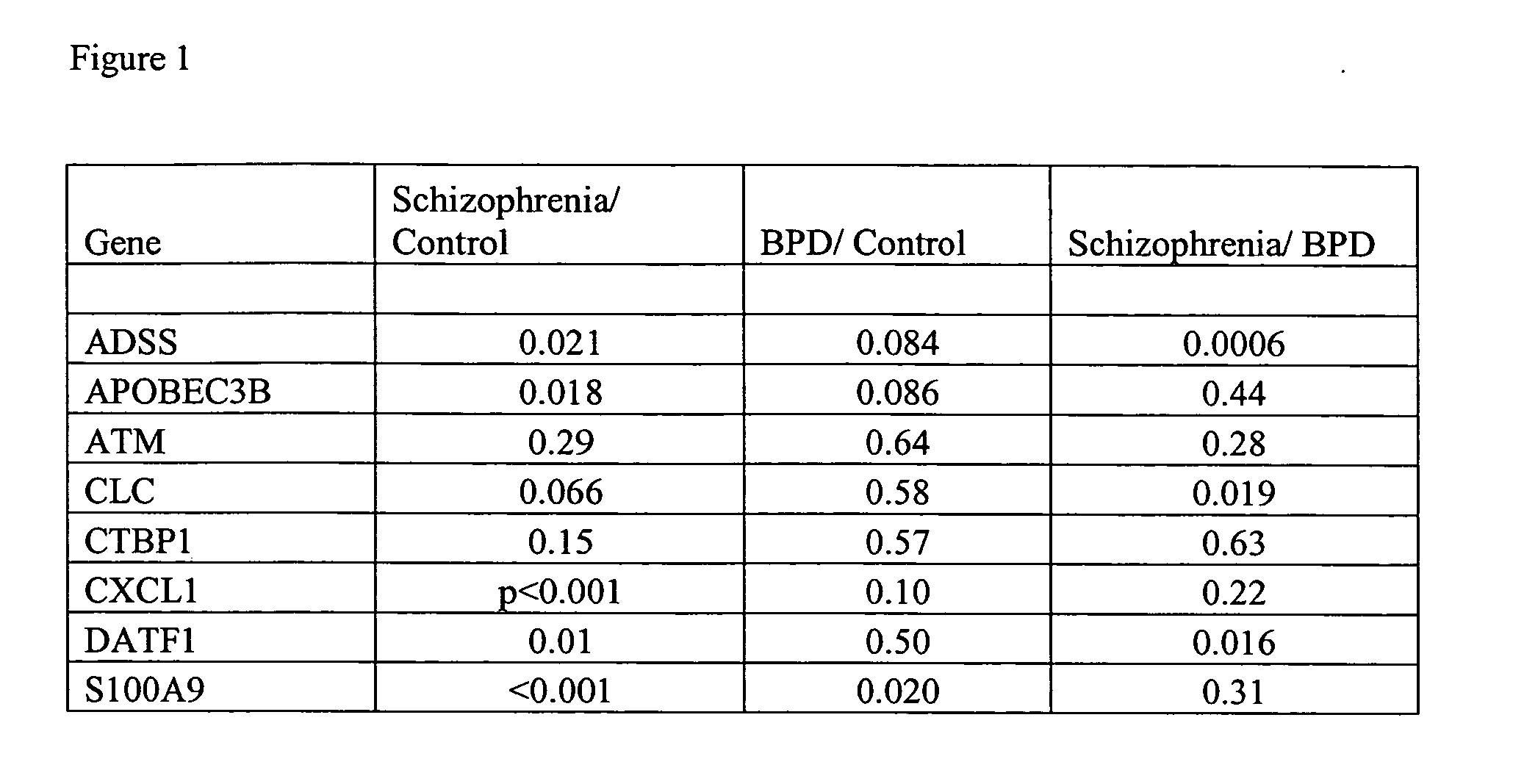
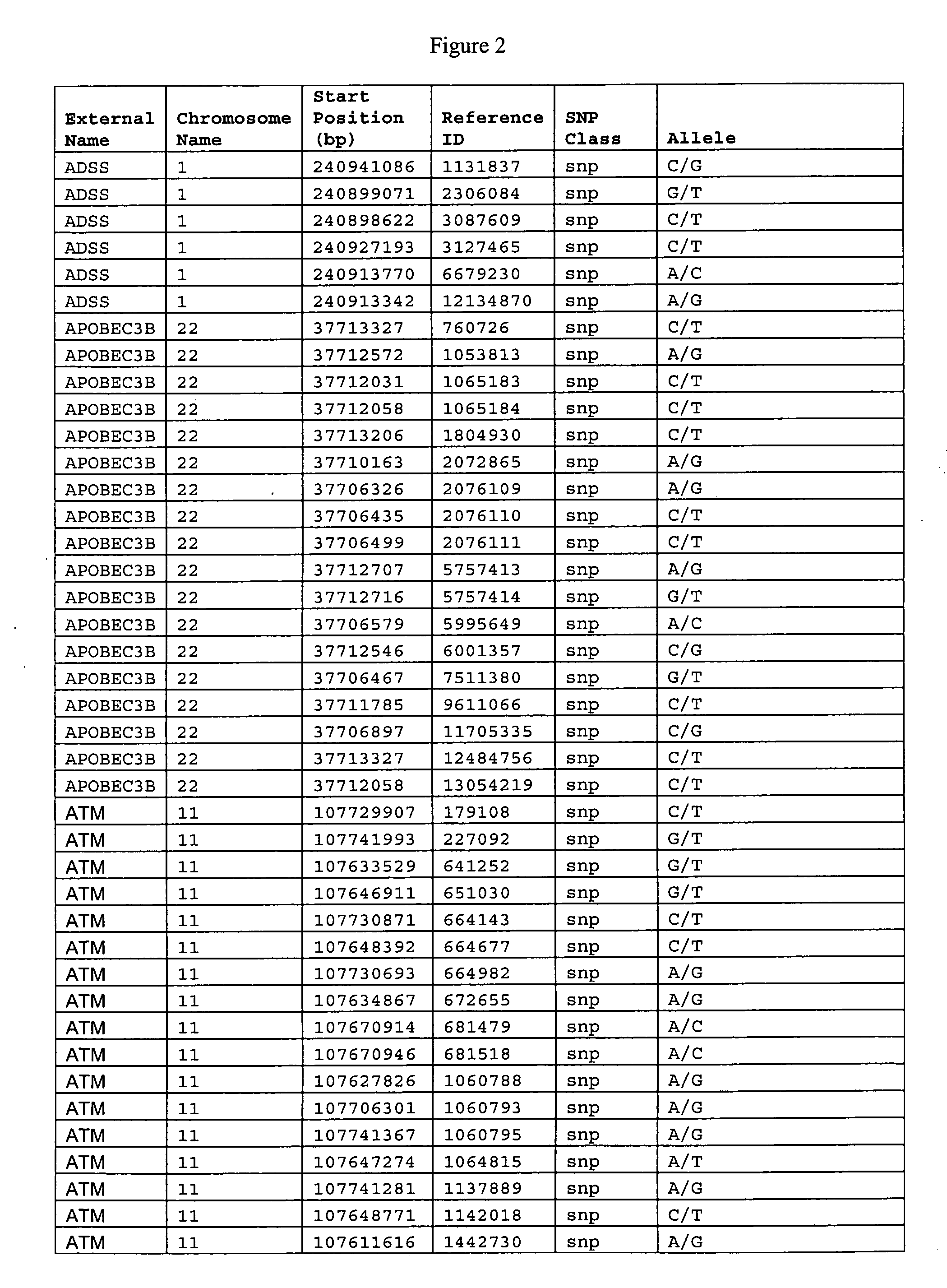
The invention relates to the identification and selection of novel biomarkers and the identification and selection of novel biomarker combinations which are differentially expressed in blood and useful in diagnosing schizophrenia and / or bipolar disorder as well as monitoring therapeutic efficacy of treatment for schizophrenia or bipolar disorder. The measurement of expression levels of the products of the biomarkers and combinations of biomarkers of the invention can be used to diagnose schizophrenia and / or bipolar disorder. Measurement of the expression level of products of biomarkers of the invention using polynucleotides and proteins which specifically and / or selectively hybridize to the products of the biomarkers of the invention are also encompassed within the scope of the invention as are compositions and kits containing said polynucleotides and proteins. Further encompassed by the invention is the use of the polynucleotides and proteins to monitor the efficacy of therapeutic regimens. The invention also provides for the identification of methods of using the products of the biomarkers of the invention in the identification of novel therapeutic targets of schizophrenia and / or bipolar disorder and a method of screening the genes of said biomarkers for additional markers of disease.
Owner:GENENEWS
Methods for treating bipolar disorder
ActiveUS20120004300A1Easily subdividedNovel and unique pharmacological propertiesBiocideNervous disorderBipolar mood disorderCarbamate
Owner:BIOPHARM
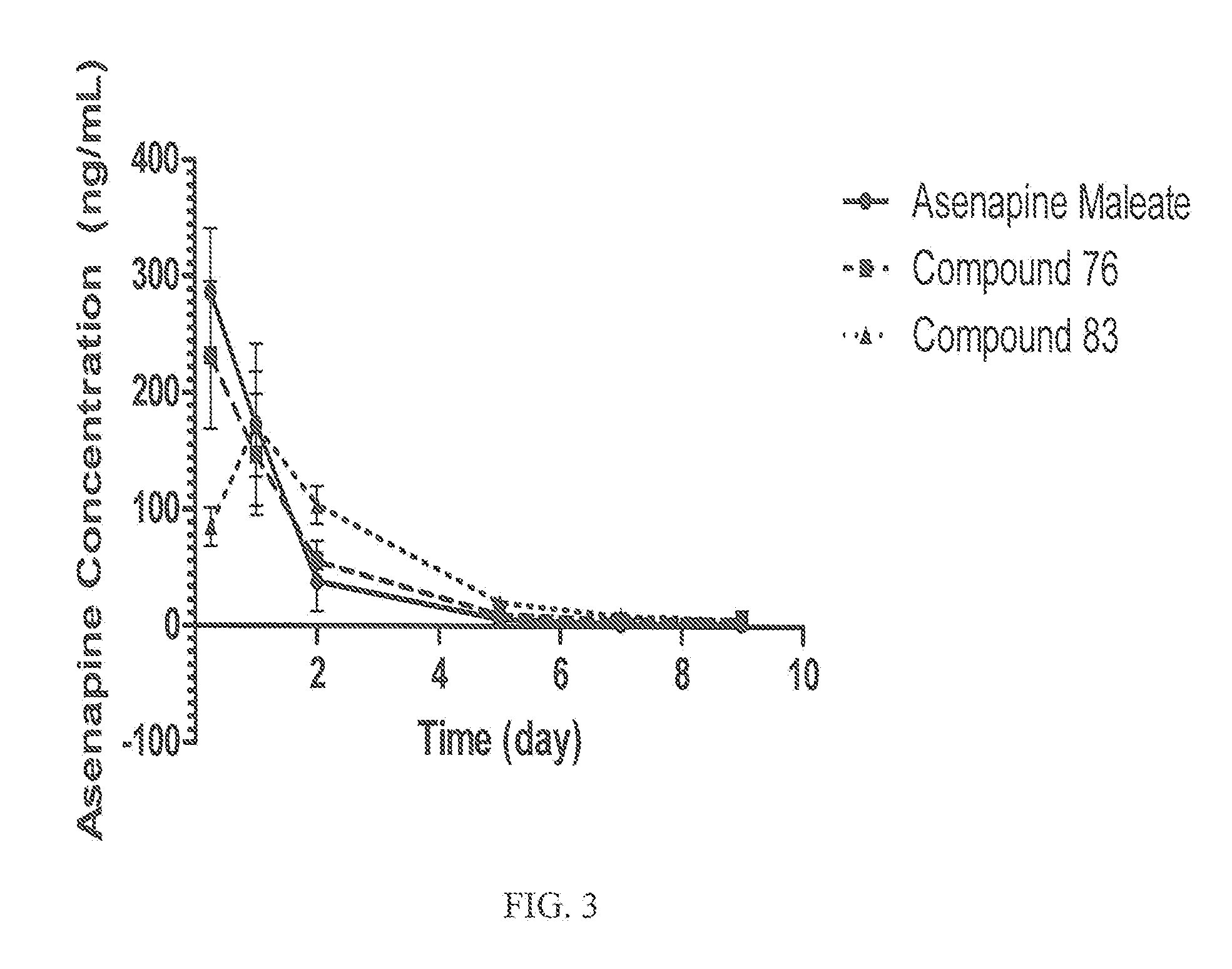
Asenapine Prodrugs
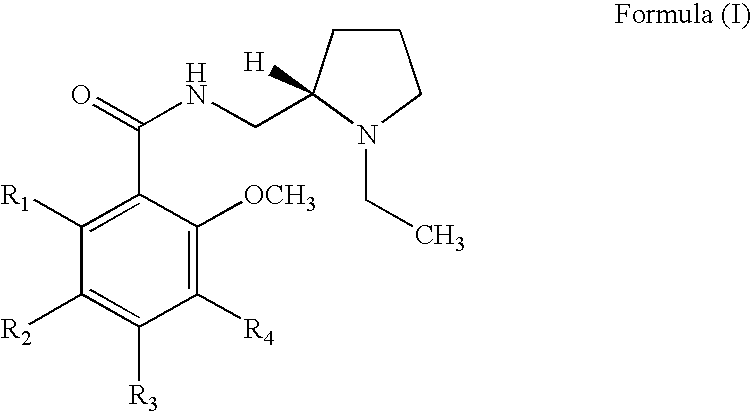
InactiveUS20110166194A1Minimize exposureMinimize diffusionBiocideNervous disorderBipolar type I disorderBipolar I disorder
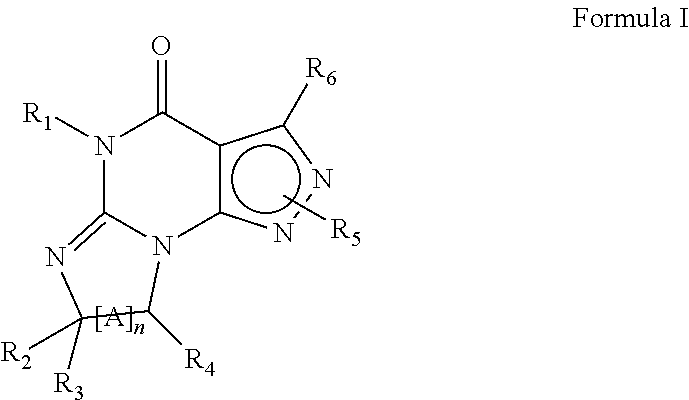
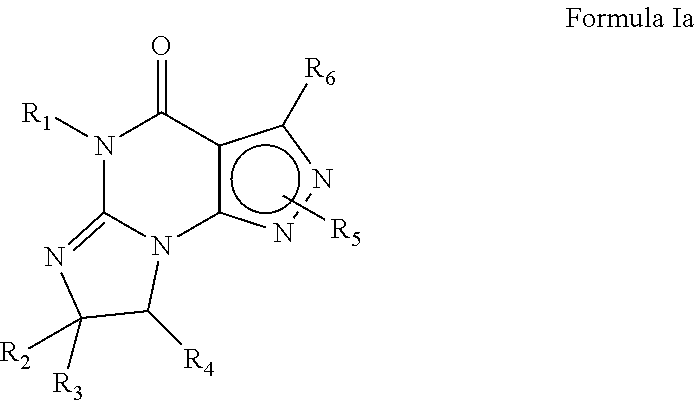
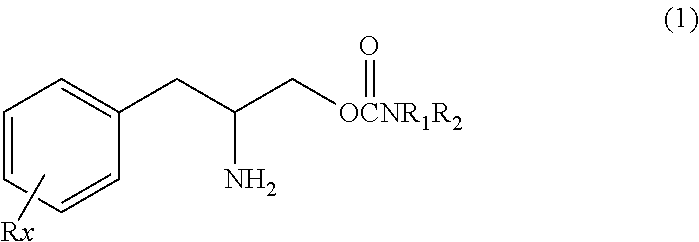
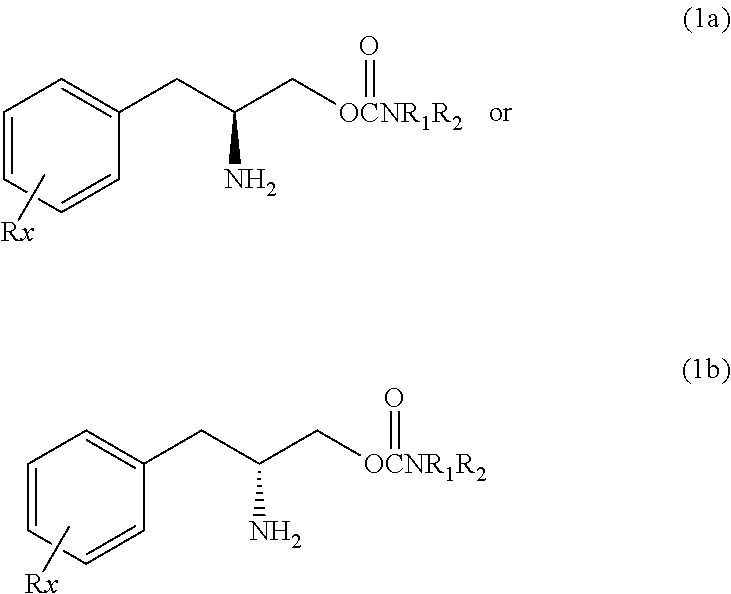
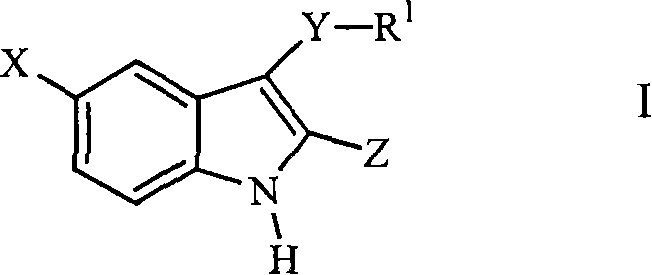
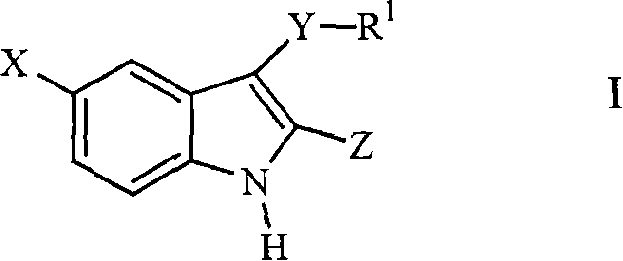
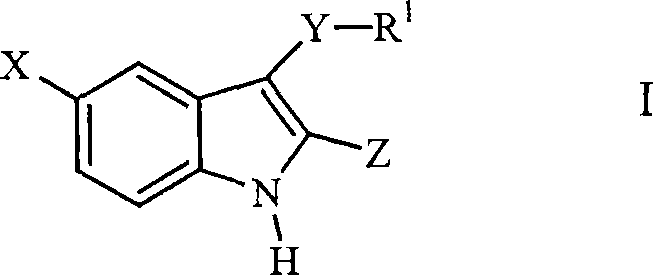
Compounds of Formula I and their use for the treatment of neurological and psychiatric disorders including schizophrenia and manic or mixed episodes associated with bipolar I disorder with or without psychotic features is disclosed:wherein R1-R8, G, N and A− are as defined in the written description.
Owner:ALKERMES INC
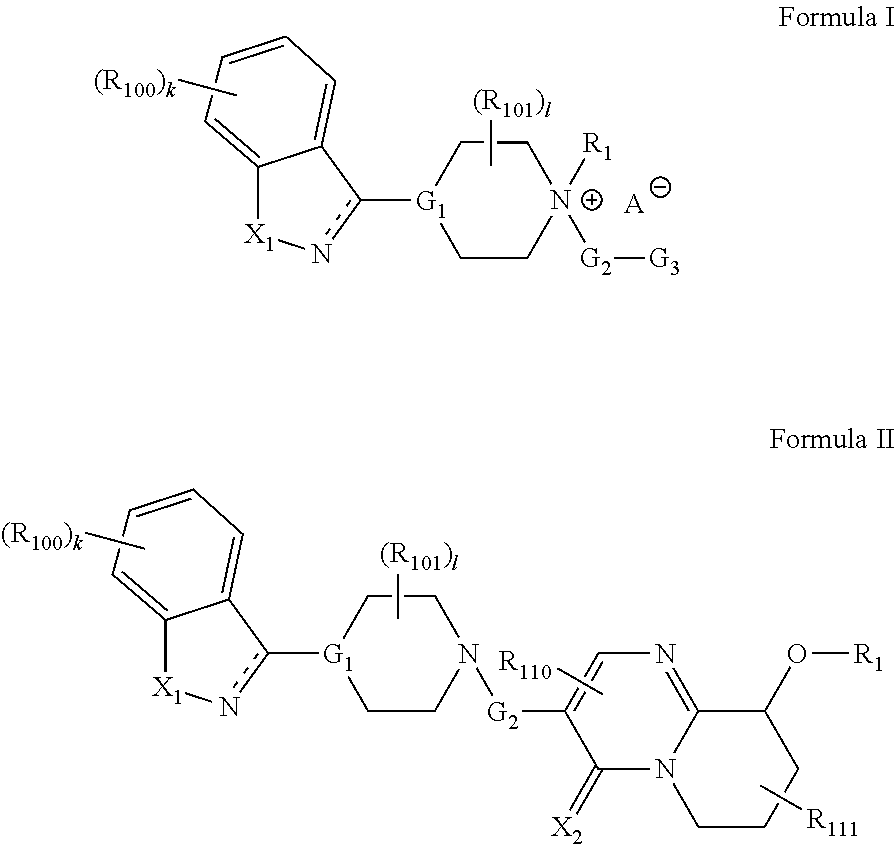
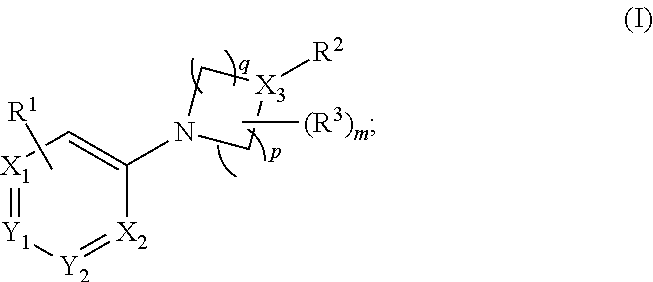
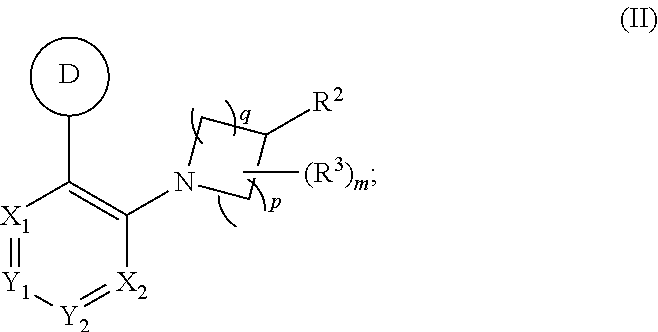
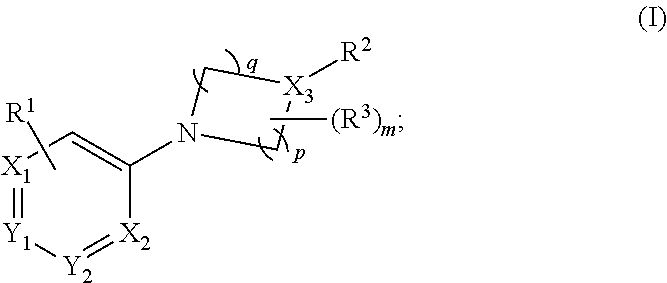
Prodrugs for the Treatment of Schizophrenia and Bipolar Disease
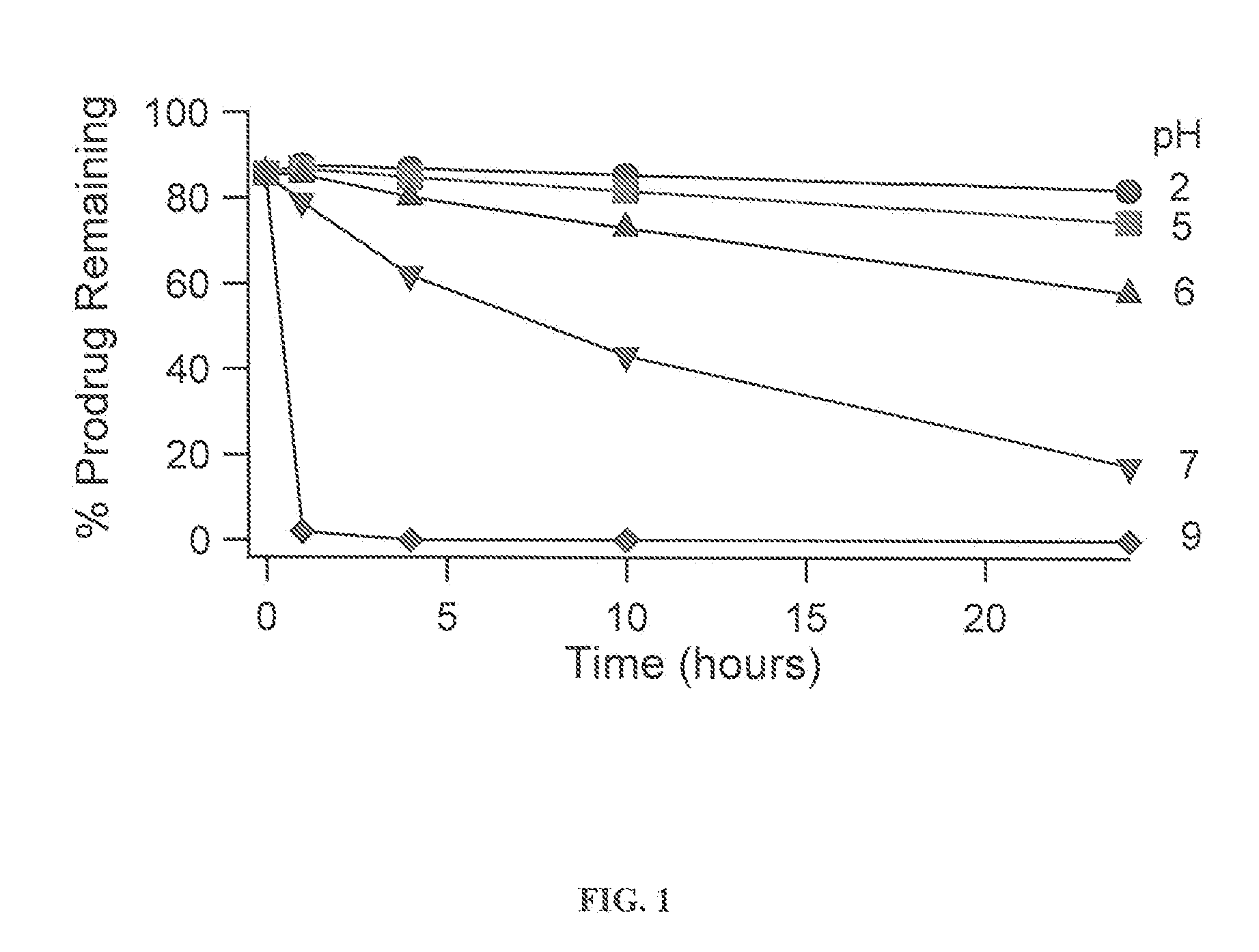
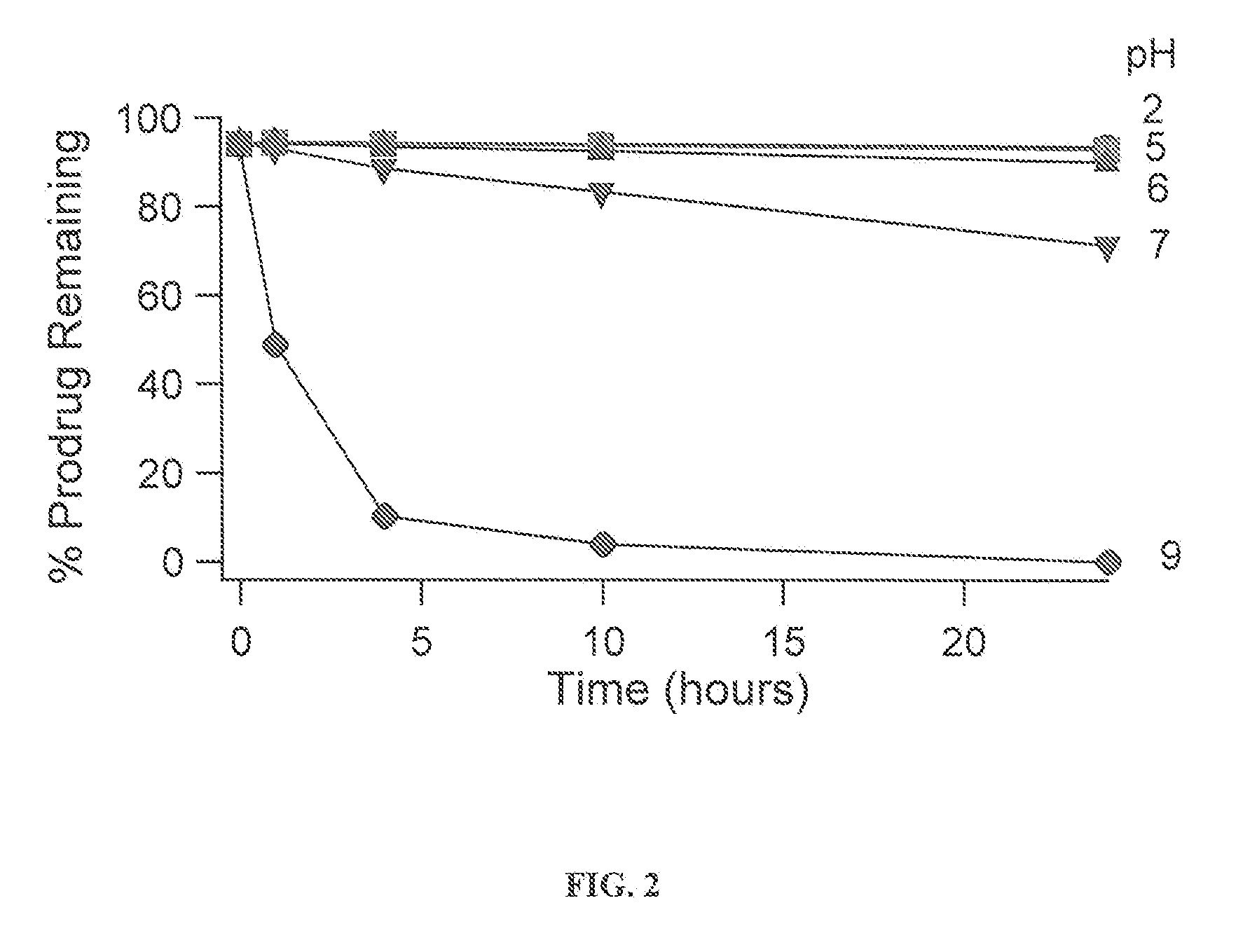
InactiveUS20110166156A1Useful in treatmentSufficient amountBiocideNervous disorderBipolar type I disorderBipolar I disorder
Compounds of Formula I and Formula II and their use for the treatment of neurological and psychiatric disorders including schizophrenia and manic or mixed episodes associated with bipolar I disorder with or without psychotic features is disclosed.
Owner:ALKERMES INC
Method of treating bipolar depression with a benzamide derivative
InactiveUS20080188537A1Effective treatmentBiocideNervous disorderBipolar mood disorderBipolar I disorder
A benzamide derivative, especially amisulpride, is used to prevent or treat bipolar depression of a patient suffering from bipolar disorder I or bipolar disorder II.
Owner:COPHARM
Aryl- and heteroaryl- nitrogen-heterocyclic compounds as pde10 inhibitors
ActiveUS20110306590A1Useful in treatmentBiocideNervous disorderHuntingtons choreaInsulin dependent diabetes
Owner:AMGEN INC
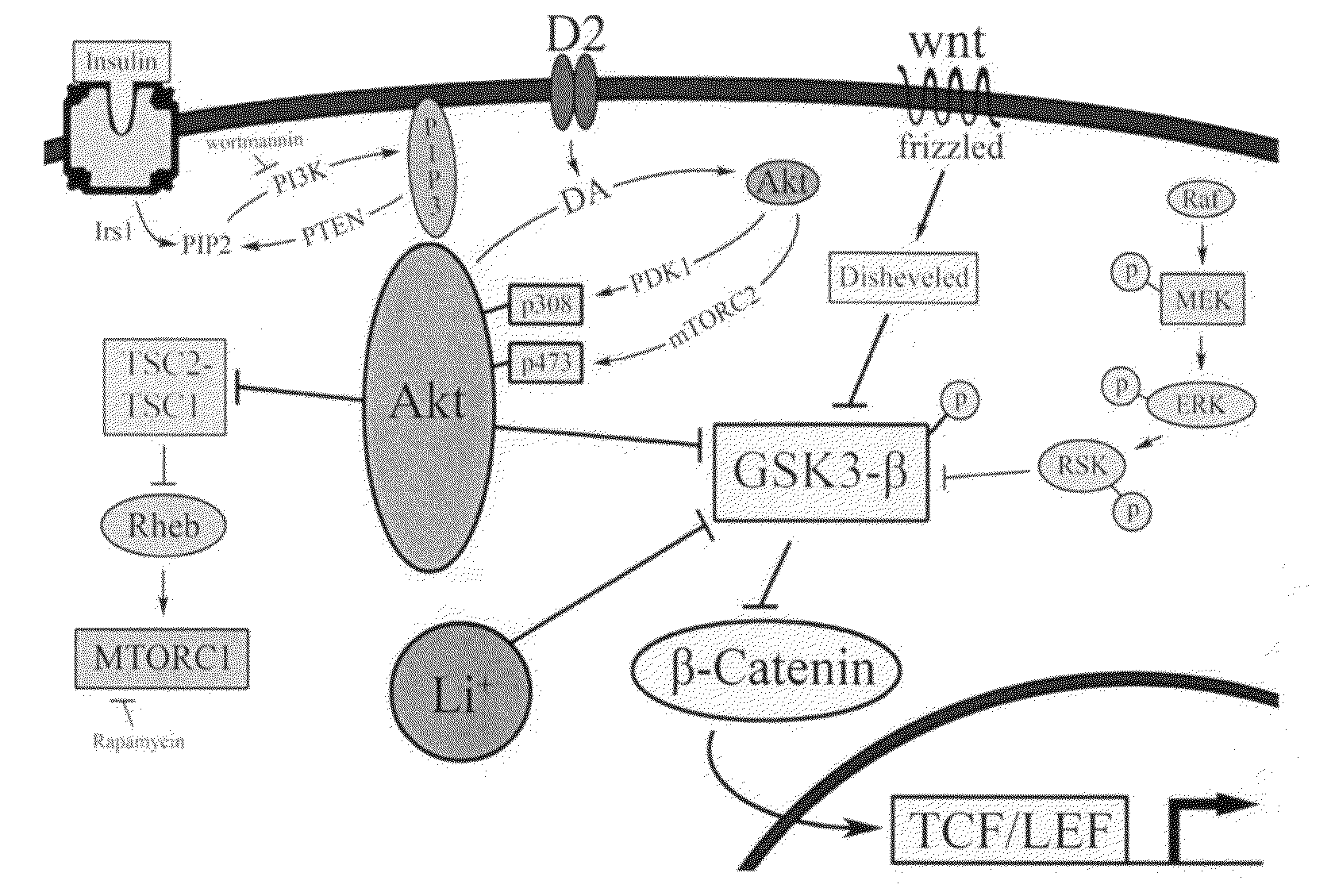
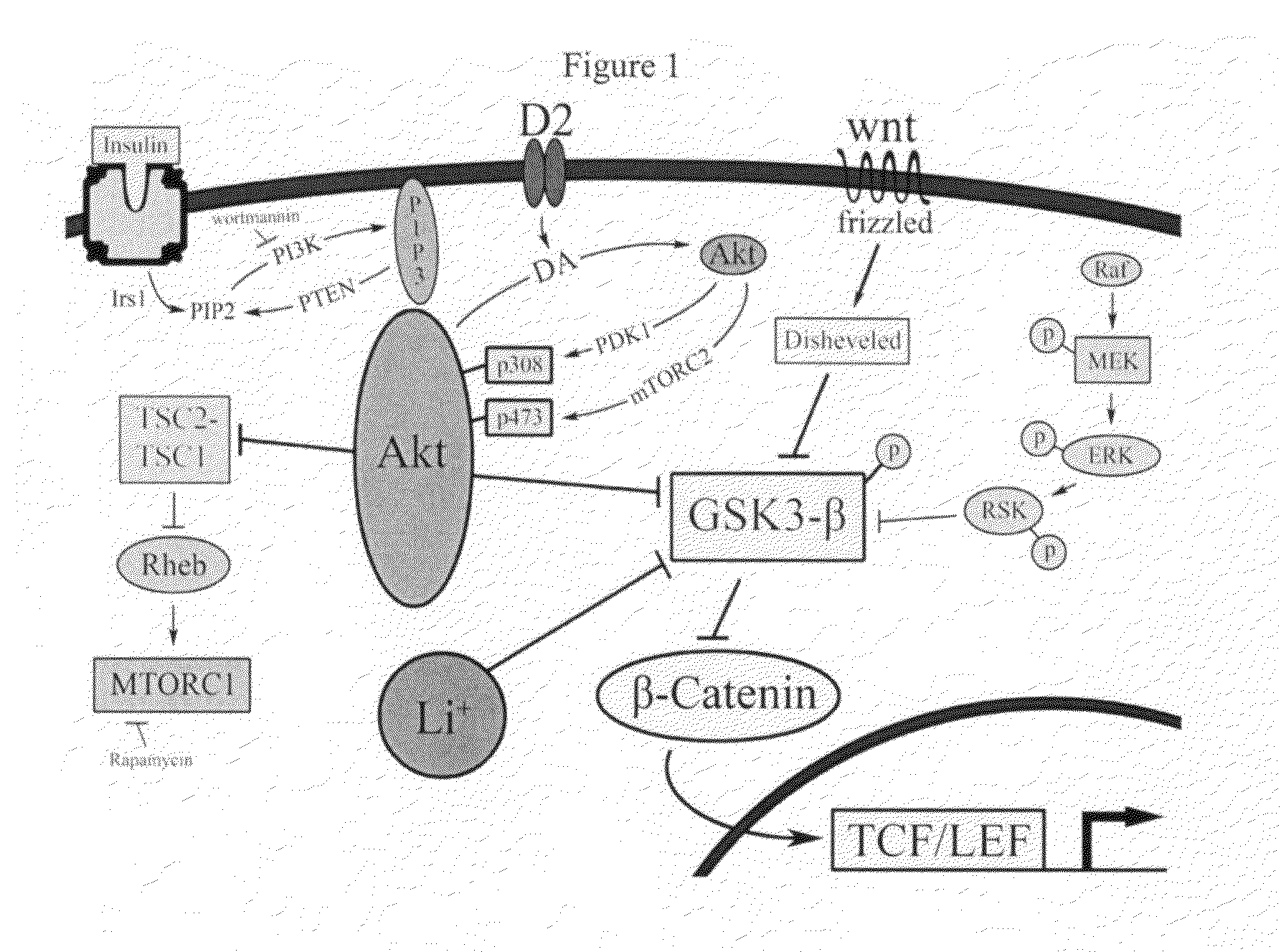
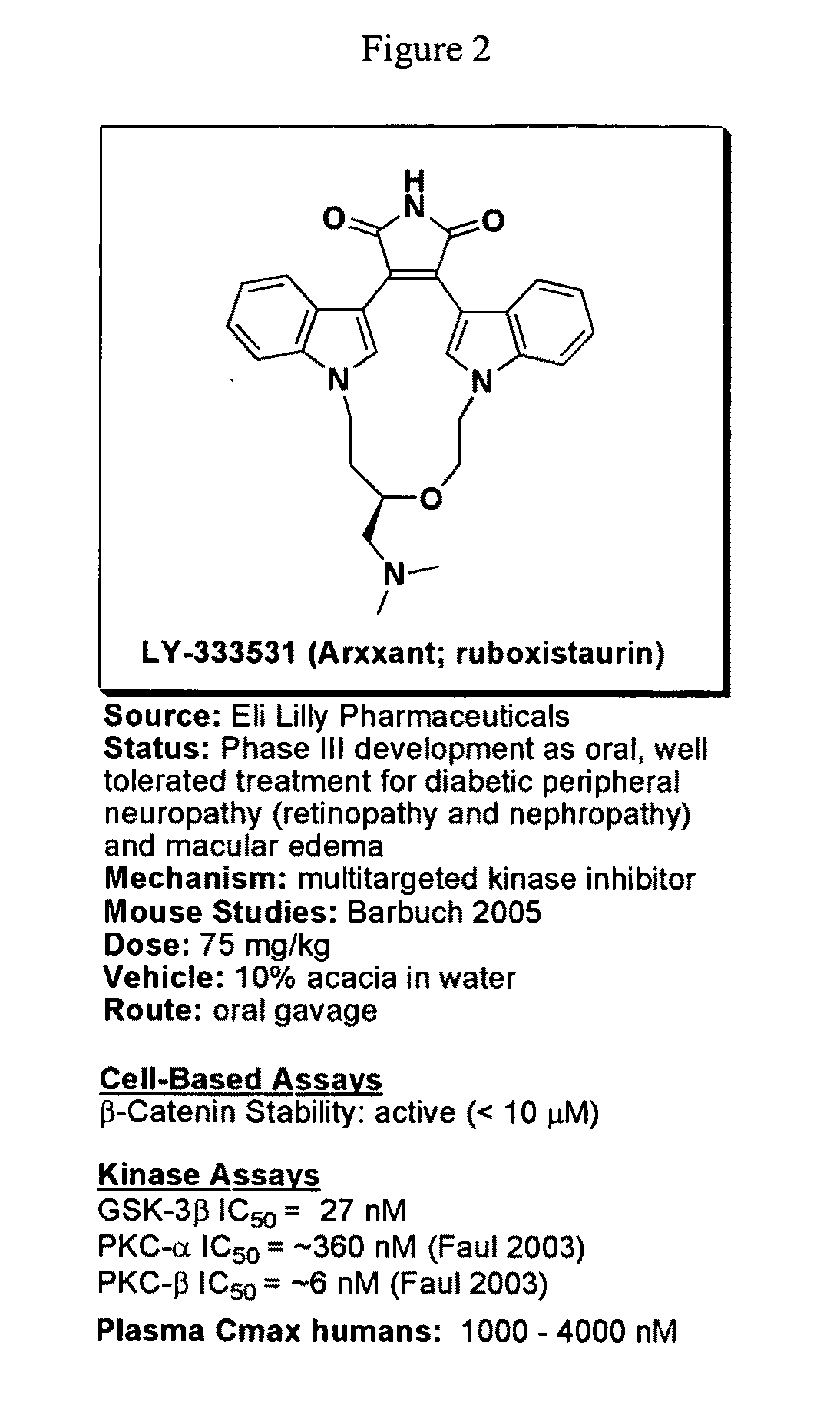
Uses of chemicals to modulate GSK-3 signaling for treatment of bipolar disorder and other brain disorders
Aspects of this invention are related, at least in part, to the use of chemical compounds able to inhibit GSK-3 and / or to stabilize β-catenin and formulations thereof. Some aspects of this invention relate to compositions comprising such compounds. Some aspects of the invention provide methods of using such compounds and / or compositions in the treatment of subjects having a neurological disease and / or psychiatric disorder. Some aspects of this invention provide methods of using ruboxistaurin, enzastaurin, sunitinib, midostaurin, lestaurtinib, 7-hydroxystaurosporine, and / or Chir99021 in the treatment of subjects having a neurological disease and / or psychiatric disorder. In some embodiments, compounds are administered in combination with Lithium.
Owner:MASSACHUSETTS INST OF TECH +1
Genes associated with schizophrenia adhd and bipolar disorders
InactiveUS20060172295A1High stringency conditionMicrobiological testing/measurementDiseaseBipolar mood disorder
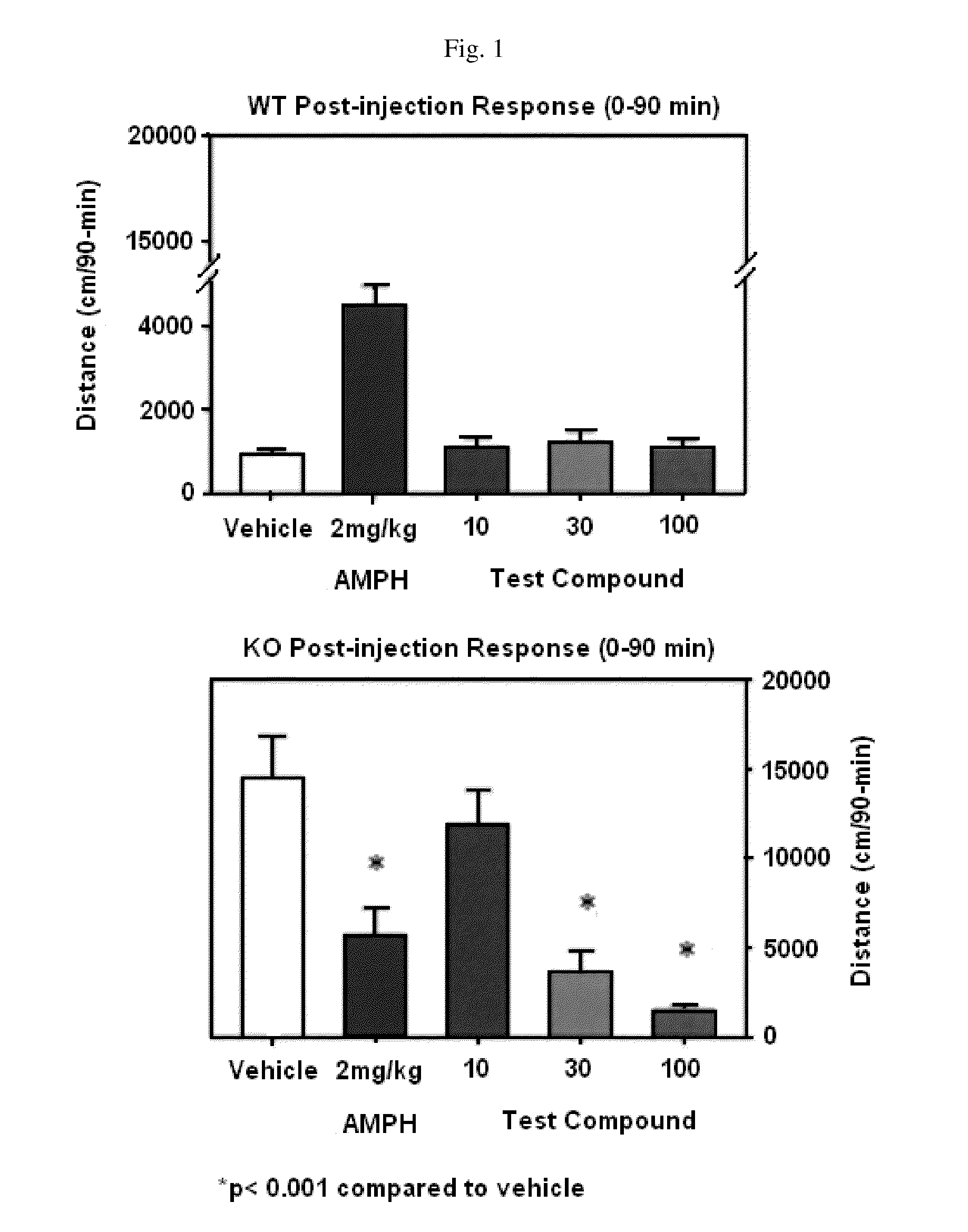
Disclosed are methods for diagnosing, monitoring the progression of, and treating schizophrenia, bipolar disorder, and / or ADHD based upon genes that are differentially expressed in said disorders at baseline, or at different timepoints following an acute stress exposure. Also disclosed are methods for identifying agents useful in the treatment of schizophrenia, bipolar disorder, and / or ADHD, methods for monitoring the efficacy of a treatment for schizophrenia, bipolar disorder, and / or ADHD, methods for preventing and treating schizophrenia, bipolar disorder, and / or ADHD, and an animal model for schizophrenia, bipolar disorder, and / or ADHD.
Owner:UNIV OF MARYLAND
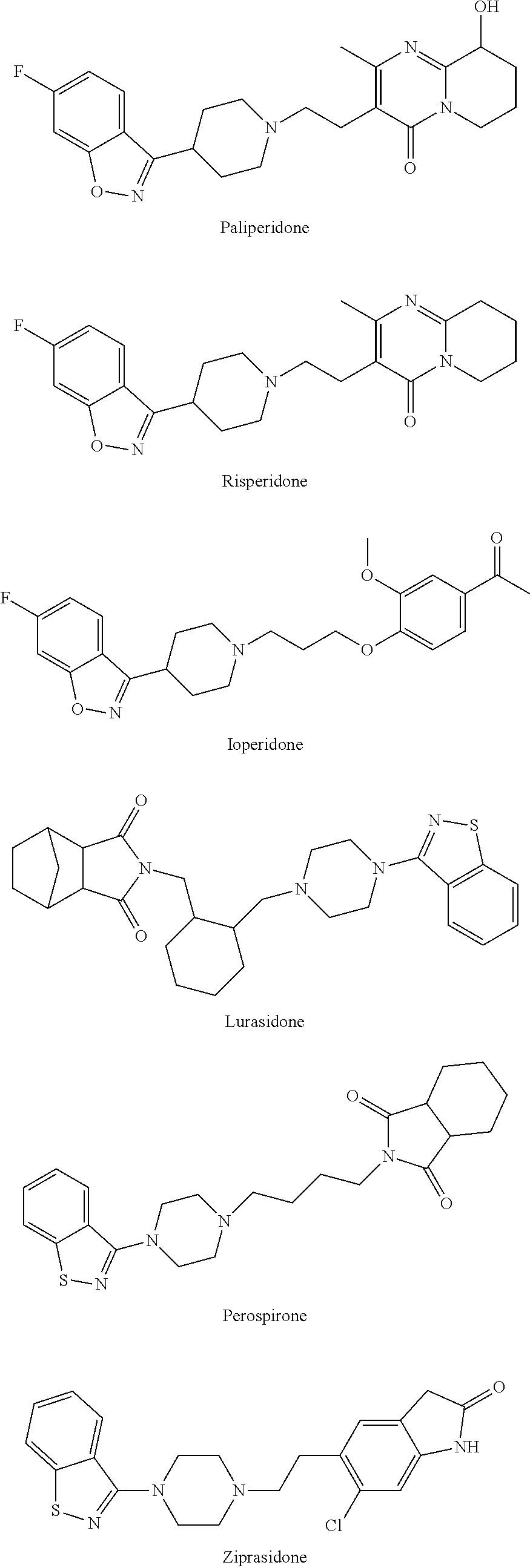
Pharmaceutical compositions of Lurasidone and Process for preparation thereof
Pharmaceutical compositions comprising an atypical antipsychotic as an active agent, process of preparation thereof and method of using the same are provided. Particularly the present invention relates to pharmaceutical compositions comprising lurasidone, process of preparation thereof and method to treat various psychotic disorders such as schizophrenia, positive and negative symptoms of schizophrenia, memory or learning dysfunctions caused by schizophrenia, senile dementia, attention deficit / hyperactivity disorder (ADHD), central nervous system (CNS) disorder responsive to modulation of glutamate levels, major depressive episodes associated with bipolar I disorder and other associated CNS disorders.
Owner:AUROBINDO PHARMA LTD
Methods For Diagnosing Bipolar Disorders and Attention-Deficit/Hyperactivity Disorder
InactiveUS20080160554A1Microbiological testing/measurementArtificial cell constructsBipolar mood disorderLymphoblast
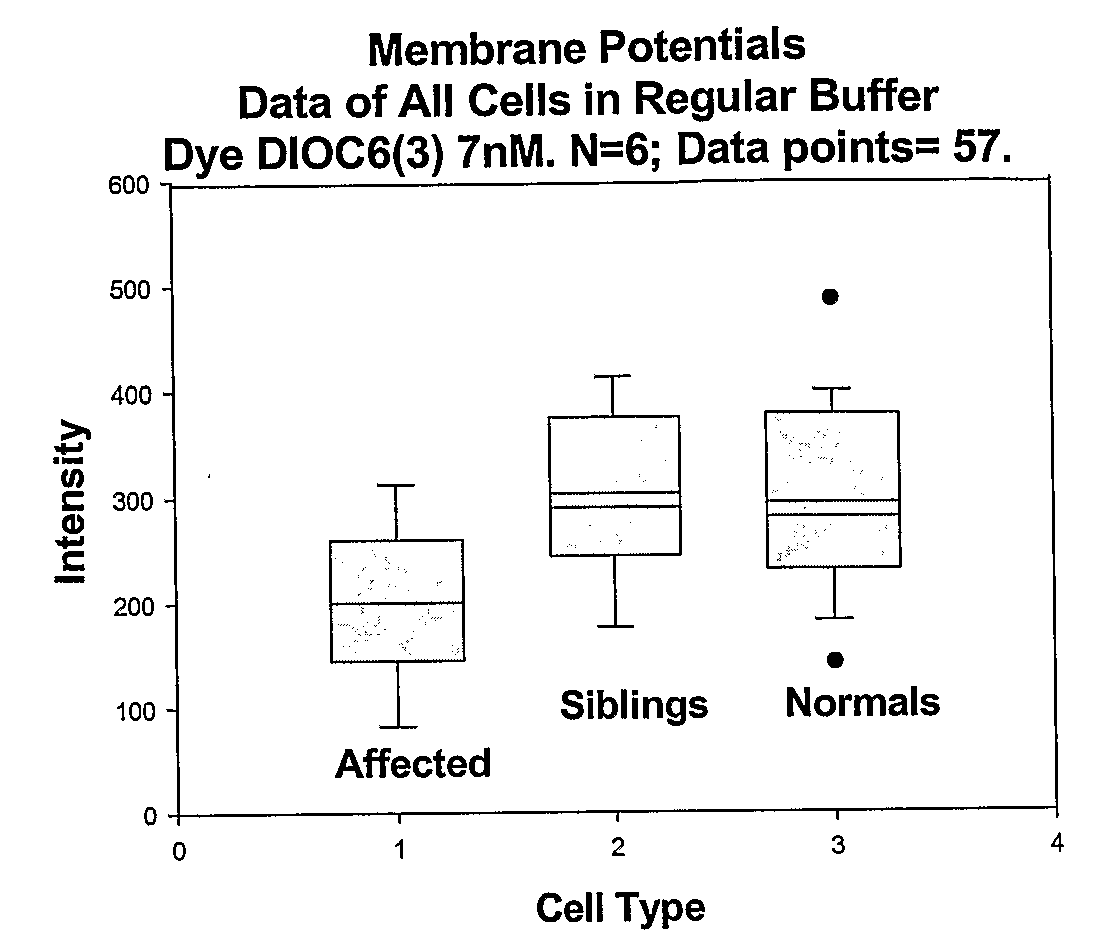
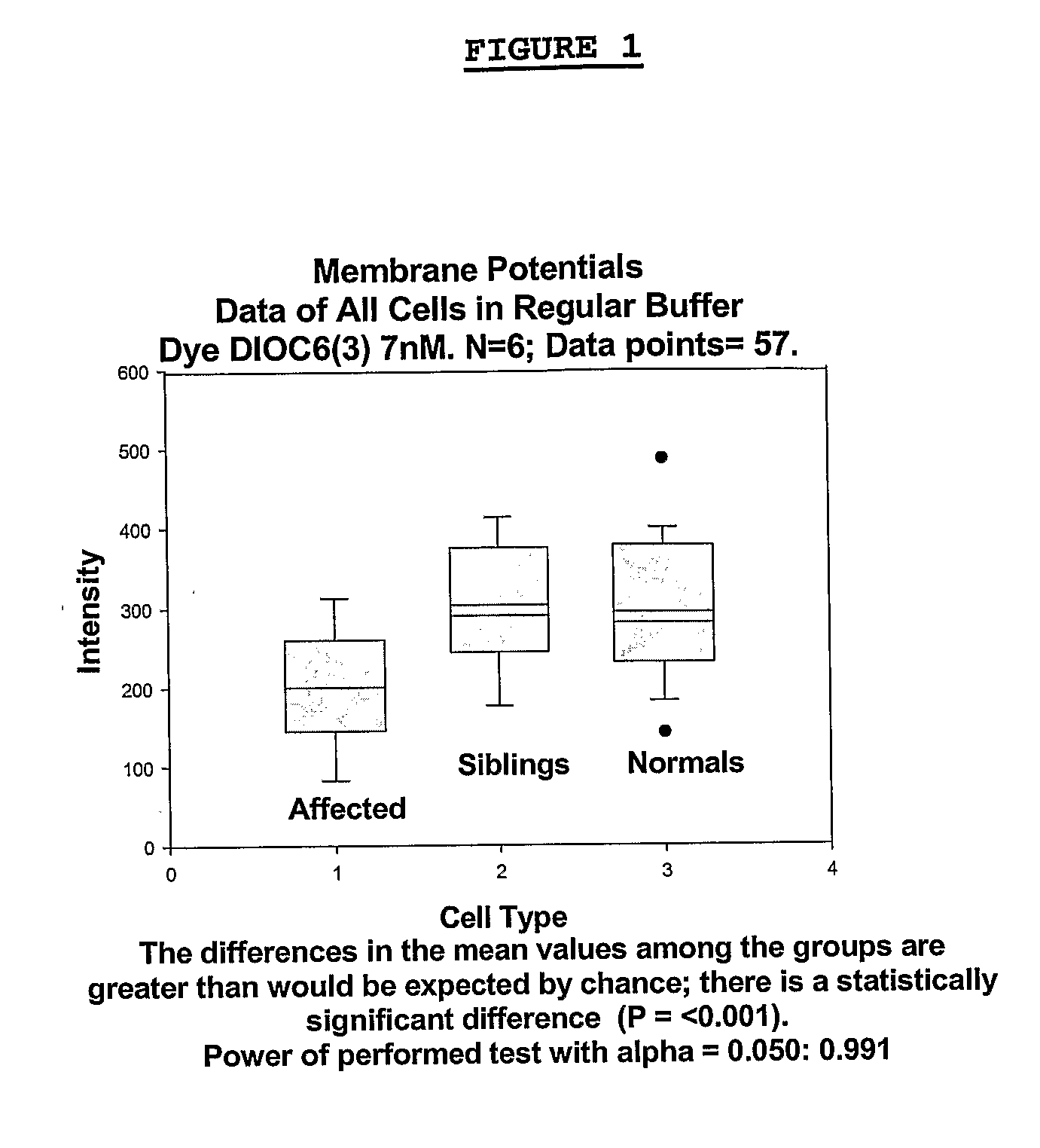
Changes that occur in Na+K+ ATPase regulation and therefore in the membrane potential in cells from bipolar individuals, as compared to cells from unaffected control individuals, are utilized to provide a diagnostic assay for bipolar I and bipolar II disorders. The diagnostic assay may also or instead exploit the similarity of cells from new patients to those of people already known to have bipolar I or bipolar II disorder. A similar diagnostic assay is provided for diagnosing attention-deficit / hyperactivity disorder (ADHD) and unipolar disorder. The diagnostic assays may further involve manipulation of membrane potential by incubation of cells in K+-free buffer and / or incubation with one or more compounds that alter Na+K+ ATPase activity. Although a variety of cells may be used, the diagnostic assays preferably employ lymphoblasts or whole blood cells.
Owner:PSYCHNOSTICS
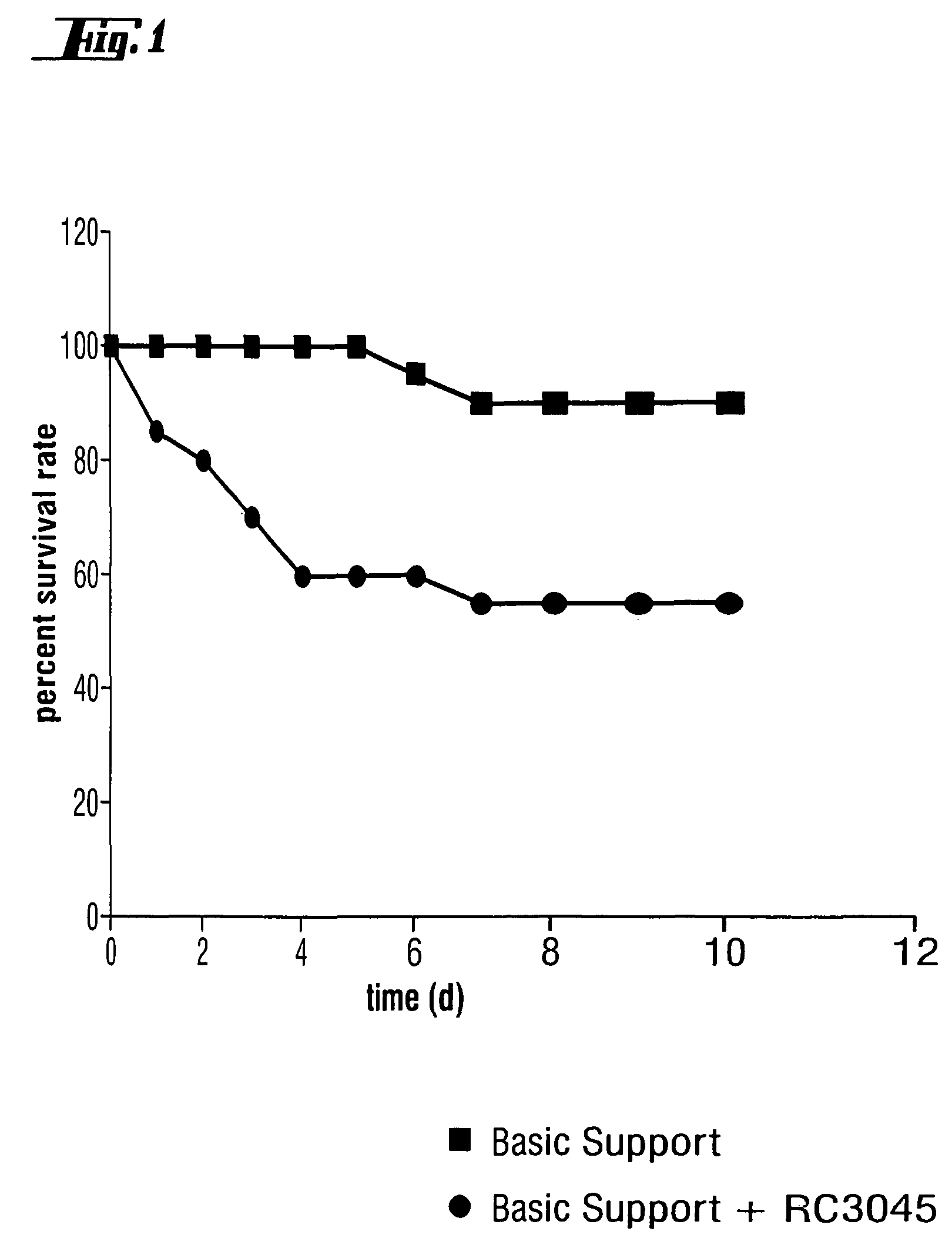
Use of bombesin/gastrin-releasing peptide antagonists for the treatment of inflammatory conditions, acute lung injury and bipolar disorder
InactiveUS7795385B2Lessen lung damageImprove survivalAntibacterial agentsNervous disorderModerate depressionGastrin-releasing peptide
The invention concerns the use of a bombesin / gastrin releasing peptide antagonist in the treatment of inflammatory and immune-mediated inflammatory conditions, in particular sepsis, acute lung injury and rheumatoid arthritis as well as for the treatment or prophylaxis of brain disorders, preferably bipolar disorder, and in particular the different forms and / or subforms of bipolar disorder, such as mania, acute mania, severe mania, hypomania, depression, moderate depression, dysthymia, severe depression, episodes of mania and / or depression, psychosis / psychotic symptoms (e.g. hallucinations, delusions), mixed bipolar state, bipolar I disorder, bipolar II disorder and / or rapid-cycling bipolar disorder. In particular, specific nonapeptides with antagonist properties against bombesin or bombesin-like peptides, such as the gastrin releasing peptide, may be used in the treatment of inflammatory and immune-mediated inflammatory conditions as well as brain disorders.
Owner:CRISTALIA PROD QUI FARM LTDA
1,7-naphthyridine derivatives
The present invention relates to compounds of general formula I wherein R1, R2, R3 and R4 are as defined herein which maybe used for the treatment of schizophrenia, obsessive-compulsivepersonality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, cognitive impairment, chemotherapy-induced cognitive dysfunction (“chemobrain”), Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, and disturbances due to radiation therapy, chronic stress, optic neuropathy or macular degeneration, or abuse of neuro-active drugs, selected from alcohol, opiates, methamphetamine, phencyclidine and cocaine.
Owner:F HOFFMANN LA ROCHE & CO AG
Combination treatments for bipolar disorders
InactiveUS20150306043A1Reduce delay timeReduce recurrenceBiocideHydroxy compound active ingredientsBipolar mood disorderValproic Acid
The invention relates generally to novel compositions and methods comprising a scyllo-inositol compound and one or both of valproic acid compound and lamotrigine. The compositions and methods provide beneficial effects in the treatment of bipolar disorders.
Owner:TRANSITION THERAPEUTICS IRELAND LTD
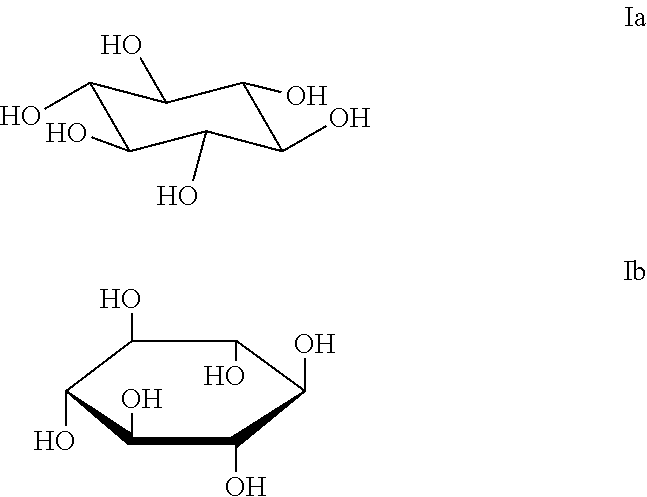
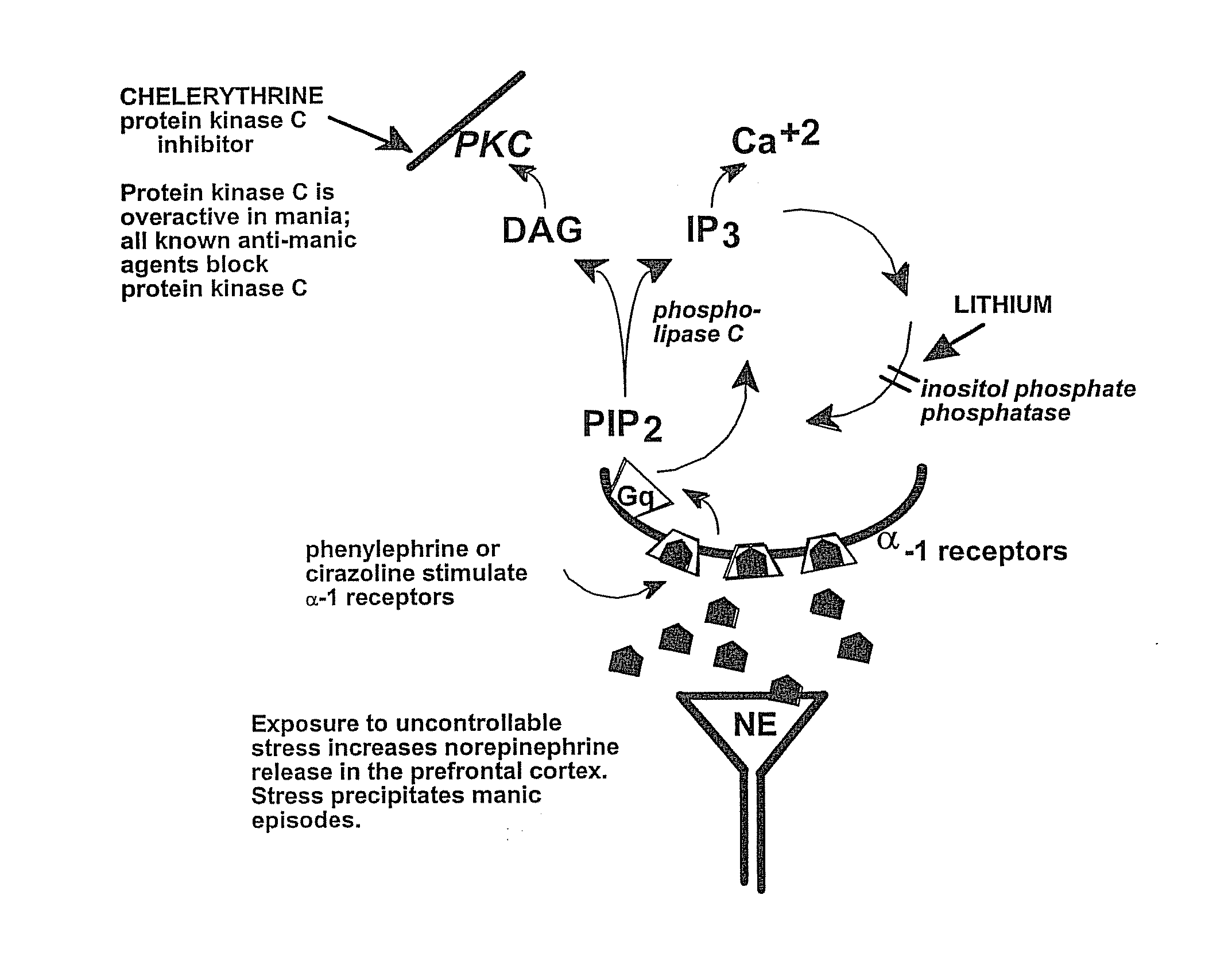
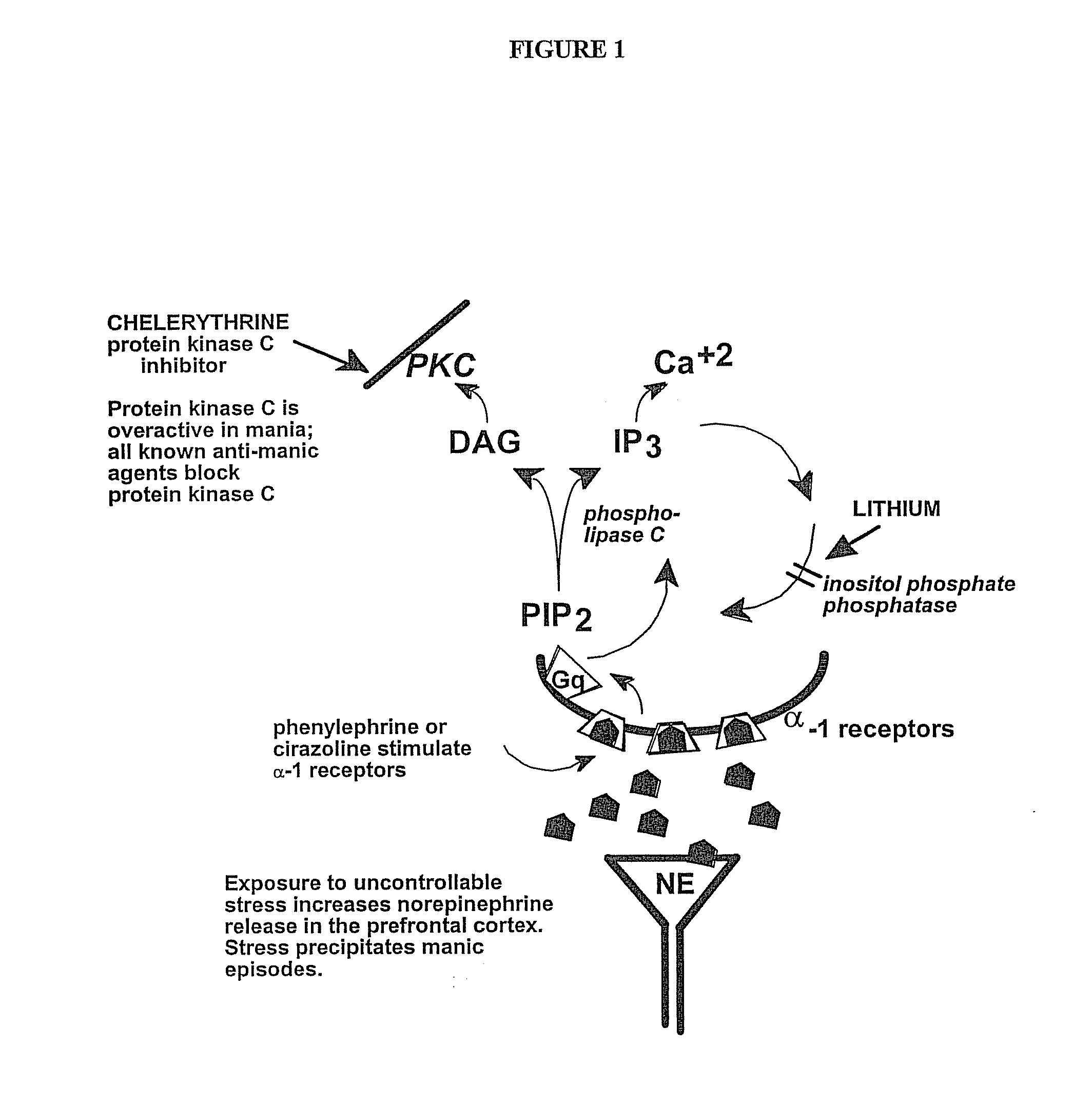
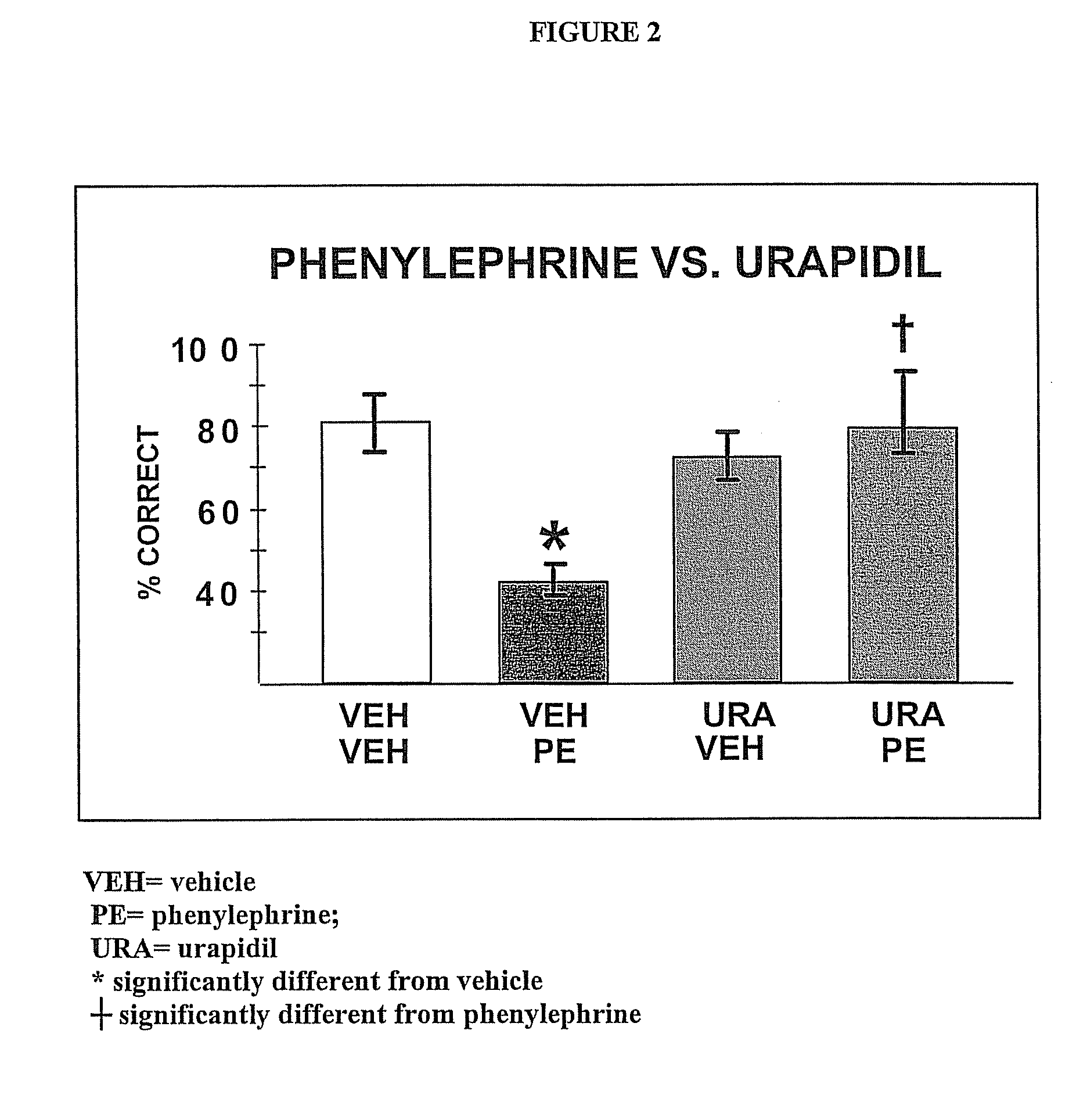
Chelerythrine, analogs thereof and their use in the treatment of bipolar disorder and other cognitive disorders
InactiveUS20100222376A1Protecting a subject's cognitive performanceBiocideNervous disorderBipolar mood disorderChelerythrine
The present invention relates to the use of chelerythrine and chelerythrine analogs in pharmaceutical compositions for the treatment of prefrontal cortical cognitive disorders, including bipolar disorder, among others.
Owner:YALE UNIV
Pharmaceutical combination
InactiveUS20130345201A1Eliminate side effectsReduce morbidityBiocideNervous disorderBipolar mood disorderSide effect
The present invention relates to a pharmaceutical combination for the treatment of schizophrenia and acute manic episodes associated with bipolar disorders, which comprises a compound which is active on a trace amine-associated receptor 1 (TAAR1 agonist) and an antipsychotic drug. This combination can reduce metabolic side effects which appear if using an antipsychotic drug alone.
Owner:F HOFFMANN LA ROCHE INC
Use of bombesin/gastrin-releasing peptide antagonists for the treatment of inflammatory conditions, acute lung injury and bipolar disorder
InactiveCN101090731AAntibacterial agentsNervous disorderModerate depressionGastrin-releasing peptide
The present invention relates to the use of bombesin / gastrin releasing peptide antagonist in the preparation of pharmaceutical composition, said pharmaceutical composition is used for treating inflammatory diseases and immune-mediated inflammatory diseases, especially sepsis , acute lung injury and rheumatoid arthritis and for the treatment or prevention of brain disorders, preferably bipolar disorders, and in particular of different types and / or subtypes of bipolar disorders such as mania, acute mania, Severe mania, hypomania, depression, moderate depression, dysthymia, major depressive disorder, manic and / or depressive episodes, psychotic / psychotic symptoms (e.g., hallucinations, delusions), mixed bipolar states, Bipolar I disorder, bipolar II disorder, and / or rapid cycling bipolar disorder. In particular, specific nonapeptides with antagonist properties against bombesin or bombesin-like peptides such as gastrin-releasing peptide can be used in the treatment of inflammatory and immune-mediated inflammatory diseases and brain disorders .
Owner:CRISTALIA PROD QUI FARM LTDA
Animal models of neurological disorders
ActiveUS20120180144A1Reduce expressionReduce functionNervous disorderVectorsDiseaseCompulsive disorders
The present invention relates to the field of neurological disorders and more particularly to the field of neuropsychiatric disorders. The invention provides non-human, transgenic animal models for brain disorders such as schizophrenia, bipolar disorders, compulsive disorders, addictive disorders and the like. The animals also have applications in the field of GABA neuro-transmission and other disorders in which GABA-dependent gene regulation has a role.
Owner:ANN & ROBERT H LURIE CHILDRENS HOSPITAL OF CHICAGO
3-arylthioindole-2-carboxamide derivatives and analogs thereof as inhibitors of casein kinase i
InactiveCN101039666AOrganic active ingredientsNervous disorderDiseaseRecurrent major depressive episodes
Owner:AVENTIS PHARMA INC
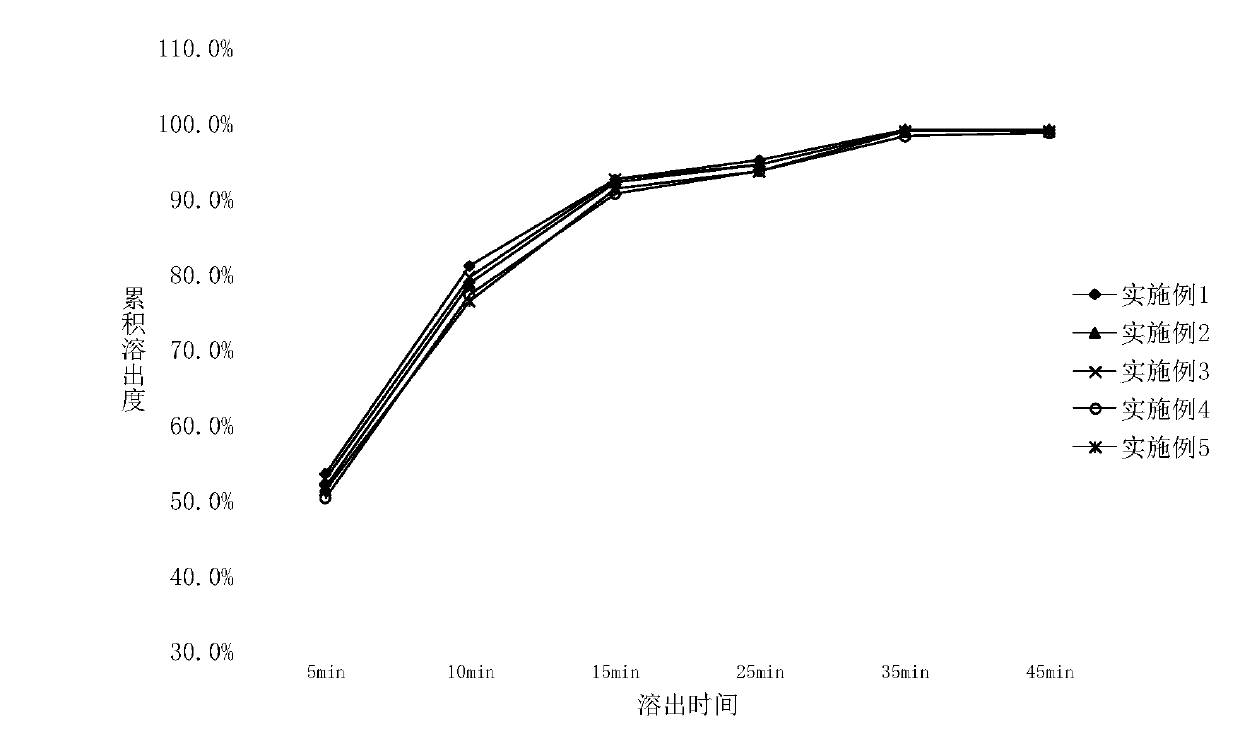
Fast-release lurasidone hydrochloride tablet and preparation process thereof
InactiveCN110833532AOrganic active ingredientsNervous disorderPediatric patientLurasidone Hydrochloride
The invention relates to a lurasidone hydrochloride tablet which is used for treating severe depression related to bipolar I disorder of pediatric patients and can realize rapid and complete drug release and a preparation process of the lurasidone hydrochloride tablet. The drug and a part of diluent are dissolved in a mixed solvent of an organic solvent and water, then the drug and the part of diluent are mixed with remaining auxiliary materials at a high speed, dried, granulated and tableted to obtain the lurasidone hydrochloride tablet. Compared with the prior art, the lurasidone hydrochloride tablet is simple in preparation process, low in energy consumption, rapid to release and good in release repeatability, and the preparation is stable after accelerated test.
Owner:赵洁
Enhancement of camp signaling as a combination drug strategy for the treatment of depression and related conditions
PendingUS20220226302A1Enhanced signalNervous disorderHeterocyclic compound active ingredientsAdrenergic receptor agonistsDepressant
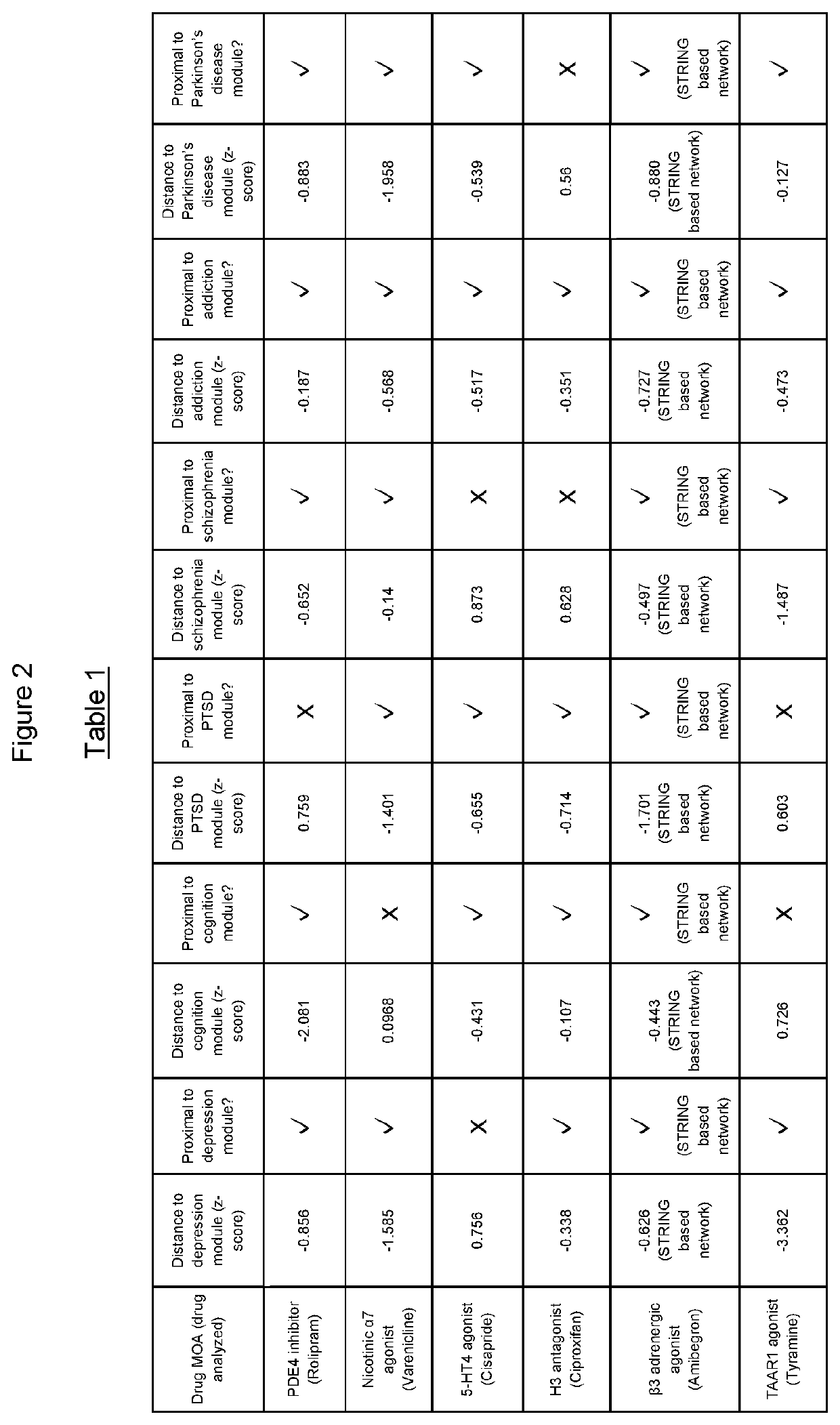
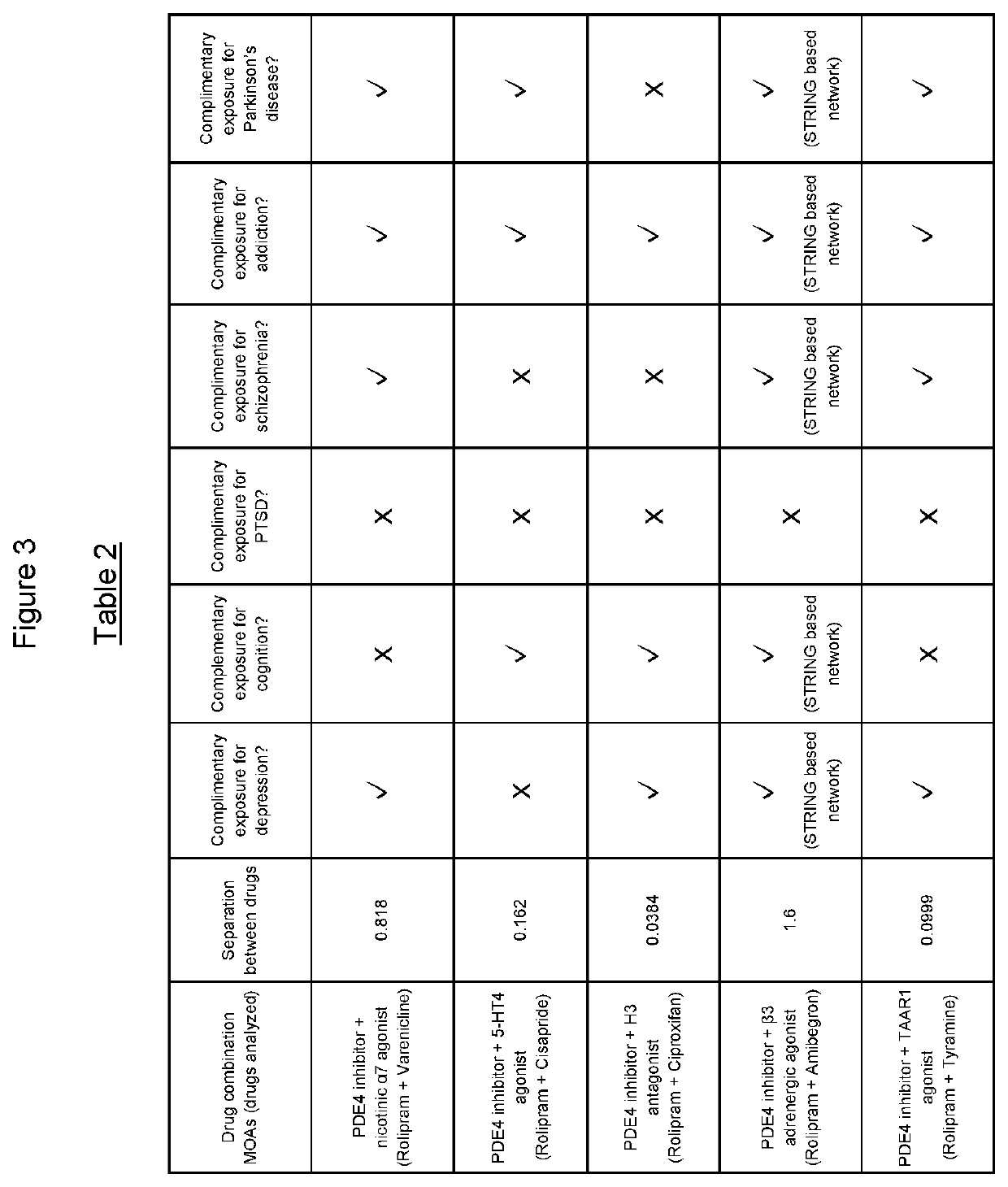
The present invention relates to the use of a combination of a phosphodiesterase 4 (PDE4) inhibitor and one or more of 5-HT4 agonist, an H3 antagonist or inverse agonist, a nicotinic α7 receptor agonist, a β3 adrenergic agonist or a TAAR1 agonist for the treatment of psychiatric or neurological disorders in which depressive, anhedonia, motivation-related or cognition-related dysfunction exists (such as major depressive disorder, bipolar I disorder, post-traumatic stress disorder, addiction, anhedonia or motivation-related aspects of schizophrenia (e.g. negative and cognitive symptoms), as well as Parkinson's disease (e.g. non-motor features such as depression, apathy and cognitive impairment)).
Owner:ALTO NEUROSCIENCE INC
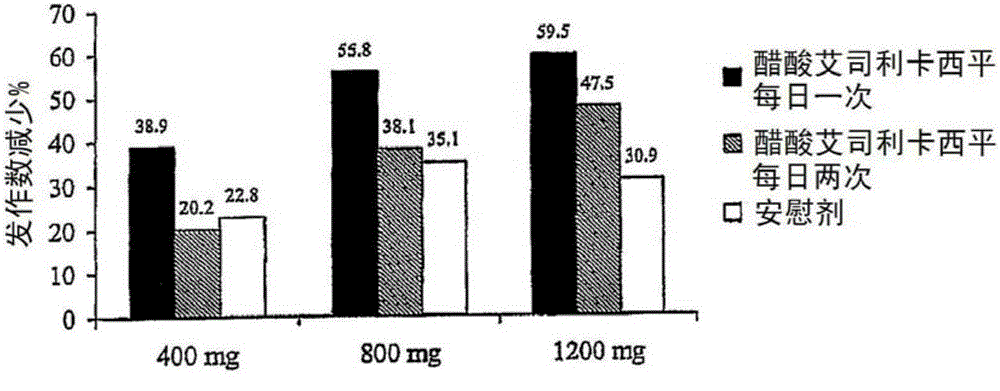
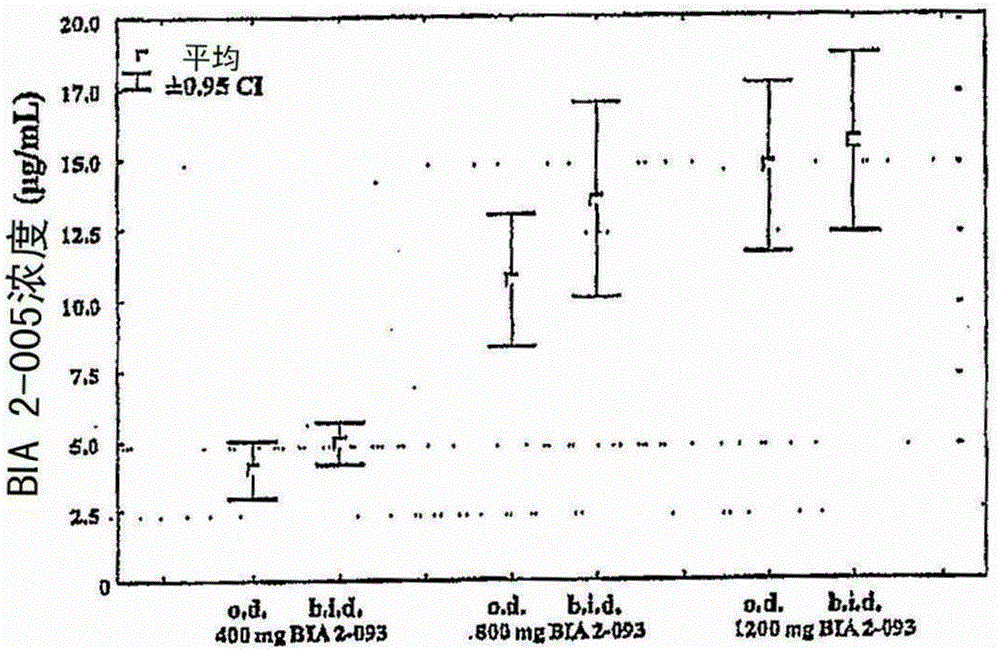
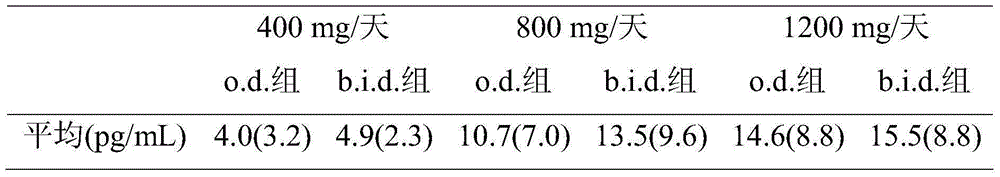
Eslicarbazepine acetate and application method thereof
InactiveCN105193822AIncrease exposureNervous disorderAnhydride/acid/halide active ingredientsDiseaseEslicarbazepine acetate
The invention relates to a method for using eslicarbazepine acetate for treating various diseases and symptoms such as affective disorder, emotional schizophrenia, bipolar disorder, attention disorder, anxiety disorder, neuropathic pain, disorder related to neuropathic pain, sensorimotor dysfunction, vestibular disorder and neurological function alternation caused by retrogressive and post-ischemic diseases. The invention further relates to application of eslicarbazepine acetate to a method for reducing or relieving epileptic seizure of a patient, and a method for improving the exposing degree of eslicarbazepine acetate to a patient. The invention further relates to a method for preparing a medicine composition containing eslicarbazepine acetate.
Owner:BIAL PORTELA & CA SA
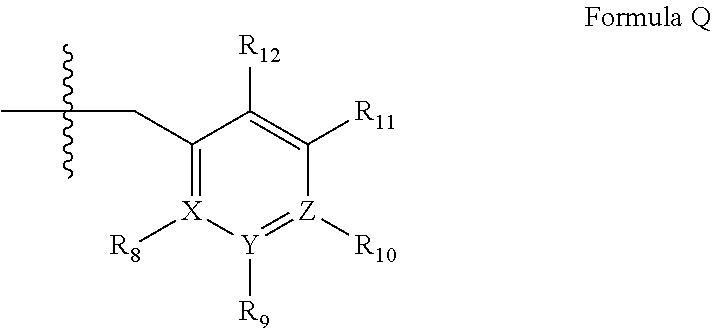
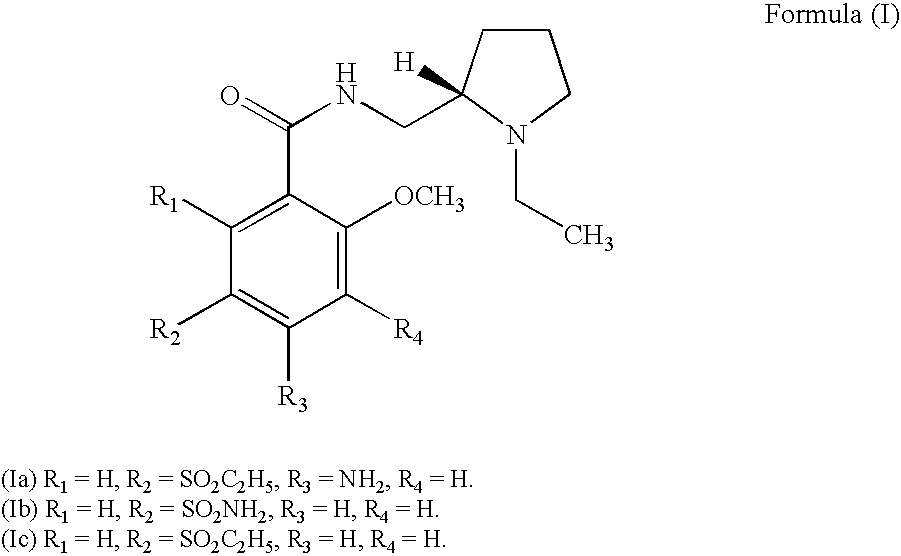
4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine
The compound 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine according to the structure (formula I), and pharmaceutically acceptable salts thereof are provided for the treatment of CNS related disorders, such as: depressive disorder, dysthymic disorder; mood disorder due to a general medical condition; atypical depression; seasonal affective disorder; melancholia; treatment resistant depression; partial responders; depression associated with bipolar disorder, pain, Alzheimer's disease, psychosis, Parkinson's disease, Lewy body disease, Huntington's disease, multiple sclerosis or anxiety; general anxiety disorder, social anxiety disorder, panic attacks; phobia; social phobia, obsessive compulsive disorder; post traumatic stress disorder, acute stress; ADHD; and pain.
Owner:H LUNDBECK AS
Combination therapies for treating bipolar disorder, and methods for using the same
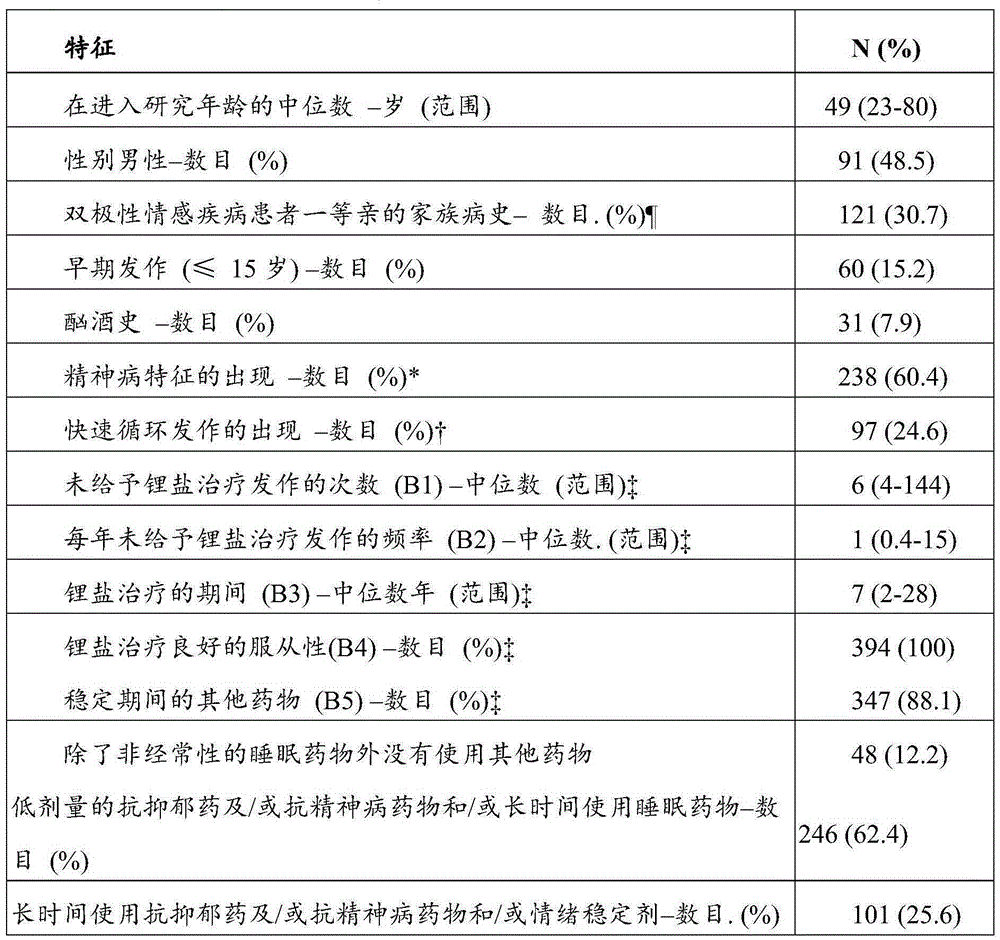
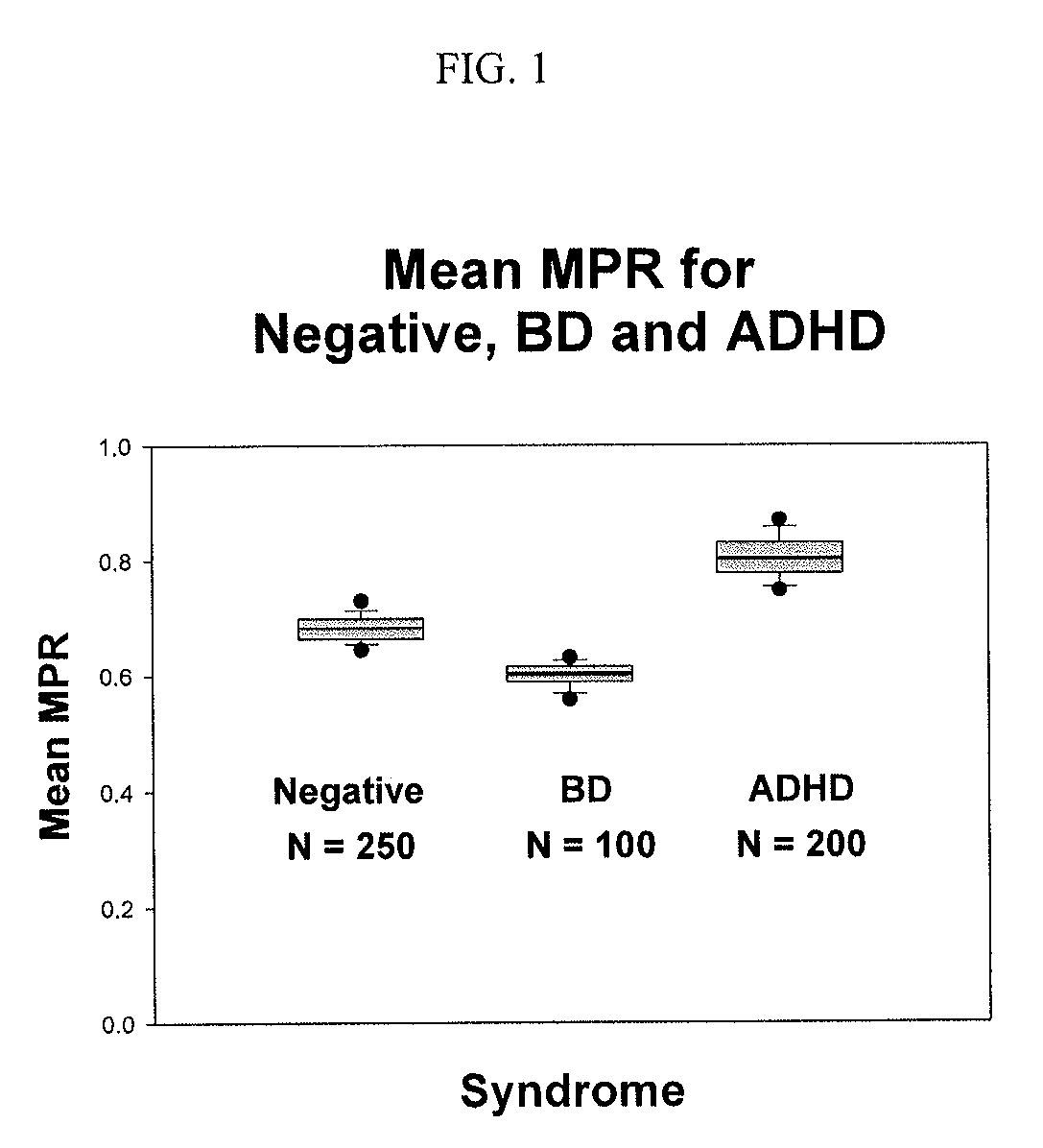
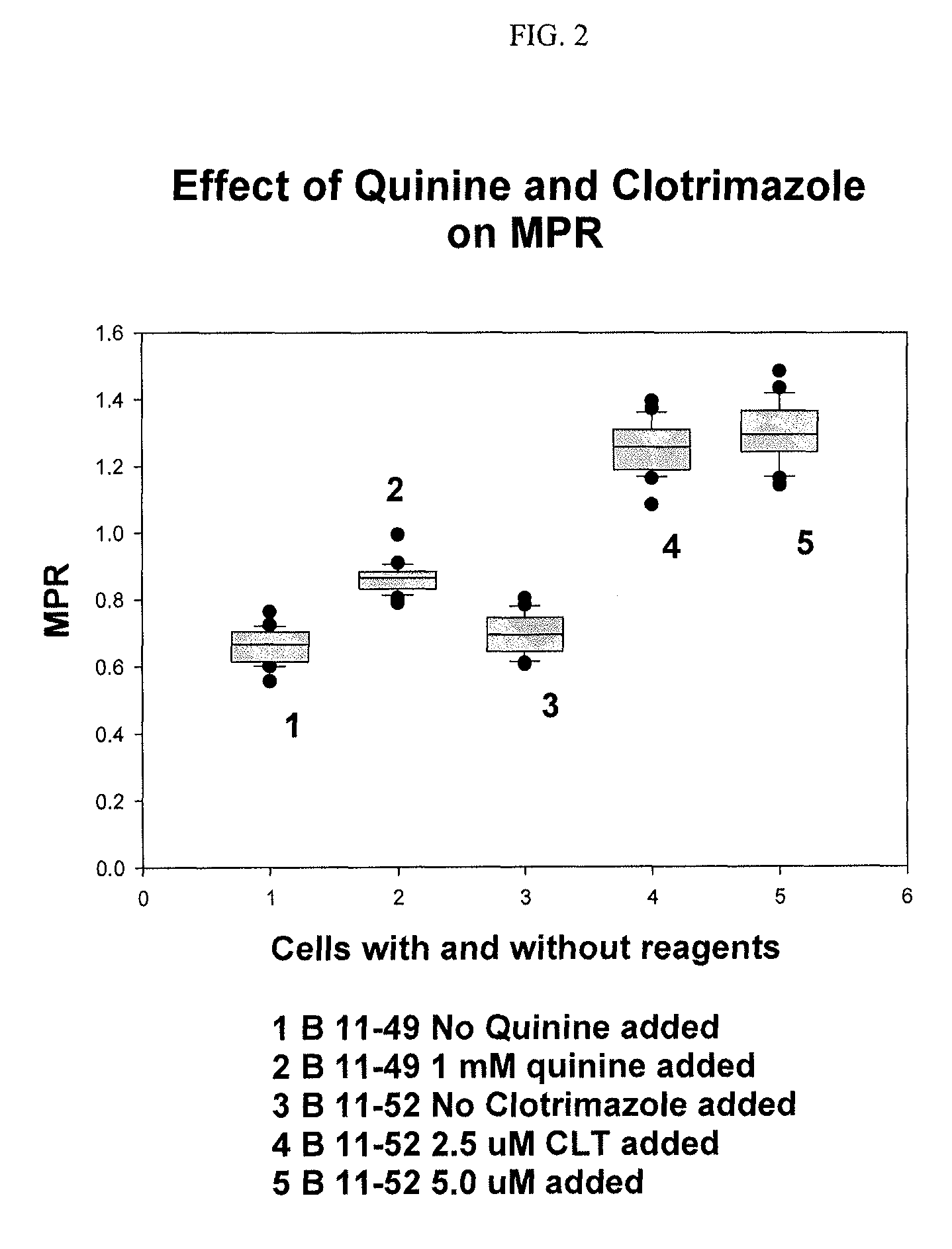
InactiveUS20190302102A1Good curative effectDrug and medicationsDisease diagnosisCombined Modality TherapyCurative effect
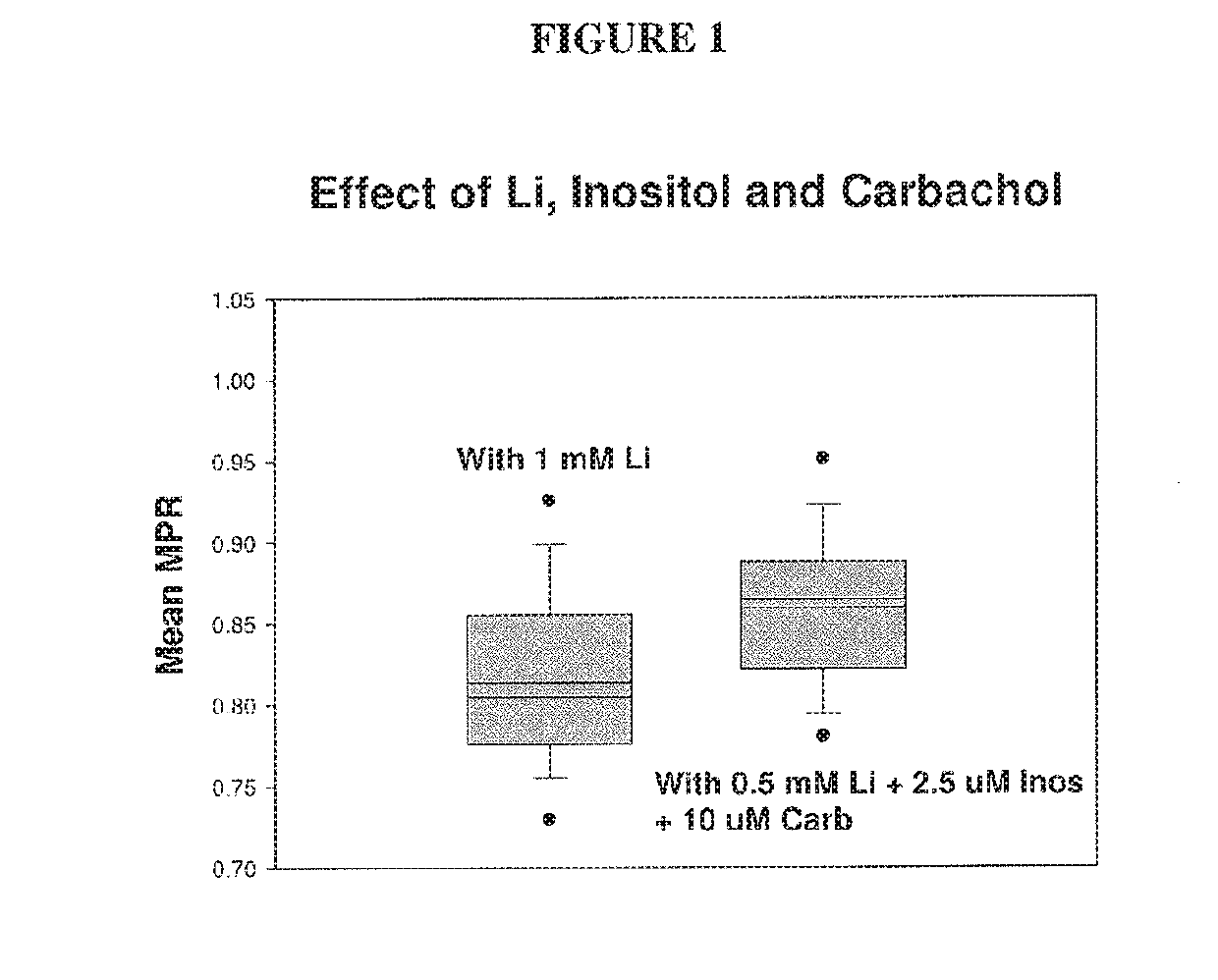
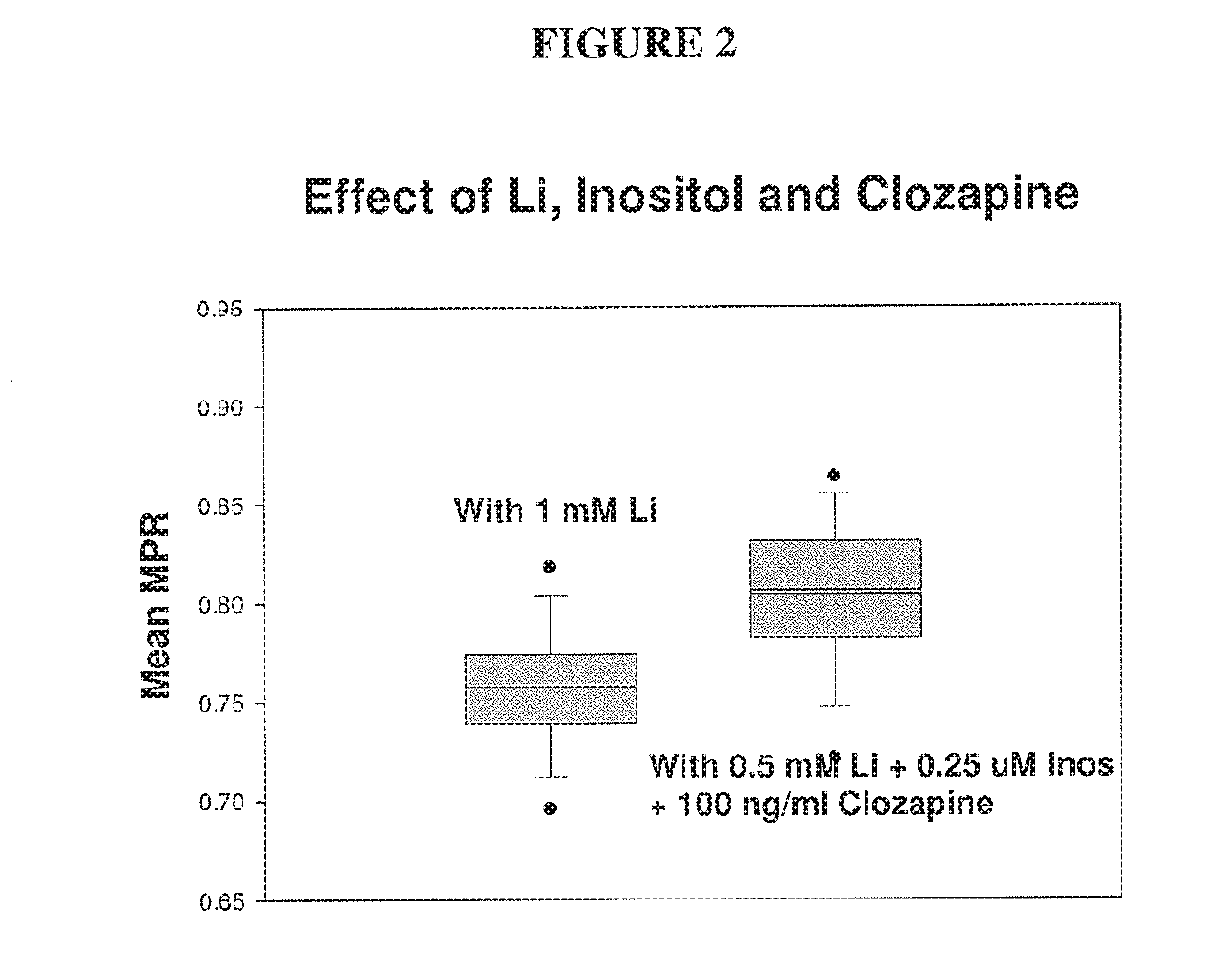
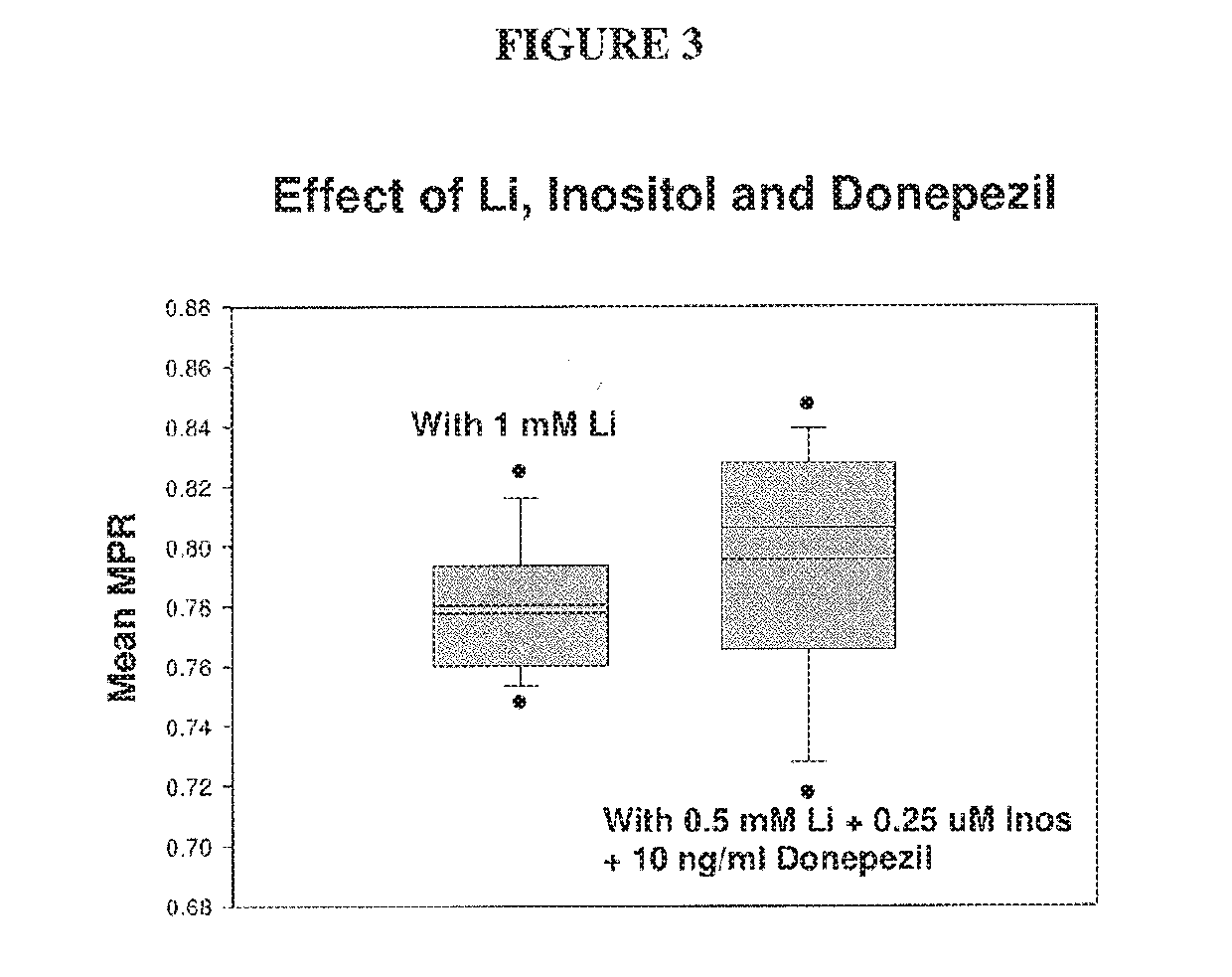
The present invention relates to pharmaceutical combinations and compositions, and methods of using the same for treatment of Bipolar Disorder (BD). More specifically, the invention relates to combination therapies for the treatment of BD, and methods for treating BD using such therapies. The present invention also relates to methods of determining an optimal combination drug treatment therapy for BD, methods of optimizing a combination drug treatment therapy for BD, methods of optimizing dosage of a drug in a combination drug treatment therapy for BD, as well as methods for monitoring the efficacy of a combination therapy for the treatment of BD. The present invention involves analyzing the membrane potential of cells isolated from a BD patient treated with the combination therapy, and calculating a membrane potential ratio therefrom.
Owner:PSYCHNOSTICS
Genetic variants associated with lithium response in bipolar disorder
InactiveCN105378105AInorganic active ingredientsMicrobiological testing/measurementDiseaseBipolar mood disorder
Owner:ACAD SINIC
Methods for diagnosing and identifying modulators of membrane potentials in bipolar disorder and attention deficit hyperactivity disorder
ActiveUS9523673B2Increase and decrease valueDecrease or increase the membrane potential ratio (MPR™)Disease diagnosisBiological testingAttention deficitsDrug target
The present invention provides methods to modulate key elements along the DAG signaling pathway as well as a diagnostic assay, device and methods of using the same to diagnose bipolar disorder (BD) and attention deficit hyperactivity disorder (ADHD). Methods to identify diagnostic markers and drug targets for BD and ADHD. Methods of identifying effective compounds responsible for membrane potentials and excitabilities influencing bipolar disorder (BD) and attention deficit hyperactivity disorder (ADHD). Methods of identifying an effective compound that modulates the activity of Ca2+ / CaM enzyme and compounds involved in changing the K+ gradient across the plasma membrane thereby increasing or decreasing the membrane potential ratio (MPR™) values. The invention provides methods of identifying a compound that modulates the activity of PKC which is an important protein of the DAG signaling pathway. Methods of identifying a compound that modulates DAG and its related enzymes along the DAG signaling pathway are provided. These compounds decrease or increase the membrane potential ratio (MPR™) in BD and ADHD patients.
Owner:PSYCHNOSTICS
Methods and systems for detecting psychotic disorders associated with serotonin receptor deficiencies
PendingUS20220023312A1Relieve symptomsOrganic active ingredientsDrug and medicationsSerotoninSleep quantity
Methods for detecting psychotic disorders such as but not limited to schizophrenia or bipolar I disorder featuring administering to a patient a dose of an antipsychotic medication; and subjecting the patient to an evaluation at a time point following administration of the dose of the antipsychotic medication. The evaluation is adapted to determine responsiveness or sleepiness (or amount of sleep) resulting from the dose of the antipsychotic medication.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
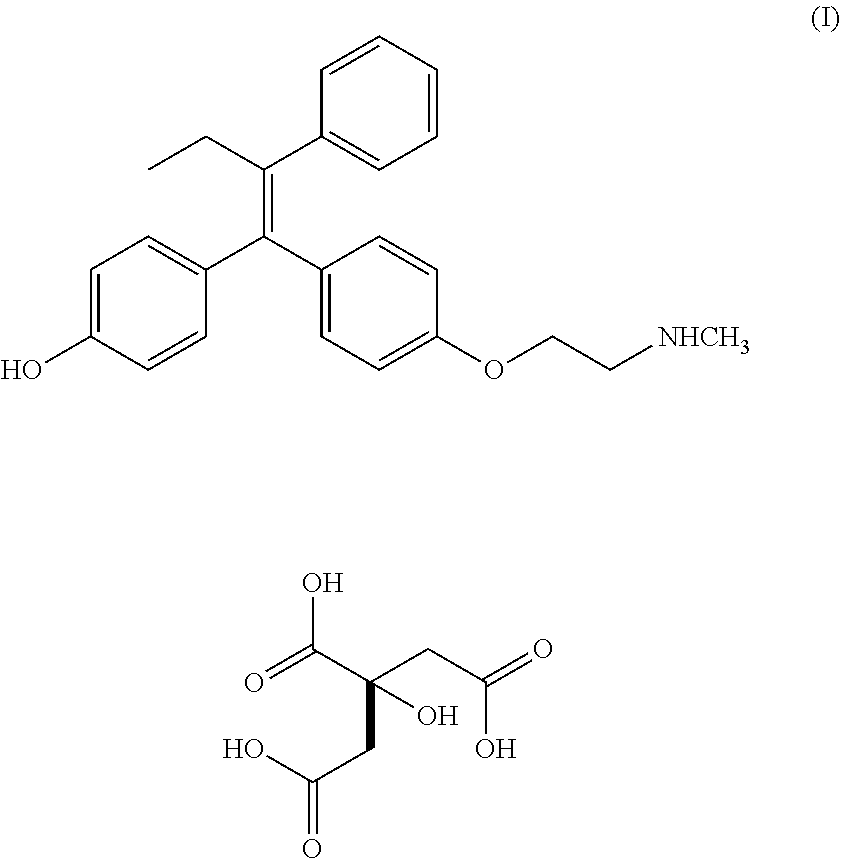
Endoxifen for the treatment of bipolar i disorder
PendingUS20210315845A1Stay focusedPatient compliance is goodOrganic active ingredientsNervous disorderBipolar type I disorderPharmaceutical drug
A method for maintaining a therapeutically effective concentration of endoxifen for treatment of a patient with bipolar I disorder is provided. The said method includes administering to the patient, a dose of 2 mg to 16 mg of endoxifen citrate in an enteric coated tablet once per day for at least 21 days. Further, the patient is not required to be administered rescue medication during the administration of the endoxifen citrate.
Owner:JINA PHARMA
Methods and systems for detecting psychotic disorders associated with serotonin 2A receptor deficiencies
ActiveUS11147888B2Relieve symptomsCompounds screening/testingOrganic active ingredientsBipolar type I disorderSedation
Methods for detecting resistance to sedation caused by antipsychotic drugs including methods for detecting psychotic disorders such as but not limited to schizophrenia or bipolar I disorder featuring administering to a patient a dose of an antipsychotic medication; and subjecting the patient to an evaluation at a time point following administration of the dose of the antipsychotic medication. The evaluation is adapted to determine a level of sedation resulting from the dose of the antipsychotic medication. A resulting level of sedation may be one of the following: completely sedated, significantly sedated, moderately sedated, not significantly sedated and completely alert. If the individual is not completely sedated or significantly sedated, or is completely alert, then the patient may have a likelihood of having a psychotic disorder such as schizophrenia.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13264ff2-4046-40b5-8301-1bda1efbba26/US20110039890A1-20110217-C00001.png)
![4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13264ff2-4046-40b5-8301-1bda1efbba26/US20110039890A1-20110217-C00002.png)
![4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/13264ff2-4046-40b5-8301-1bda1efbba26/US20110039890A1-20110217-C00003.png)