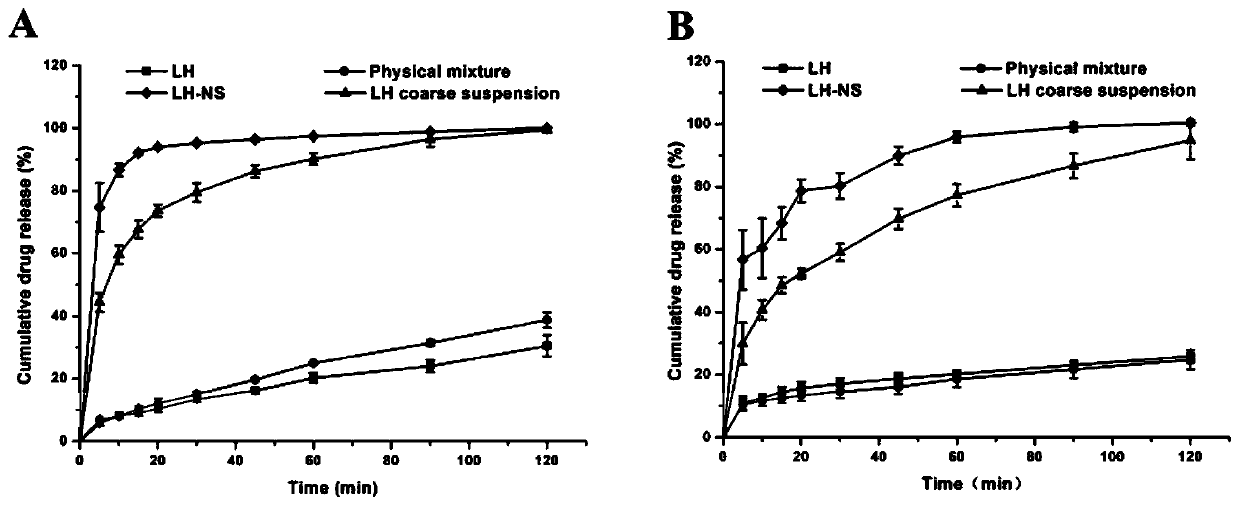
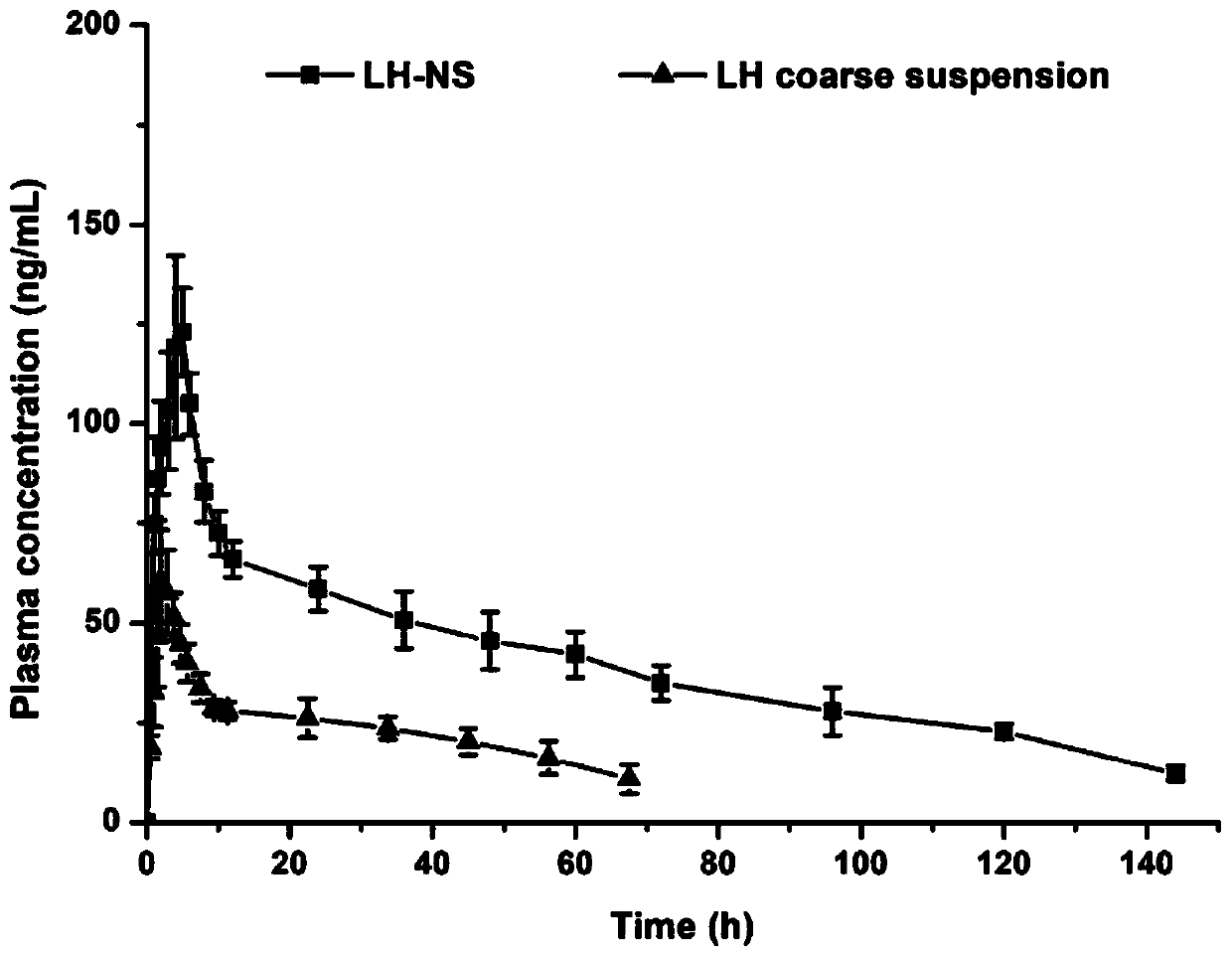
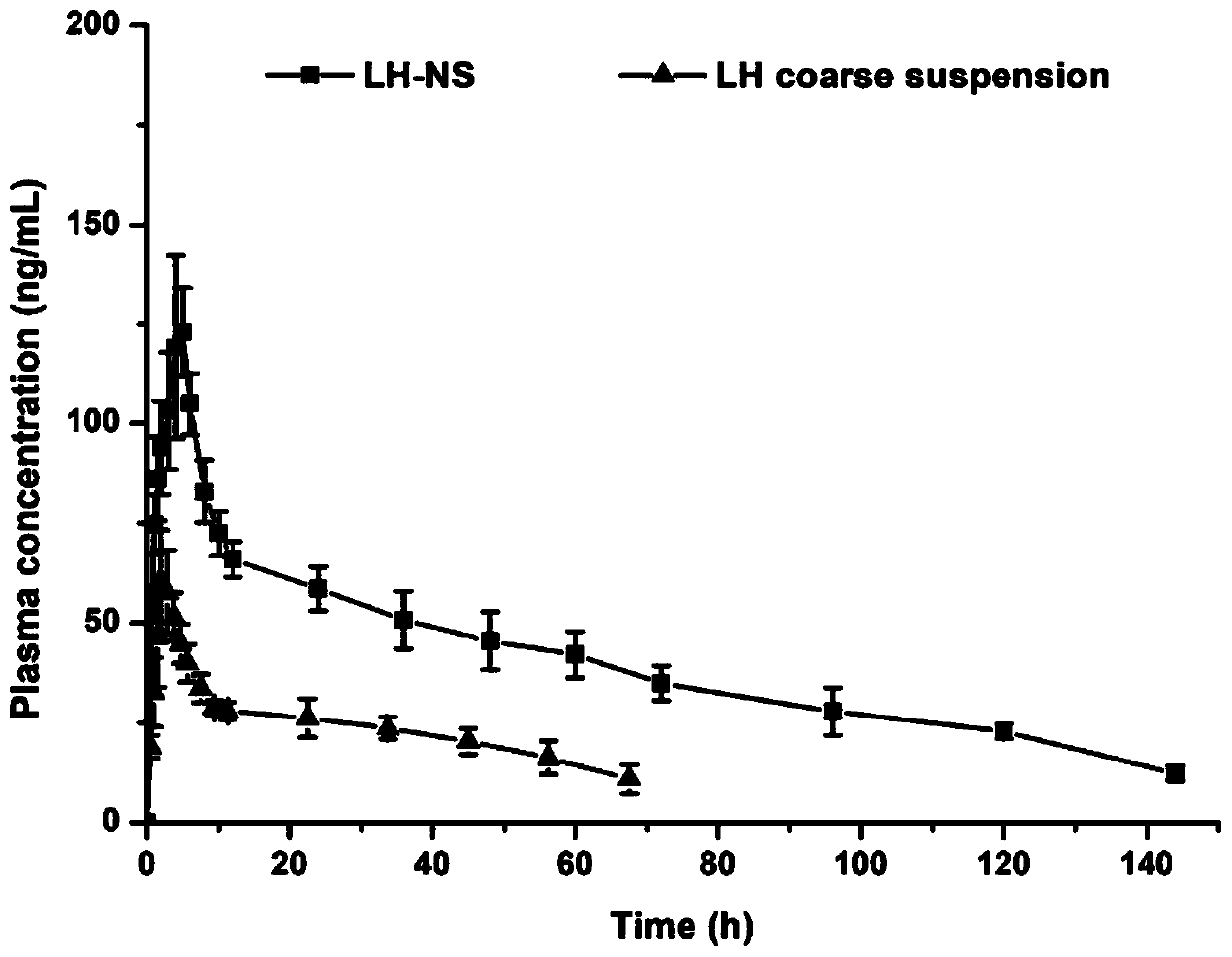
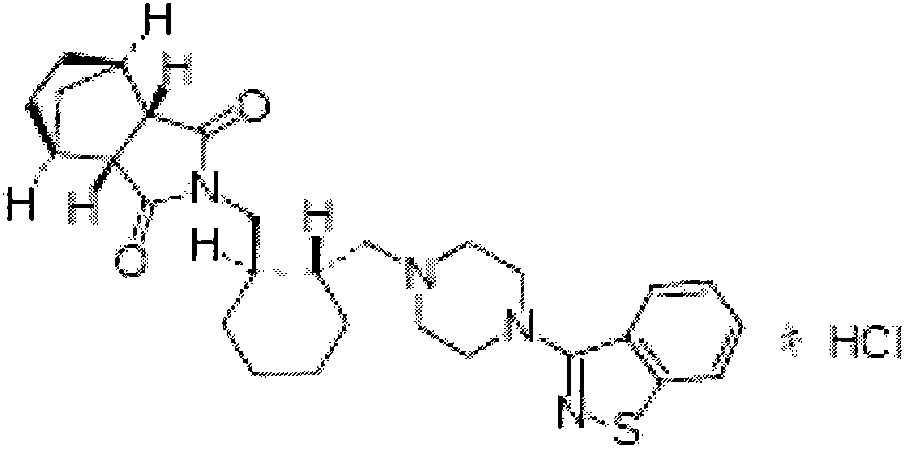Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Lurasidone Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lurasidone hydrochloride (Latuda) is an antipsycotic medication used for treating schizophrenia and bipolar disorder. Side effects, drug interactions, and use during pregnancy should be reviewed prior to taking any medication.
Lurasidone hydrochloride orally-disintegrating tablet preparation and preparation method thereof
InactiveCN103054824AOrganic active ingredientsNervous disorderLurasidone HydrochlorideOrally disintegrating tablet
The invention belongs to the technical field of medicine, and relates to a lurasidone hydrochloride orally-disintegrating tablet preparation and a preparation method thereof. The orally-disintegrating tablet comprises the following components in mass percent: 5-60 percent of lurasidone hydrochloride, 25-80 percent of filling material, 5-20 percent of disintegrating agent, 0.02-0.15 percent of wetting agent, 0.2-5 percent of flavoring agent and 0.5-2 percent of lubricating agent. The invention aims to provide a lurasidone hydrochloride orally-disintegrating tablet with simple preparation process, low cost, convenience in use and quick action to the indications; and compared with the conventional preparation for oral use, the lurasidone hydrochloride orally-disintegrating tablet preparation can reduce the difficulty in swallowing, improve the compliance, is suitable for special populations such as the elderly, children, patients with difficulty in swallowing and mental patients, can be fully disintegrated within 60 s, can be disintegrated into extremely fine powder, is higher in drug dissolution rate and quick in absorption, and can improve the bioavailability of insoluble drugs, for example, lurasidone hydrochloride.
Owner:BEIJING VENTUREPHARM BIOTECH
Orally disintegrating tablets containing lurasidone and preparation method thereof
ActiveCN103536568ASolve liquidity problemsSolve the problem of bad tasteOrganic active ingredientsNervous disorderLurasidone HydrochlorideOrally disintegrating tablet
The invention relates to orally disintegrating tablets containing lurasidone and a preparation method thereof. The orally disintegrating tablets comprise lurasidone hydrochloride, filler, disintegrant, flavoring agent and lubricant, wherein the grain diameter D90 of the lurasidone hydrochloride is less than or equal to 75 mu m. The lurasidone orally disintegrating tablets have good dissolution in vitro and excellent taste; the orally disintegrating tables are simple in preparation process, and industrial production can be realized by adopting conventional granulation and tabletting equipment.
Owner:CHENGDU KANGHONG PHARMA GRP
Preparation containing lurasidone hydrochloride and preparation method thereof
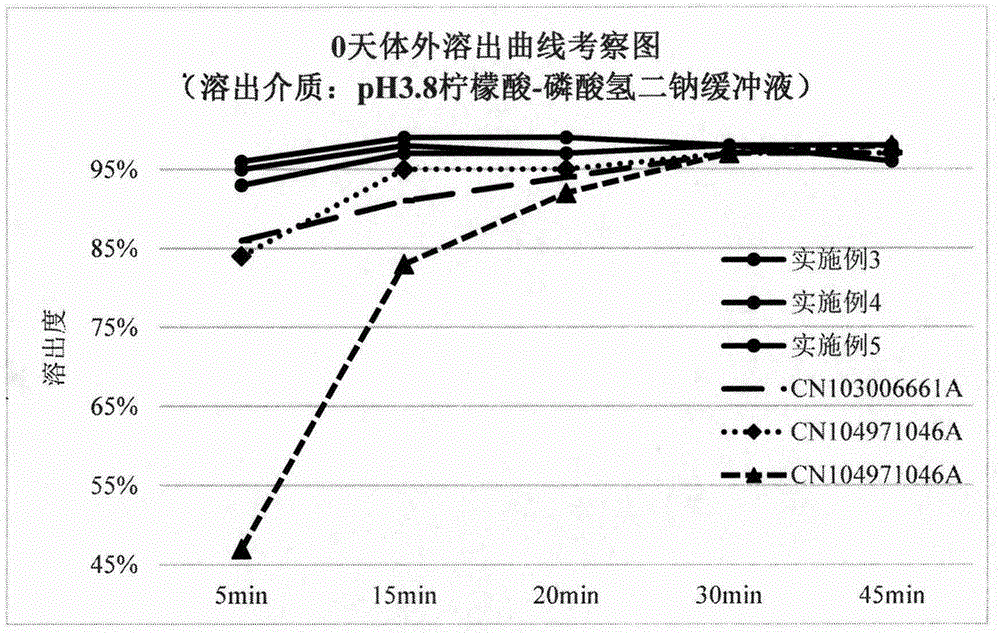
ActiveCN103006661AFast Dissolution PropertiesOrganic active ingredientsNervous disorderLurasidone HydrochlorideActive component
The invention relates to an oral preparation and a preparation method thereof. The preparation contains lurasidone hydrochloride, a micronized particle of a dispersion supporter, microcrystalline cellulose and a water-soluble polymer adhesive. More specifically, the invention relates to the oral preparation and especially to a tablet. The preparation has a fast dissolution characteristic, and dissolution characteristics of an active component are identical; even though the content of the active component in the preparation changes in a certain range, the dissolution characteristics of the active component are still identical.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD
Lurasidone hydrochloride tablets
ActiveCN105395493AHigh yieldSimple production processOrganic active ingredientsNervous disorderLurasidone HydrochlorideAdditive ingredient
The invention belongs to the novel technical field of medicine preparation, and particularly relates to lurasidone hydrochloride tablets and a preparing method thereof. The lurasidone hydrochloride tablets are prepared with lurasidone hydrochloride as the main ingredient through direct compression of lurasidone hydrochloride solid dispersion particles and auxiliaries. The lurasidone hydrochloride solid dispersion particles are prepared in the way of heating potassium citrate and sorbitol in a hot melt extruder to be molten, then adding lurasidone hydrochloride for melting, and extruding melt liquid for pelleting, wherein the weight ratio of lurasidone hydrochloride to potassium citrate is 1 : (0.5-2), and the weight ratio of lurasidone hydrochloride to sorbitol is 1 : (1-5). By the adoption of the lurasidone hydrochloride tablets, the problem that the in vitro dissolution rate of lurasidone hydrochloride is low is solved, the curative effect is improved, and a more ideal curative effect is realized.
Owner:NANJING ZENKOM PHARMA
Preparation method for lurasidone hydrochloride
InactiveCN102746289AImprove stabilityImprove reliabilityOrganic chemistryCarbon numberLurasidone Hydrochloride
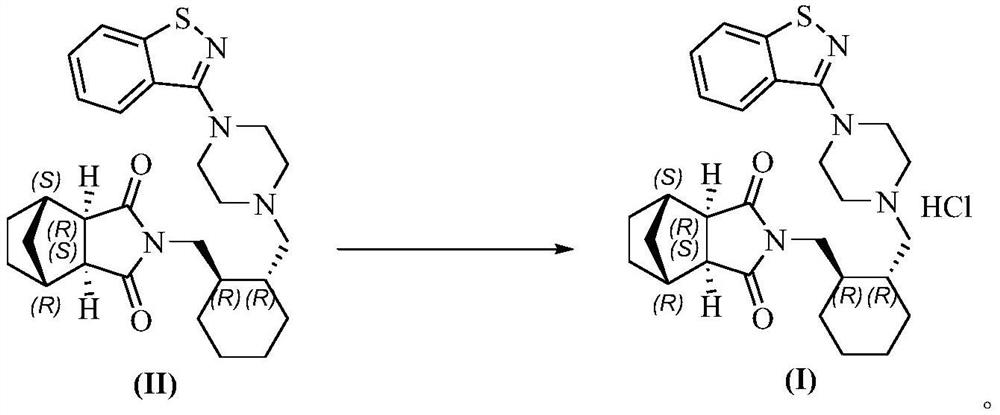
The present invention discloses a preparation method for lurasidone hydrochloride represented by a formula (I). The method comprises the following steps: adopting a mixture to treat a solution formed by dissolving a compound (II) in a dialkyl ketone solvent having carbon number not more than 6, and carrying out crystallization to obtain the product of the present invention, wherein the mixture comprise an organic solvent capable of mixing and dissolving with water and hydrochloric acid, a mass ratio of the organic solvent to the hydrochloric acid is 0.5:1-100:1, a molar ratio of hydrogen chloride in the hydrochloric acid to the compound II is 10:1-0.9:1. The preparation method of the present invention has the following advantages that: durability is significantly improved, the prepared lurasidone hydrochloride (the compound I) has characteristics of high purity, low residual acetone, and high yield, and the method is economical and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Lurasidone hydrochloride oral preparation and preparing method of lurasidone hydrochloride oral preparation
ActiveCN104337790ARapid dissolutionReduce lossesOrganic active ingredientsNervous disorderLurasidone HydrochlorideLow-substituted hydroxypropylcellulose
The invention provides a lurasidone hydrochloride oral preparation and a preparing method of the lurasidone hydrochloride oral preparation. The lurasidone hydrochloride oral preparation comprises lurasidone hydrochloride accounting for 20 to 45 weight percent of the oral preparation and cellulose derivatives accounting for 5 to 30 weight percent of the oral preparation, wherein the cellulose derivatives are a mixture of low-substituted hydroxypropyl cellulose and croscarmellose sodium. The lurasidone hydrochloride oral preparation has the advantages that the low-substituted hydroxypropyl cellulose and the croscarmellose sodium are simultaneously used as disintegrating agents, so that lurasidone hydrochloride tablets can be fast dissolved out, even when the content of active ingredients in the preparation is changed, the dissolution characteristics are also identical to the reference preparation, and the in-batch differences are small. In addition, according to the raw material processing mode, the raw material yield is higher, and the loss is less.
Owner:SHIJIAZHUANG NO 4 PHARMA
Sustained-release suspension with lurasidone and preparation method of sustained-release suspension
InactiveCN104983679AReduce releaseMask bad smellOrganic active ingredientsNervous disorderBlood concentrationLurasidone Hydrochloride
The invention belongs to the field of pharmaceutic preparations, and particularly relates to a sustained-release suspension composition with lurasidone or salt of the lurasidone and a preparation method of the sustained-release suspension composition. A sustained-release suspension comprises the lurasidone or the salt, capable of being medically accepted, of the lurasidone and a sustained-release micro-capsule coating material medicine composition. According to the preparation, the release time of lurasidone hydrochloride is effectively prolonged, and the blood concentration peak valley phenomenon caused by quick release preparations is avoided. The sustained-release suspension with the lurasidone is low in production cost and has the good taste and medicine release character, the preparation process of the sustained-release suspension with the lurasidone is convenient, and the aim of providing the preparation with the lurasidone or the salt, capable of being medically accepted, of the lurasidone for a patient is achieved, wherein the preparation is convenient to take, high in bioavailability and mild in medicine release.
Owner:AVENTIS PHARMA HAINAN
Lurasidone hydrochloride orally disintegrating tablet and preparation method thereof
ActiveCN106539768ADisintegrates quicklyInconvenient to take medicineOrganic active ingredientsNervous disorderLurasidone HydrochlorideOrally disintegrating tablet
The invention provides a lurasidone hydrochloride orally disintegrating tablet containing indissolvable drugs. The lurasidone hydrochloride orally disintegrating tablet comprises 8%-24% of lurasidone hydrochloride, 8%-48% of a carrier, 0.8%-4% of adhesive, 24%-76.2% of filler, 4%-10% of a disintegrating agent, 2%-6% of flavoring agent and 1%-4% of a lubricating agent. The lurasidone hydrochloride orally disintegrating tablet can be disintegrated quickly, and convenience is provided for medicine taking of patients particularly like the aged, children, coma patients and the patients who cannot take water conveniently. Moreover, the dissolving-out rate can reach 85% or above within 5 minutes. In addition, the lurasidone hydrochloride orally disintegrating tablet can be disintegrated and absorbed quickly in the oral cavity, so that the bioavailability is greatly improved. Operation is easy in production technology, the production cost is low, and the lurasidone hydrochloride orally disintegrating tablet is suitable for industrial mass production.
Owner:CHENGDU KANGHONG PHARMA GRP
A kind of lurasidone hydrochloride tablet
ActiveCN105395493BHigh yieldSimple production processOrganic active ingredientsNervous disorderLurasidone HydrochlorideCurative effect
The invention belongs to the novel technical field of medicine preparation, and particularly relates to lurasidone hydrochloride tablets and a preparing method thereof. The lurasidone hydrochloride tablets are prepared with lurasidone hydrochloride as the main ingredient through direct compression of lurasidone hydrochloride solid dispersion particles and auxiliaries. The lurasidone hydrochloride solid dispersion particles are prepared in the way of heating potassium citrate and sorbitol in a hot melt extruder to be molten, then adding lurasidone hydrochloride for melting, and extruding melt liquid for pelleting, wherein the weight ratio of lurasidone hydrochloride to potassium citrate is 1 : (0.5-2), and the weight ratio of lurasidone hydrochloride to sorbitol is 1 : (1-5). By the adoption of the lurasidone hydrochloride tablets, the problem that the in vitro dissolution rate of lurasidone hydrochloride is low is solved, the curative effect is improved, and a more ideal curative effect is realized.
Owner:NANJING ZENKOM PHARMA
Lurasidone hydrochloride crystal form as well as preparation method and application thereof
InactiveCN104016976AThe crystal form is stableGood repeatabilityOrganic active ingredientsNervous disorderLurasidone HydrochlorideDrug development
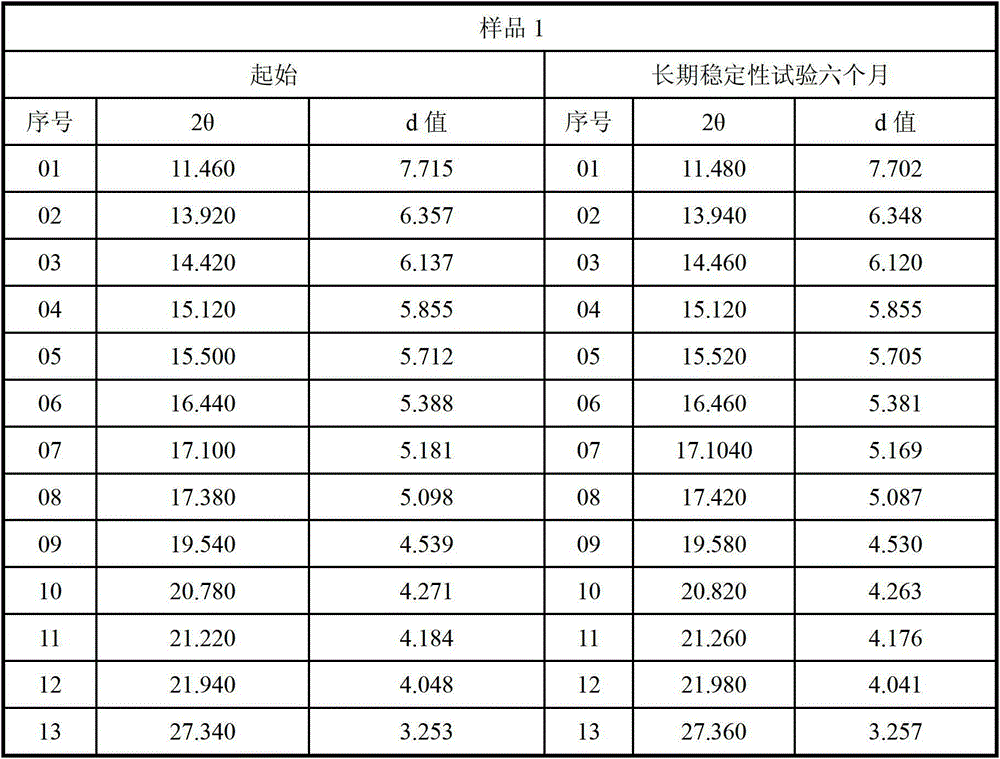
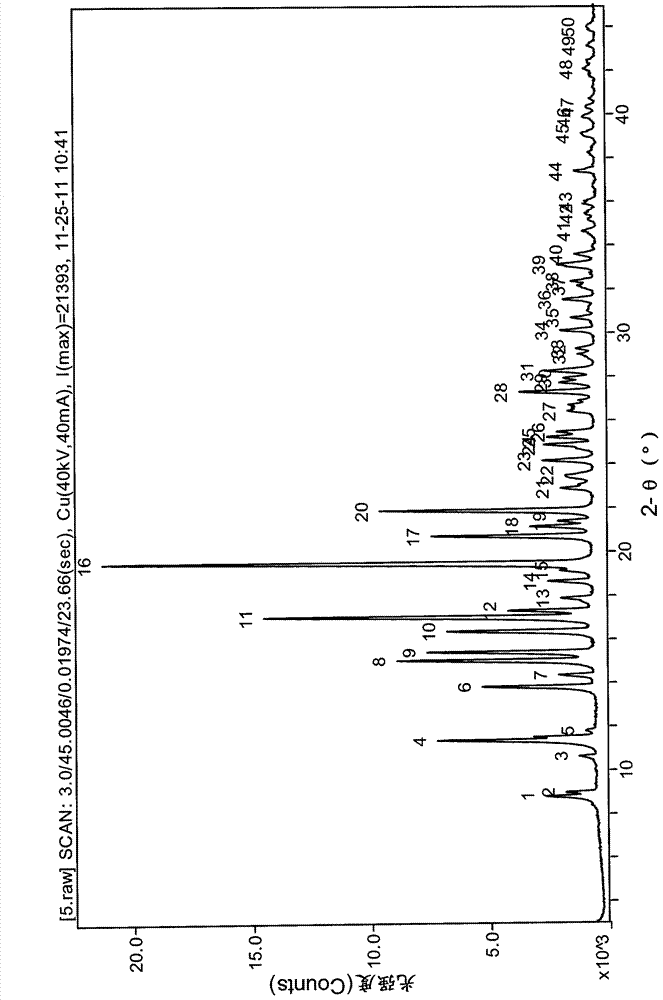
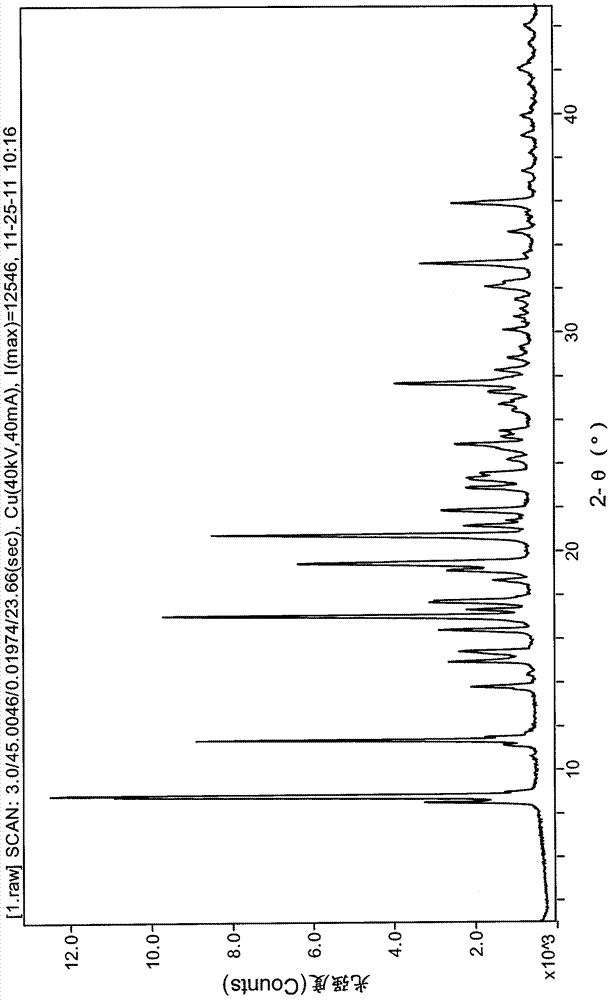
The invention relates to a lurasidone hydrochloride crystal form as well as a preparation method and application thereof. Specifically, the invention discloses a lurasidone hydrochloride crystal form, and a preparation method, a pharmaceutical composition and a pharmaceutical purpose thereof. An XRD characteristic peak of the lurasidone hydrochloride crystal form is as shown in figure I. The lurasidone hydrochloride crystal form provided by the invention is stable in character, good in repeatability and suitable for drug development.
Owner:JIANGSU HANSOH PHARMA CO LTD
Preparation method of lurasidone hydrochloride nanocrystals based on solubility pH-dependent property
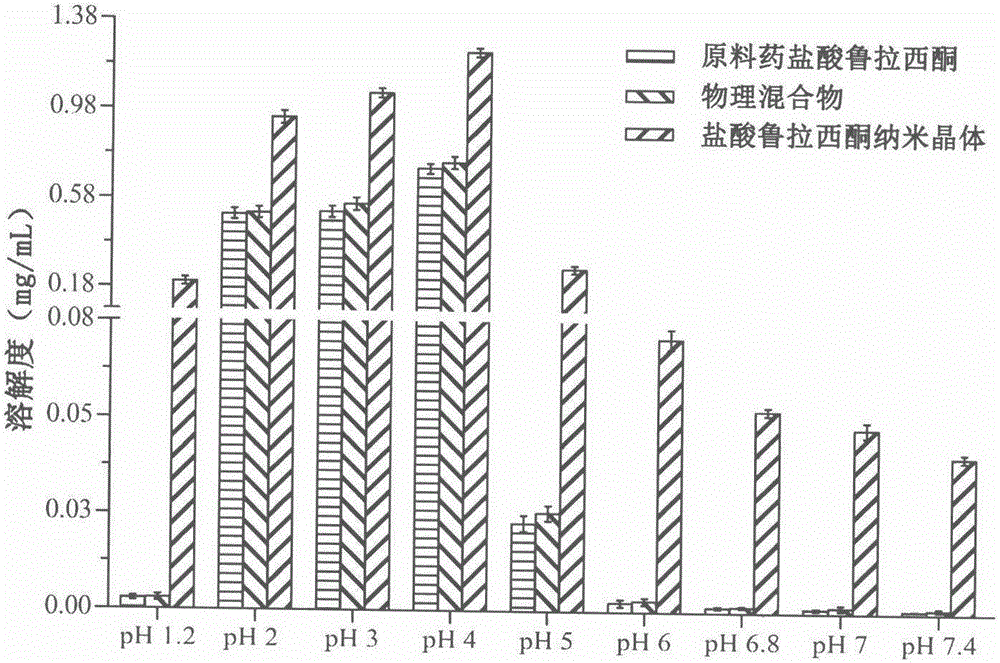
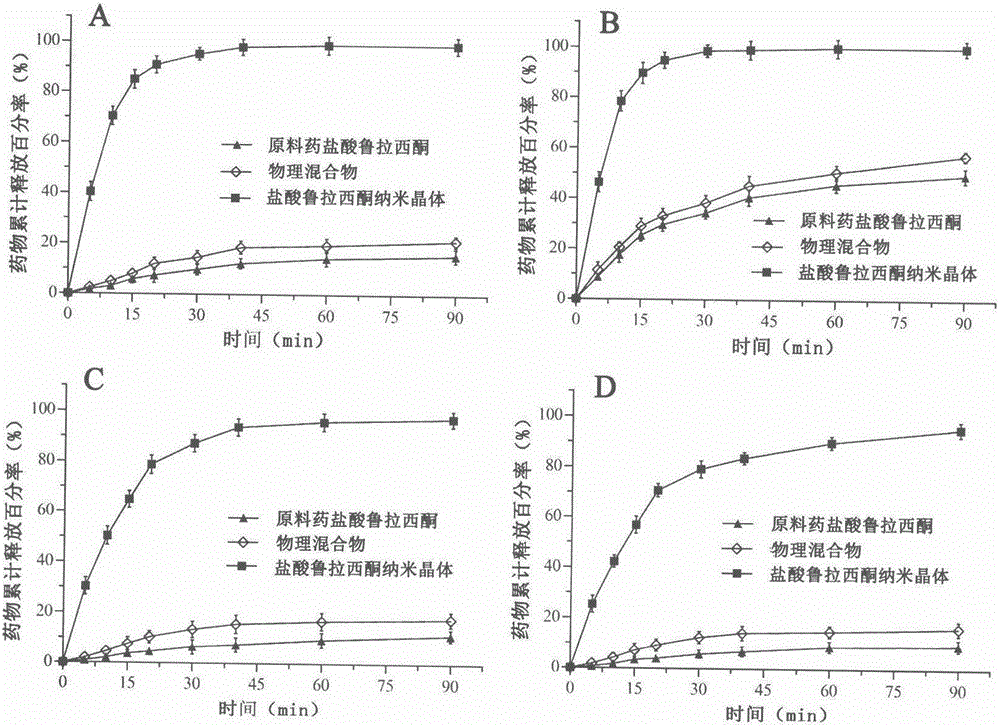
The invention relates to a preparation method of lurasidone hydrochloride nanocrystals based on the solubility pH-dependent property and belongs to the technical field of medicine. The preparation method of the lurasidone hydrochloride nanocrystals includes the steps that 1, lurasidone hydrochloride is dissolved in a mixed solvent of a pH4 hydrochloric acid solution and a latent solvent, and the mixture serves as a good solvent; 2, a stabilizer is dissolved in deionized water, and the mixture serves as a poor solvent; 3, the good solvent with lurasidone hydrochloride dissolved is added into the poor solvent, magnetic stirring dispersion is carried out, after the good solvent is added into the poor solvent, the materials continue to be stirred, and the nanocrystals are prepared. The prepared nanocrystals are uniform in quality and appearance, the average grain diameter is 200-600 nm, and the solubility, dissolution rate and oral bioavailability of lurasidone hydrochloride are remarkably improved. The preparation process is simple, operation is easy to carry out, cost is low, no toxic or harmful organic solvent is used, no byproducts are generated, and industrial production is convenient.
Owner:CHINA PHARM UNIV
Preparation method for lurasidone hydrochloride
InactiveCN106916151ASimple processLow toxicityOrganic chemistryLurasidone HydrochlorideCrystallization
The invention discloses a preparation method for lurasidone hydrochloride. According to the preparation method, trans-1,2-cyclohexanedicarboxylic acid (SM-1) is used as a raw material and subjected to resolution, methyl esterification, reduction, methylsulfonylation, condensation, recrystallization and salt formation so as to eventually obtain lurasidone hydrochloride. The preparation method provided by the invention greatly reduces production cost and has the characteristics of high product yield, easy operation, low toxicity and suitability for industrial large-scale production.
Owner:SUZHOU ERYE PHARMA CO LTD
Preparation method of antipsychotic drug lurasidone
InactiveCN103450172AHighly toxicHigh puritySulfonic acid esters preparationLurasidone HydrochlorideIsoindoles
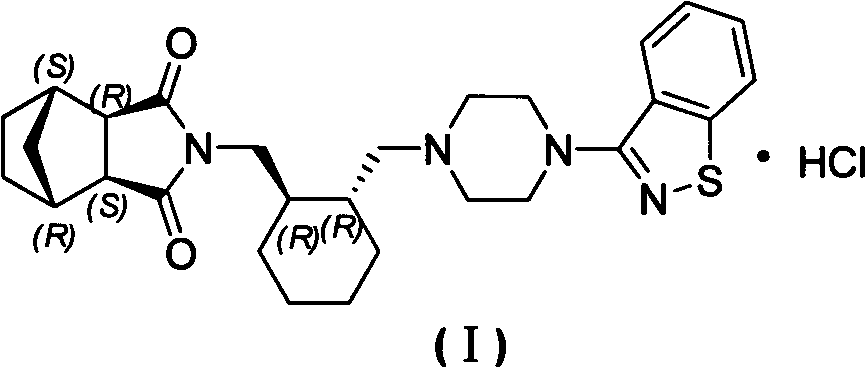
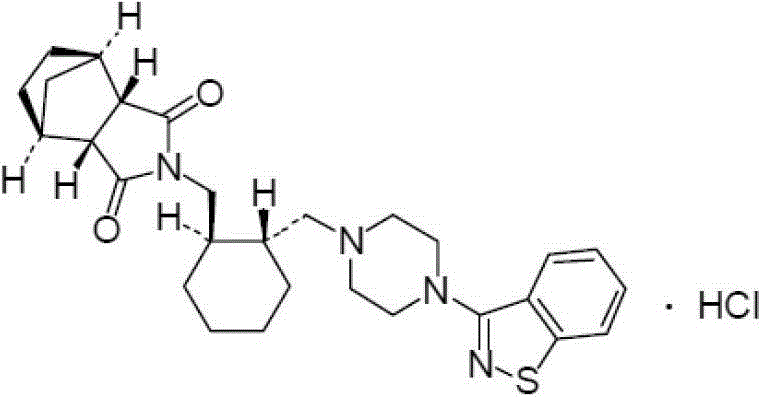
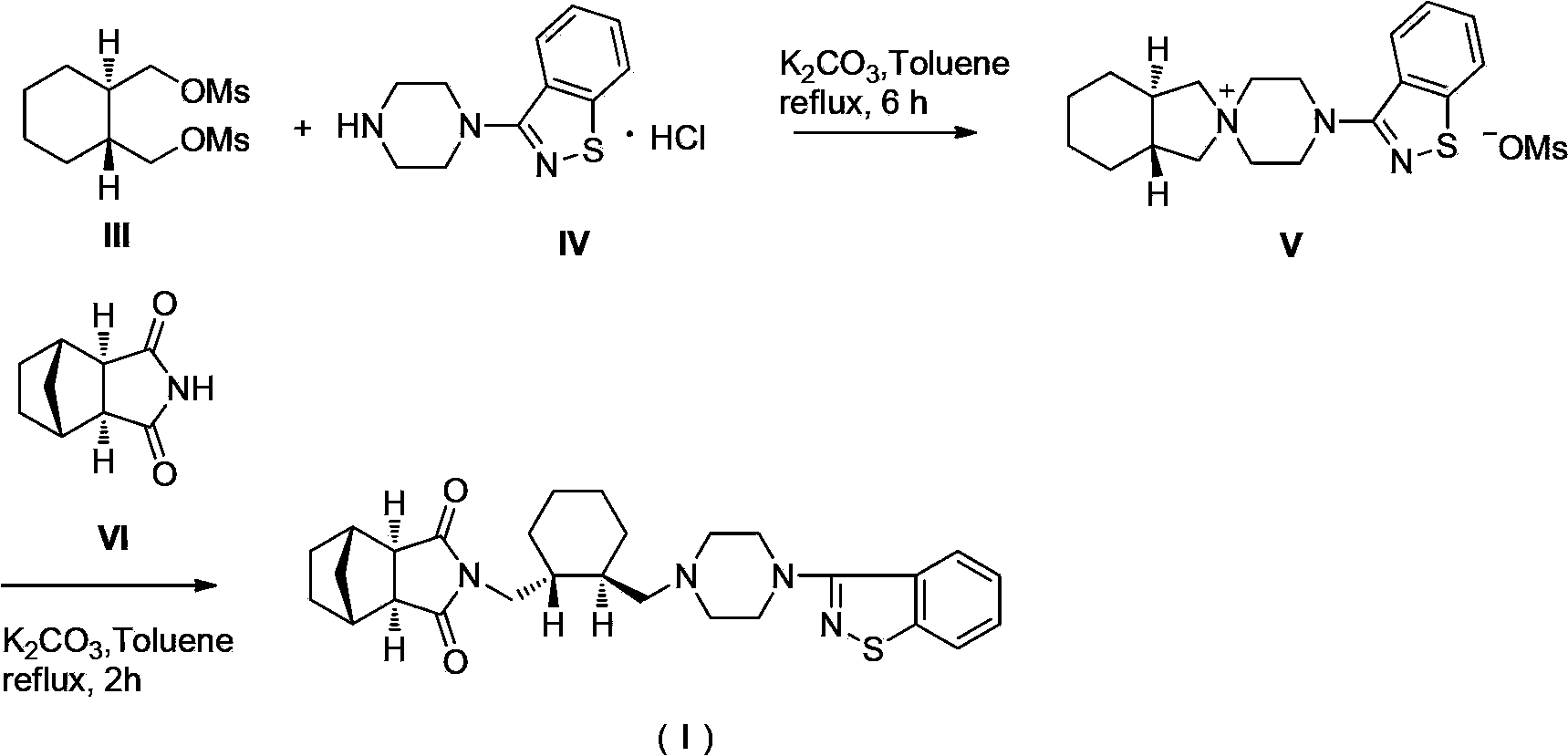
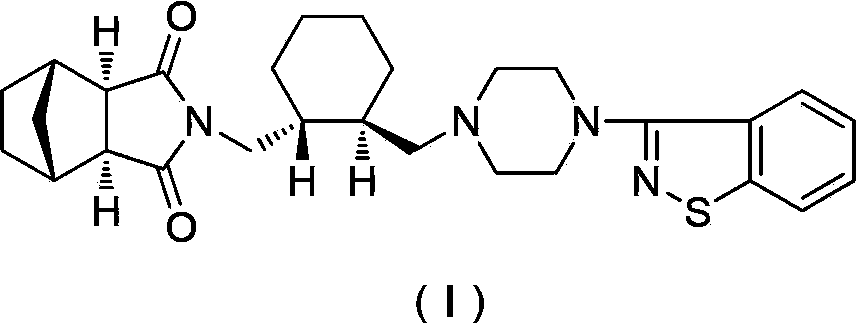
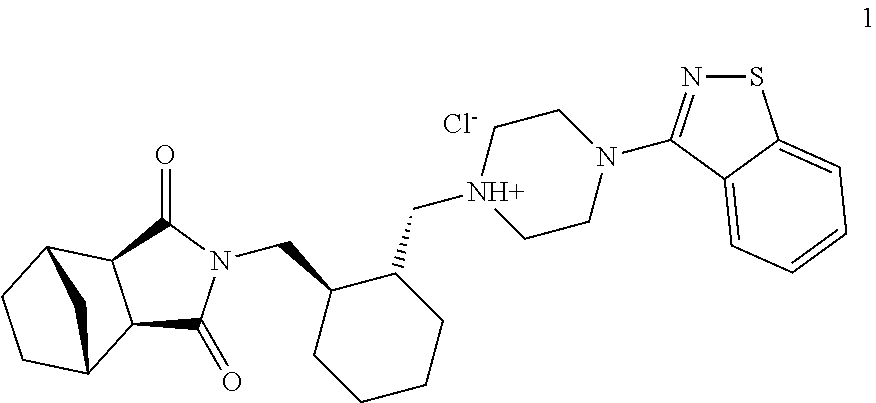
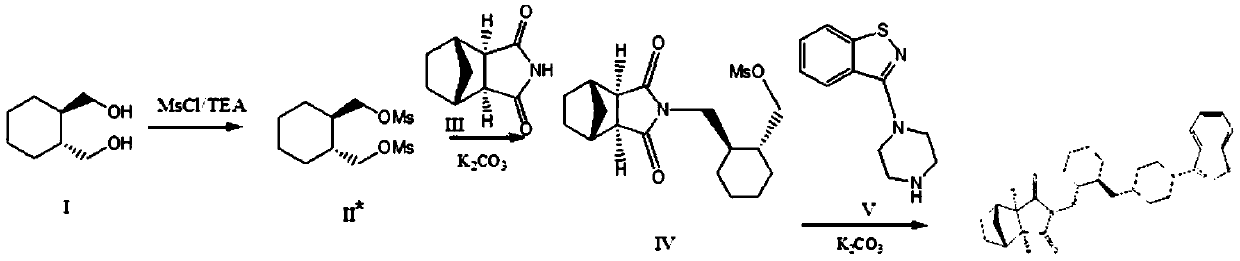
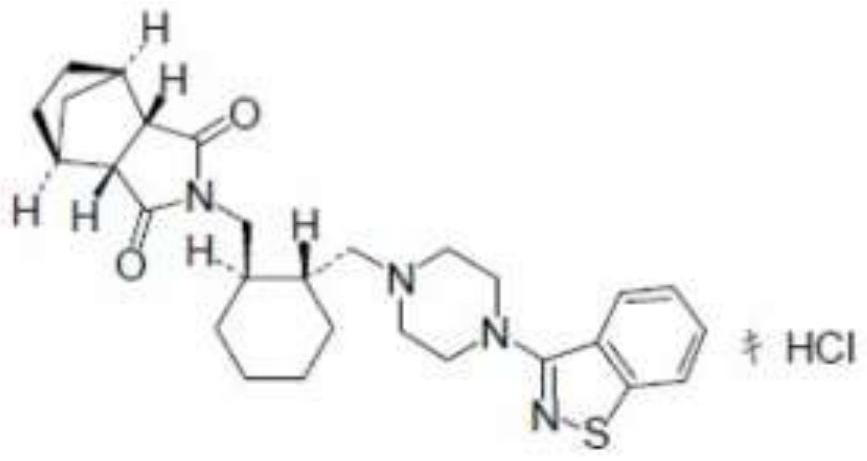
The invention relates to preparation methods of (3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzisothiazole-3-yl)piperazinyl-1-methyl]cyclohexyl methyl}hexahydro-1H-4,7-methano-isoindole-1,3-dione (a compound represented by a formula I) and intermediates thereof. A hydrochloride (lurasidone hydrochloride) of the compound represented by the formula (I) can be used as a drug for treatment of psychosis.
Owner:广东永正药业有限公司
Lurasidone hydrochloride-amino acid coamorphous material
The invention relates to a lurasidone hydrochloride-L-cysteine hydrochloride coamorphous material capable of significantly improving the solubility dissolution rate of highly-insoluble drug lurasidone hydrochloride. The coamorphous material is amorphous, is completely different from lurasidone hydrochloride crystal, and different from the lurasidone hydrochloride crystal in melting point, powder X ray diffraction spectra, DSC spectra and infrared spectroscopy. The powder X ray diffraction spectra shown by 2 theta and obtained by Cu-K alpha radiation has no sharp diffraction peak. The glass transition temperature is about 71.7 DEG C. The characteristic dissolving experiment shows that the characteristic dissolving rate of the lurasidone hydrochloride-L-cysteine hydrochloride coamorphous material is improved by about 5 times compared with that of the original lurasidone hydrochloride crystal.
Owner:CHINA PHARM UNIV
Lurasidone hydrochloride orally disintegrating tablet and preparation method thereof
InactiveCN107007565AImprove stabilitySolve the problem of long-term storage dissolution reductionOrganic active ingredientsNervous disorderLurasidone HydrochlorideOrally disintegrating tablet
The invention belongs to the field of pharmaceutic preparation and specifically relates to a lurasidone hydrochloride orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is prepared according to the following steps: taking lurasidone hydrochloride as a raw material; taking filler, disintegrating agent, corrigent and lubricating agent as ingredients; using a coating material under a specific condition, adopting a specific preparation method for preparing and adopting a tablet press for tableting. The invention can solve the problem of the reducing drug dissolution in long-term storage process and the problem of the taste of the lurasidone hydrochloride orally disintegrating tablet.
Owner:万全万特制药江苏有限公司
Method for separating and measuring relevant substance of lurasidone hydrochloride intermediate by using gas chromatographic technique
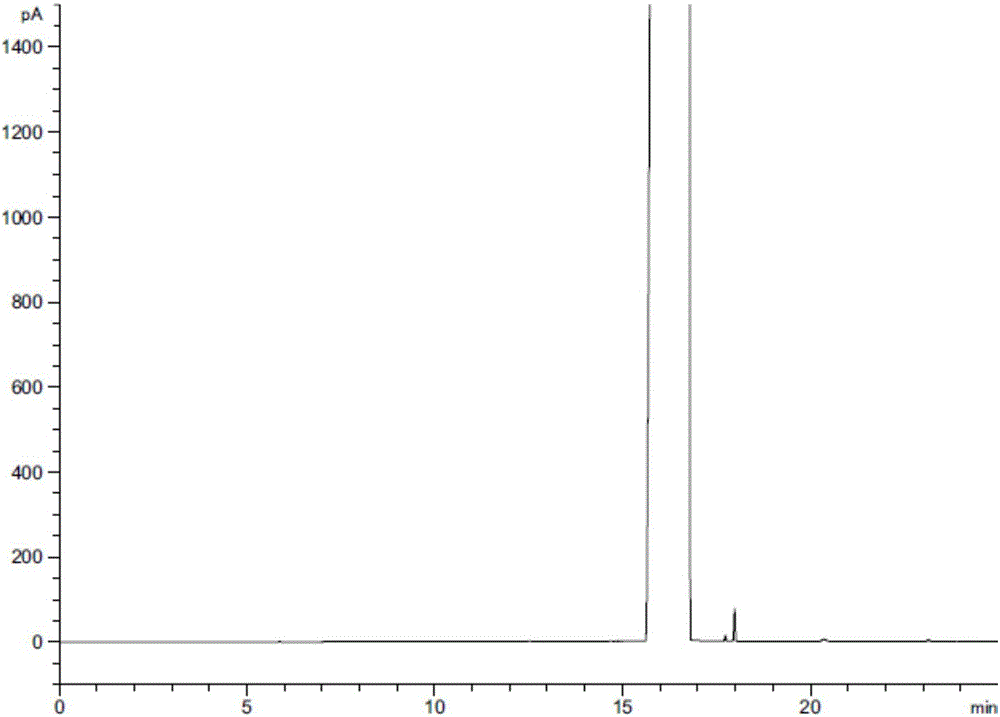
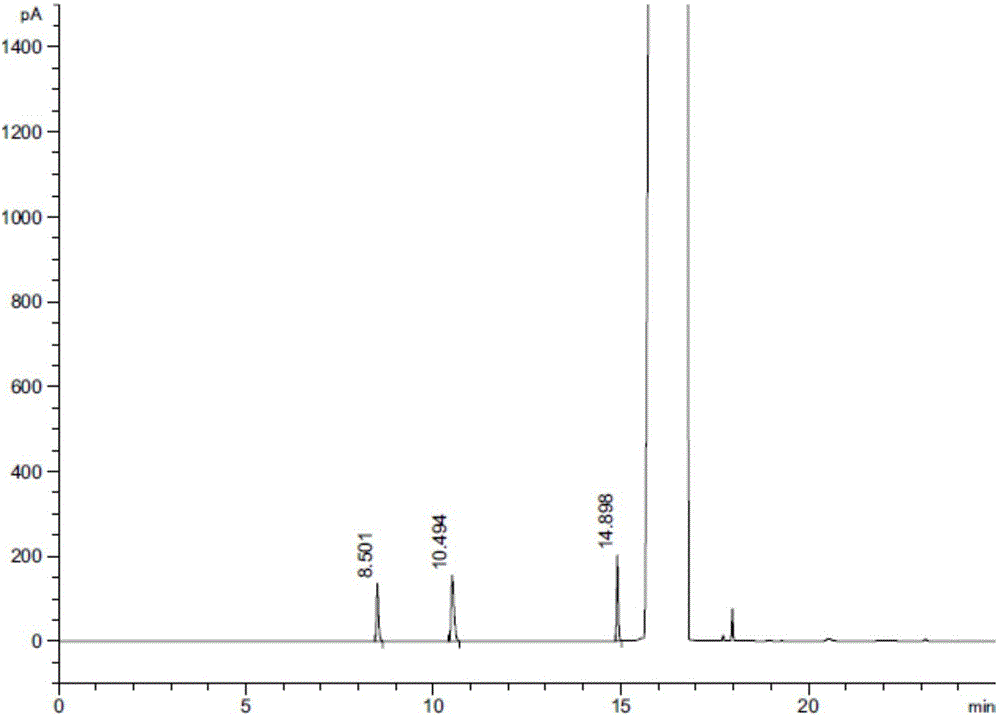
ActiveCN106525996ARapid and effective separation assaySolving Separation Assay ProblemsComponent separationGas phaseVapor phase chromatography
The invention belongs to the field of analytical chemistry, and the invention discloses a method for separating and detecting a relevant substance of a lurasidone hydrochloride intermediate 3-(1-piperazinyl)-1,2-benzisothiazole by using a gas chromatographic technique. A methylpolysilicone medium polar capillary chromatographic column and a hydrogen flame ionization detector are adopted by the method; the content of the relevant substance of the lurasidone hydrochloride intermediate can be quantitatively measured; thus, the purity of a reactant in the process of synthesizing the lurasidone hydrochloride is effectively controlled; the occurrences of side reactions and the generation of impurities are reduced; the yield of a product is improved. The method provided by the invention is quick in separation and detection, high in specificity and high in accuracy, and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Process for the preparation of lurasidone hydrochloride
Disclosed is a new and efficient process for the synthesis with high yields and purity of lurasidone hydrochloride, a medicament which is useful as a psychotropic substance. The process involves the preparation of lurasidone base in a reaction system not containing inorganic salts, followed by conversion of the latter to an addition salt with an organic carboxylic acid, which is finally converted to lurasidone hydrochloride.
Owner:EDMOND PHARMA SRL
Method for preparing new crystalline form of Lurasidone hydrochloride
InactiveCN109705112AStable in natureGood repeatabilityNervous disorderOrganic chemistry methodsDiseaseLurasidone Hydrochloride
The invention belongs to the field of compounds, and relates to a method for preparing a new crystalline form of Lurasidone hydrochloride. The Lurasidone hydrochloride crystals of types of A, B and Care used for treating and preventing diseases of the central nervous system, particularly schizophrenia.
Owner:NHWA PHARMA CORPORATION
Process for the preparation of lurasidone hydrochloride
Disclosed is a new and efficient process for the synthesis with high yields and purity of lurasidone hydrochloride, a medicament which is useful as a psychotropic substance. The process involves the preparation of lurasidone base in a reaction system not containing inorganic salts, followed by conversion of the latter to an addition salt with an organic carboxylic acid, which is finally converted to lurasidone hydrochloride.
Owner:EDMOND PHARMA SRL
Method for preparing lurasidone and salt thereof
The invention relates to a method for preparing lurasidone and a salt thereof. The method comprises the following steps: reacting (R,R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane and 3-(1-piperazinyl)-1,2-benzoisothiazole hydrochloride in acetonitrile in the presence of potassium carbonate to obtain an intermediate I; reacting the intermediate I with (3aR,4S,7R,7aS)-4,7-methylene-1H-isoindole-1,3(2H)-dione in DMF in the presence of potassium carbonate to prepare lurasidone free alkali; and reacting the lurasidone free alkali with hydrochloric acid in isopropanol to obtain lurasidone hydrochloride. The invention also relates to lurasidone and the salt thereof prepared by using the method, and application of lurasidone and the salt thereof in medicines for resisting psychosis, especially schizophrenia. The method disclosed by the invention has one or more advantages in a group consisting of simple process, high product yield, few impurities and the like.
Owner:HUNAN DONGTING PHARMA
Method for separating and determining related substances of lurasidone hydrochloride intermediate by using gas chromatography
ActiveCN105911155ARapid and effective separation assaySolving Separation Assay ProblemsComponent separationKetoneSide reaction
The invention discloses a method for separating and determining a lurasidone hydrochloride intermediate (1R,2R)-1,2-cyclohexanedimethanol and related substances thereof by using gas chromatography. The method employs a polysiloxane nonpolar capillary chromatographic column and a hydrogen flame ionization detector, can quantitatively determine the contents of the lurasidone hydrochloride intermediate and the related substances thereof, so purity of reactants in the process of synthesis of lurasidone hydrochloride is effectively controlled, occurrence of side reactions and generation of impurities are reduced, and product yield is improved. The method provided by the invention is fast in separation and detection, good in specificity, high in accuracy, and easy and simple to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Lurasidone pamoate amorphous substance as well as preparation method and application thereof
ActiveCN111454256AGood physical and chemical stabilityImprove bioavailabilityOrganic active ingredientsNervous disorderLurasidone HydrochlorideBioavailability
The invention relates to the technical field of medicines, and discloses a lurasidone pamoate amorphous substance as well as a preparation method and application thereof. The method comprises the following steps: (1) dissolving lurasidone hydrochloride and pamoic acid disodium salt, or lurasidone and pamoic acid in tetrahydrofuran to obtain a first mixed solution; (2) stirring for 2 to 3 h at 55 to 60 DEG C for reaction to obtain a second mixed solution; and (3) carrying out spin-drying on the second mixed solution at 55-60 DEG C, and carrying out precipitation by taking water as a precipitation solvent. The pamoic acid lurasidone amorphous substance prepared by the preparation method is different from lurasidone hydrochloride in the prior art in form; the equilibrium solubility of the first amorphous substance and the second amorphous substance of the pamoic acid lurasidone prepared by the method is 50.69-635 [mu]g / mL and 29-361 [mu]g / mL respectively between pH 6-7.8, so that the solubility of lurasidone hydrochloride can be remarkably improved, and the oral bioavailability of lurasidone is improved.
Owner:HUBEI UNIV OF CHINESE MEDICINE
An improved process for the preparation of lurasidone hydrochloride
ActiveUS20170246165A1Useful in treatmentCost-effective and industrially amenableOrganic active ingredientsOrganic chemistryLurasidone HydrochlorideIndustrial scale
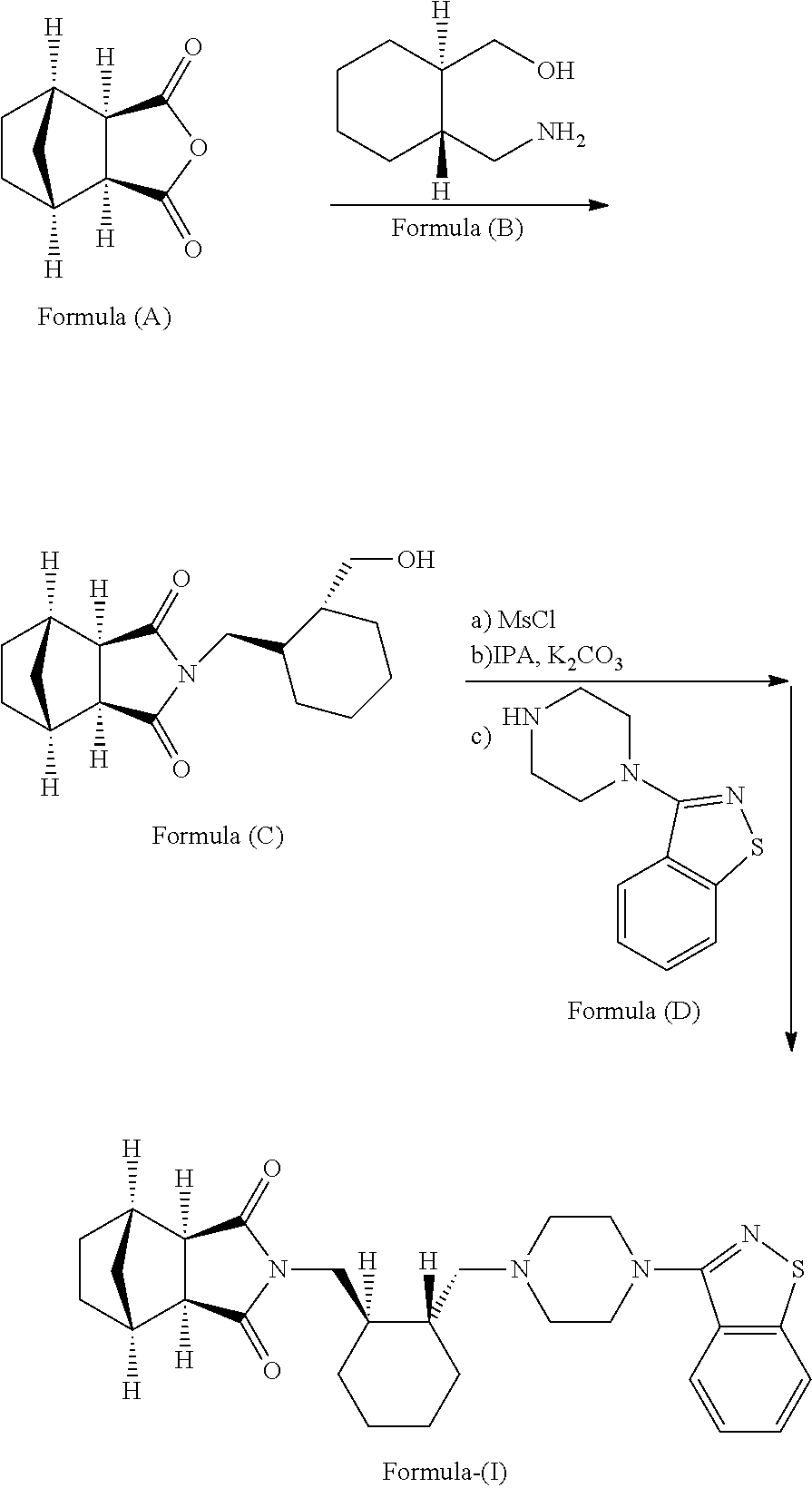
Disclosed herein is an improved process for the preparation of Lurasidone and its pharmaceutically acceptable salts via novel intermediate and use thereof for the preparation of an antipsychotic agent useful for the treatment of schizophrenia and bipolar disorder. Further, present invention provides a cost effective and eco-friendly process for producing Lurasidone hydrochloride of formula (I) substantially free of residual solvent(s) at industrial scale.
Owner:JUBILANT GENERICS
Lurasidone hydrochloride nano mixed suspension solution for durable intramuscular injection and preparation method of mixed suspension solution
InactiveCN109998991AImprove solubilityImprove dissolution rateOrganic active ingredientsNervous disorderLurasidone HydrochlorideIntramuscular injection
The invention discloses a lurasidone hydrochloride nano mixed suspension solution for durable intramuscular injection and a preparation method of the nano mixed suspension solution. The raw materialsof the nano mixed suspension solution comprise 0.1-0.5 wt.% of lurasidone hydrochloride and 0.1-0.3 wt.% of a stabilizer, and a solvent is water for injection. Through high-speed dispersion, in combination with a wet-method medium grinding method, the lurasidone hydrochloride is prepared into the nano mixed suspension solution for intramuscular injection, the first-pass effect is avoided, and thesolubility and dissolution rate of the medicine after muscle injection are improved. After muscle injection, the effect can be achieved continuously for 3-7 days, the drug administration frequency isreduced, fluctuation of the plasma drug concentration is reduced, and the compliance and safety of a patient during medicine taking are improved.
Owner:CHINA PHARM UNIV
Crystal form B of lurasidone hydrochloride and preparation method thereof
The invention discloses a crystal form B of N-[4-[4-(1,2-benzisothiazole-3-yl)-1-piperazine]-(2R, 3R)-2,3-tetramethylene-butyl]-(1'R, 2'S, 3'R, 4'S)-2,3-bicyclo[2,2,1], which is characterized in that the crystal form B displays an X-ray powder diffraction pattern having characteristic peaks represented by 2 theta degrees, namely 8.5+ / -0.2, 8.8+ / -0.2, 11.4+ / -0.2, 13.8+ / -0.2, 14.9+ / -0.2, 15.4+ / -0.2, 16.4+ / -0.2, 17.0+ / -0.2, 17.3+ / -0.2, 17.7+ / -0.2, 18.7+ / -0.2, 19.1+ / -0.2, 19.4+ / -0.2, 20.7+ / -0.2, 21.2+ / -0.2, 21.9+ / -0.2, 27.7+ / -0.2, 33.1+ / -0.2, and 35.9+ / -0.2.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Method for preparing lurasidone hydrochloride intermediate
The invention discloses a method for preparing a lurasidone hydrochloride intermediate (formula I). (S,R)-p-toluene sulfonic acid cyclohexane dimethyl ester (formula II) and benzisothiazole piperazine (formula III) are dissolved in an aprotic polar solvent, a basic catalyst is used to serve as an acid binding agent, and heating reaction is performed for 10-24 h. The proper aprotic polar solvent is adopted to serve as a reaction medium for substitution reaction, a mixed feeding method is adopted, so that the reaction is performed quickly, the content of chiral impurities is low, and a postprocessing process is simple and convenient.
Owner:BEIJING VENTUREPHARM BIOTECH
Lurasidone sublingual tablet and preparation method thereof
ActiveCN113081983AReduce typesHigh drug contentOrganic active ingredientsNervous disorderCelluloseDrug content
The invention discloses a sublingual tablet containing lurasidone hydrochloride or salt thereof and a preparation method of the sublingual tablet. The sublingual tablet is prepared from the following raw materials in parts by mass: 4 to 8 parts of lurasidone or a salt thereof, 2 to 6 parts of sorbitol, 4 to 8 parts of mannitol, 0.5 to 1.5 parts of polyvinylpolypyrrolidone, 0.1 to 0.5 part of hydroxy propyl cellulose, 0.1 to 0.2 part of aspartame and 0.1 to 0.2 part of magnesium stearate, wherein the particle size range of the lurasidone or the salt thereof is 0.1 to 10 microns. The sublingual tablet disclosed by the invention is convenient in preparation process, high in drug content, high in disintegration speed, good in permeable membrane absorption and good in in-vitro dissolution and taste, and a preparation containing lurasidone or salt thereof, which is convenient to take, quick to absorb and high in bioavailability, can be provided for patients.
Owner:北京阳光诺和药物研究股份有限公司
Preparation method of lurasidone hydrochloride
PendingCN113024535ALow costImprove processing stabilityOrganic chemistryLurasidone HydrochlorideMedicinal chemistry
The invention provides a preparation method of lurasidone hydrochloride. The method comprises the step of reacting lurasidone free alkali with hydrochloric acid in a mixed solvent of an alcohol solvent and a haloalkane solvent to obtain the lurasidone hydrochloride. The method provided by the invention has the advantages of high yield, low cost, high safety and low residual solvent content, and is very suitable for industrial production.
Owner:SHANGHAI SYNCORES TECH INC +1
Lurasidone hydrochloride tablets and preparation method thereof
InactiveCN113476415AInhibition of crystallization rateImprove bioavailabilityOrganic active ingredientsNervous disorderLurasidone HydrochlorideDrugs preparations
The invention belongs to the technical field of pharmaceutical preparations and particularly relates to lurasidone hydrochloride tablets and a preparation method thereof. The tablets take lurasidone hydrochloride as an active component and consist of the lurasidone hydrochloride, a filling agent, copovidone, cross-linked povidone and a lubricant. According to the lurasidone hydrochloride tablets and the preparation method thereof, based on physicochemical property characteristics of the lurasidone hydrochloride, the hydrogen bond association effect between the copovidone and the cross-linked povidone and the lurasidone hydrochloride is utilized, the recrystallization rate of the medicine is inhibited, the dissolution rate of the medicine is improved, and the bioavailability of the medicine is guaranteed. Simultaneously, the invention further discloses a preparation process of the lurasidone hydrochloride tablets. The preparation process is simple, has good reproducibility and is suitable for industrial production.
Owner:北京丰科睿泰医药科技有限公司
Improved process for the preparation of lurasidone and its intermediate
ActiveUS20170349601A1Increase productionNervous disorderOrganic chemistryAlcoholLurasidone Hydrochloride
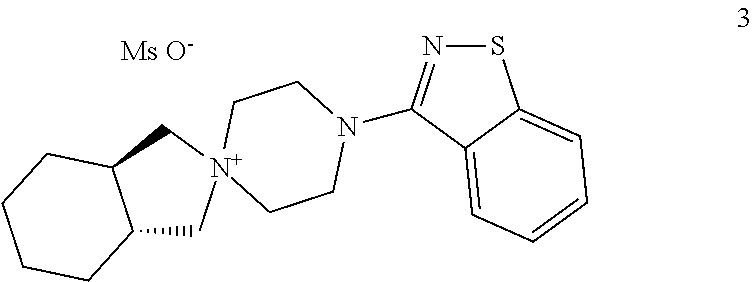
The present invention provides an improved process for preparation of the substantially pure (3aR,7aR)-4′-(benzo[d]isothiazol-3-yl)octahydrospiro[isoindole-2,1′-piperazin]-1′-ium methanesulfonate (referred to as compound-II), which is useful as a key intermediate for the synthesis of lurasidone ((3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1ylmethyl]cyclohexylmethyl}hexahydro-4,7-methano-2H-isoindole-1,3-dione). The process comprises reaction of the compound-III (as described herein) with the compound-IV (as described herein) in the presence of a solvent mixture selected from an alcohol and water; and a base The improved process for the preparation of compound II provides the product with total amount of unreacted compound-IV as impurity in less than 0.06% and the product with HPLC purity as ≧99.8%. The process further refers purification of Lurasidone hydrochloride, wherein the product contains the residual acetone <5000 ppm.
Owner:PIRAMAL PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com