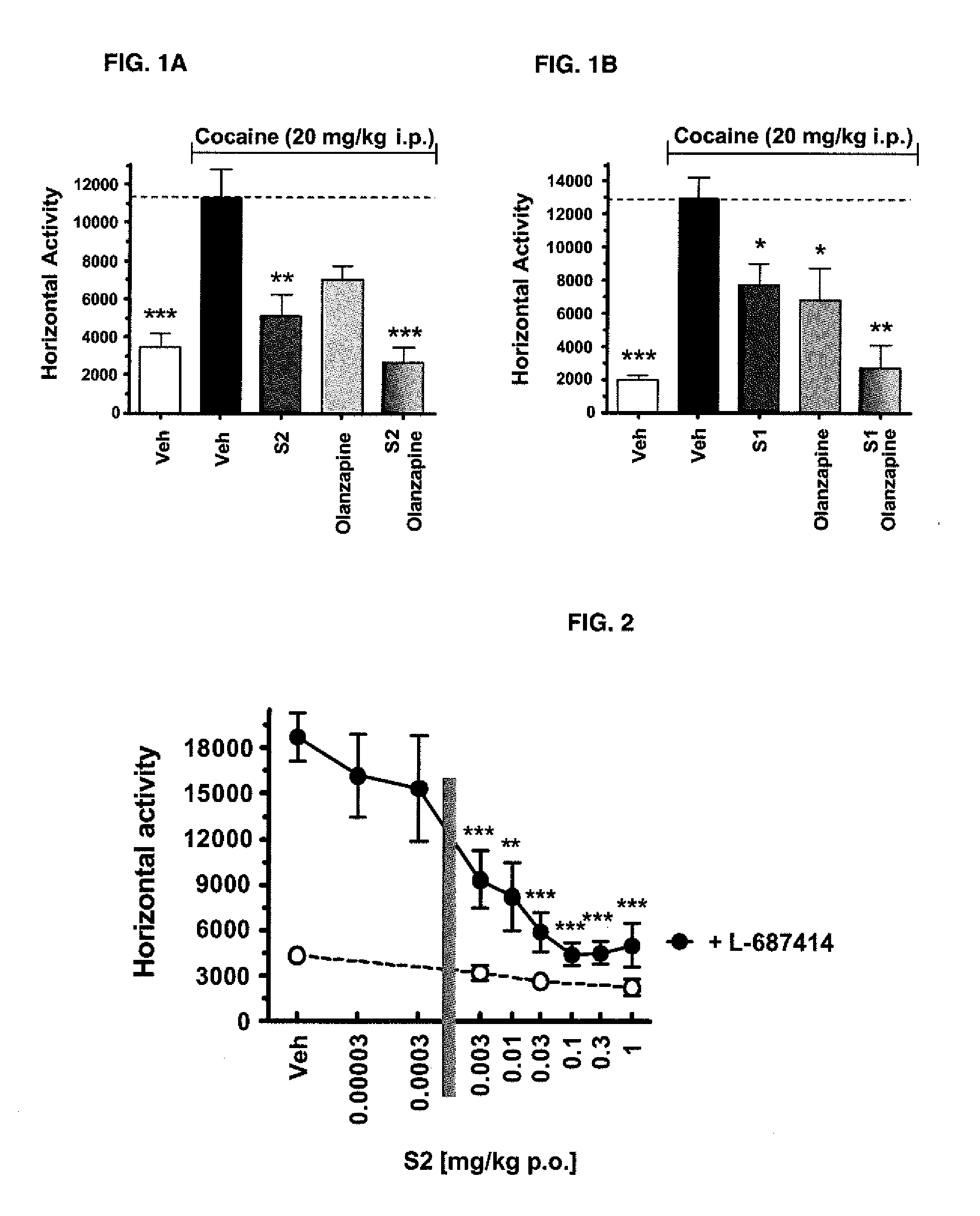
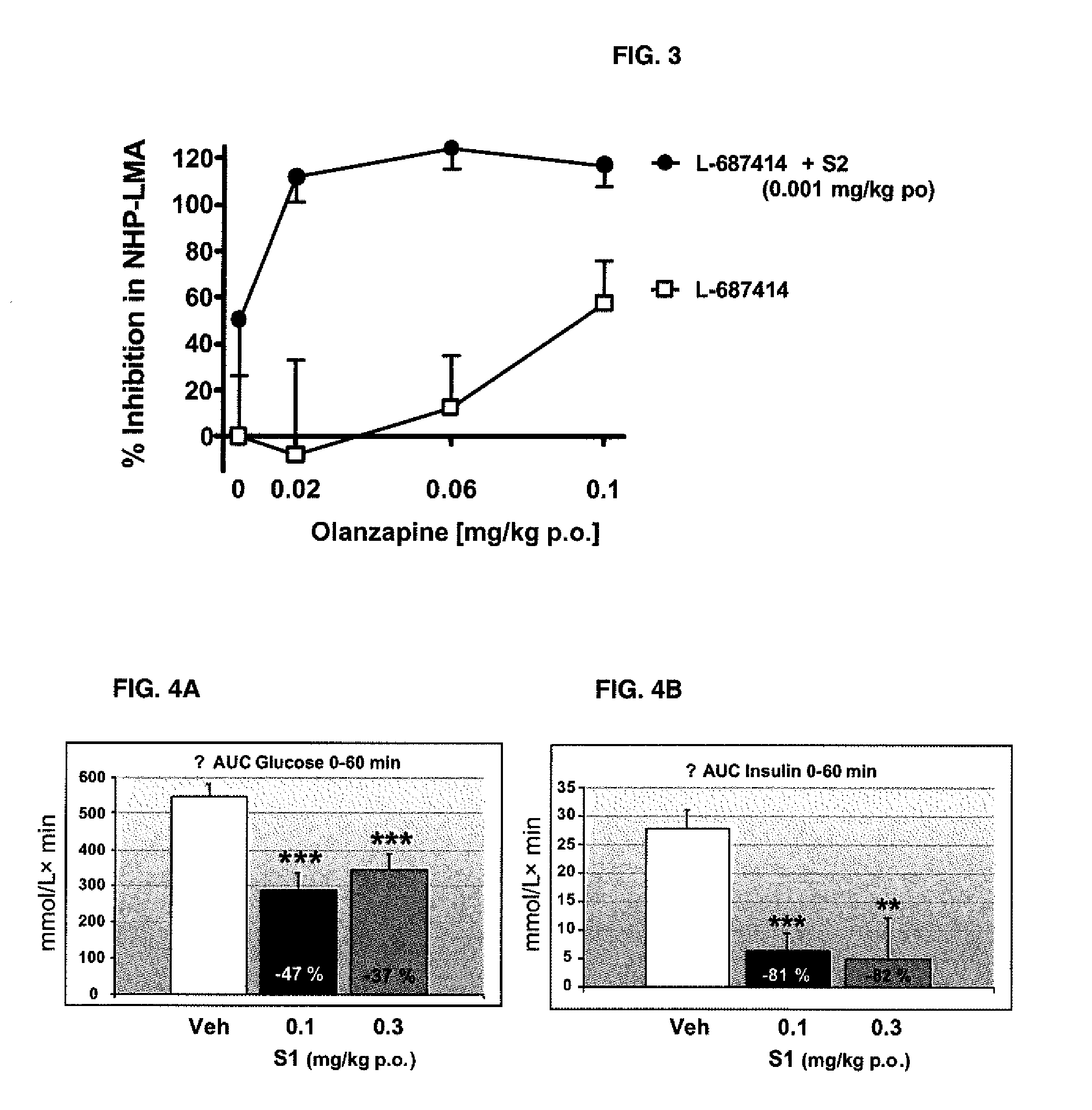
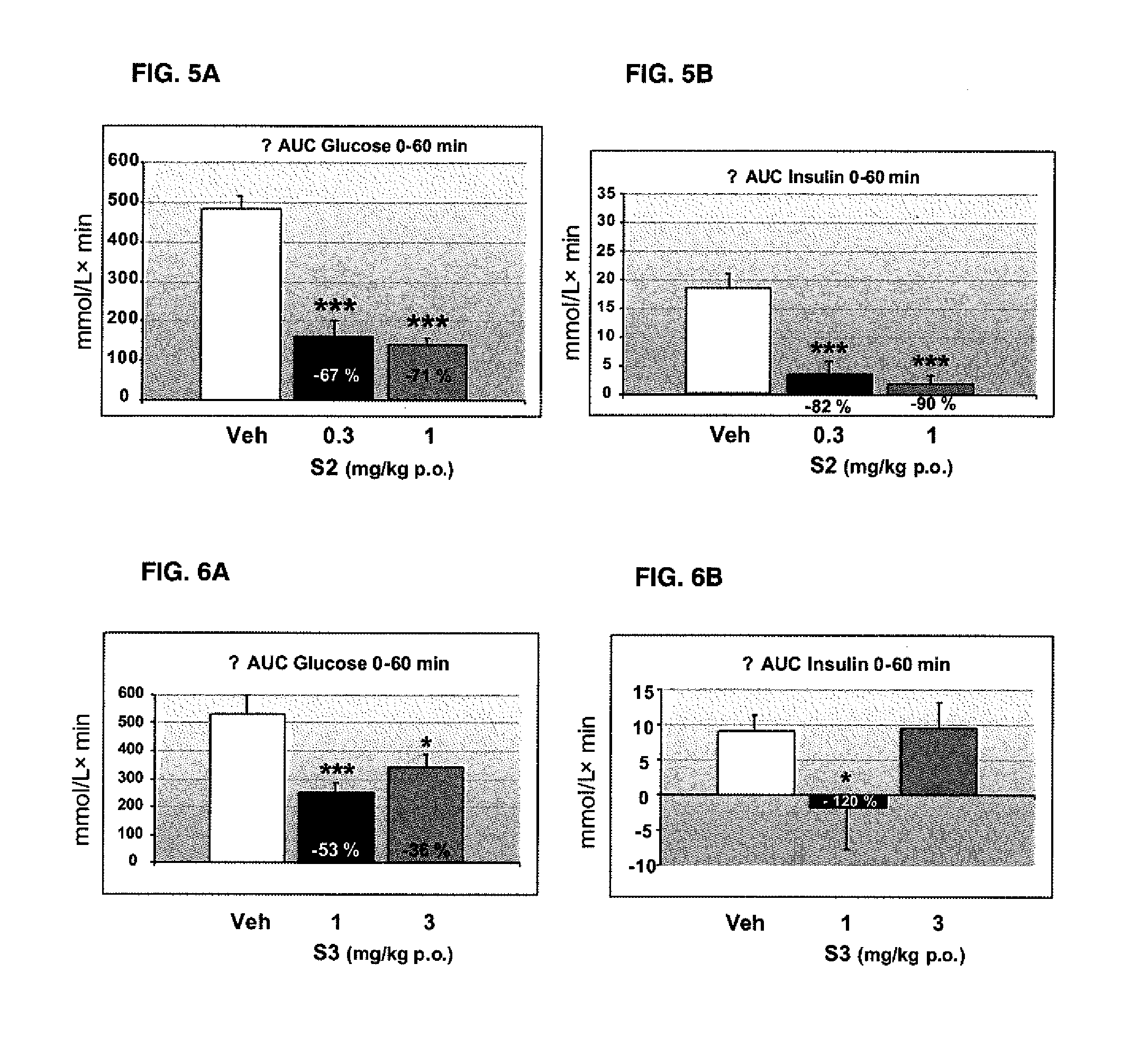
Pharmaceutical combination
a technology of combination and drugs, applied in the field of pharmaceutical combination, can solve the problems of increased risk of increased blood sugar level and diabetes, serious side effects, and adverse effects of cardiometabolic drugs, so as to reduce metabolic side effects, reduce the incidence of metabolic syndrome, and treat schizophrenic patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example s1
(S)-4-((S)-2-Phenyl-butyl)-4,5-dihydro-oxaza-2-ylamine
[0114]
a) (R)-1-Iodomethyl-propyl)-benzene
[0115]To a solution of triphenylphosphine (15.4 g, 59 mmol) and imidazole (3.99 g, 59 mmol) in dichloromethane (150 ml) at room temperature was added portionwise iodine (14.9 g, 50 mmol) at such a rate that the temperature of the reaction mixture did not rise above 30° C. To the mixture was then added a solution of (R)-2-phenyl-butan-1-ol (7.34 g, 41 mmol, CAS 16460-75-6) in dichloromethane (50 ml) and the mixture was then stirred at room temperature overnight. The mixture was then concentrated in vacuo and the residue was resuspended in ether and the resulting crystals collected by filtration. The filtrate was concentrated in vacuo and the residue was triturated in heptane. The resulting crystals were removed by filtration and the filtrate was concentrated in vacuo. The residue was purified by column chromatography (SiO2, heptane / EtOAc) to yield a colourless oil, (6.38 g, 60%).
b) (2R,5S)-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com