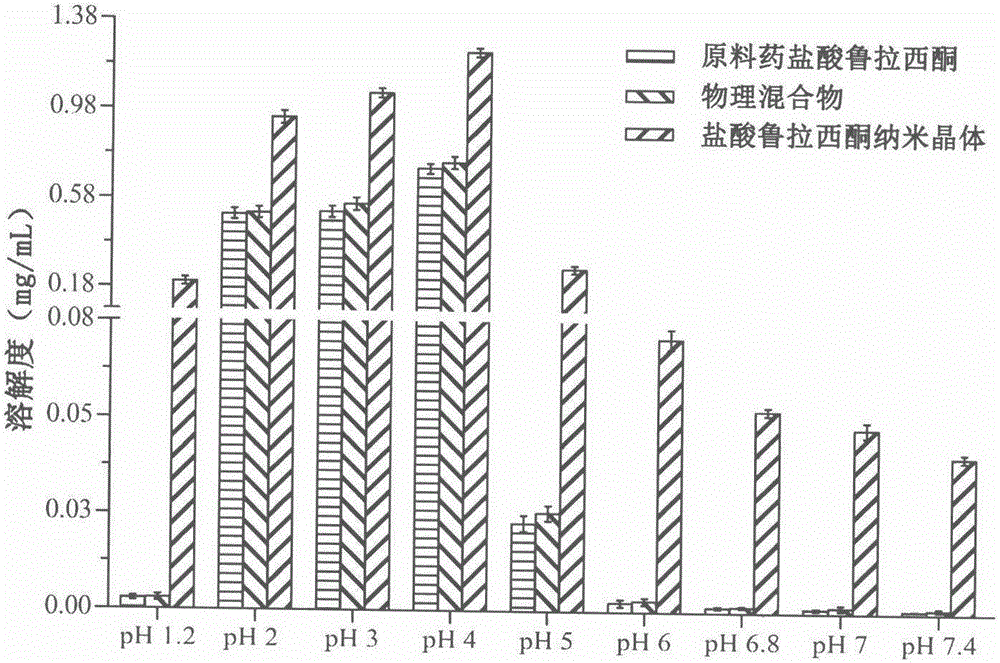
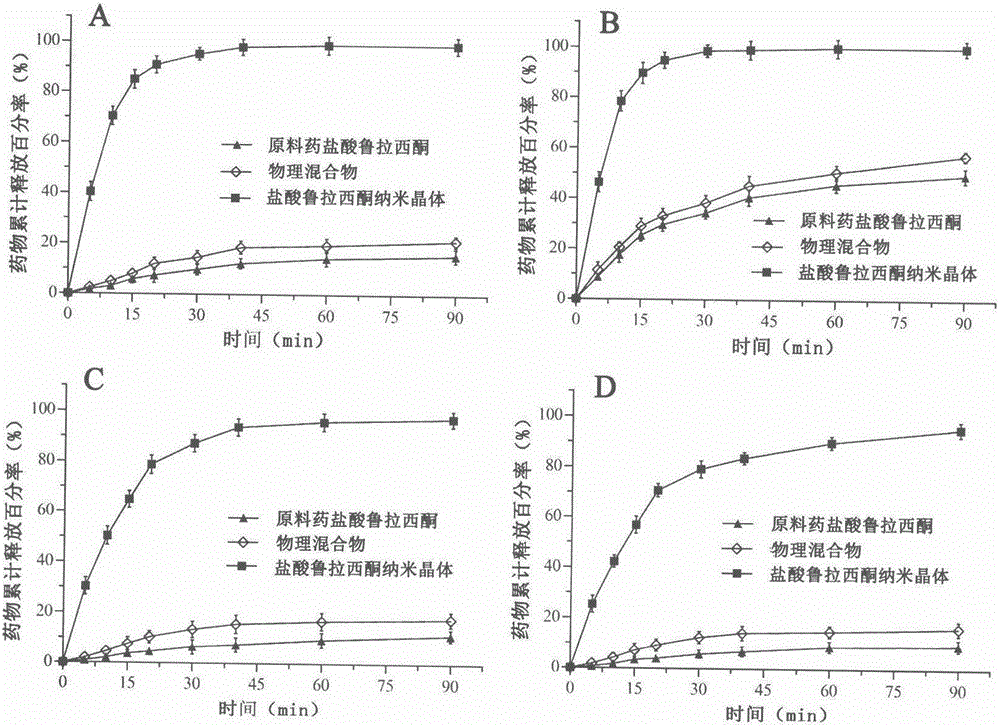
Preparation method of lurasidone hydrochloride nanocrystals based on solubility pH-dependent property
A technology of lurasidone hydrochloride and nanocrystals, which is applied in the field of medicine, can solve the problems of low solubility of lurasidone hydrochloride, poor oral bioavailability, slow dissolution rate, etc., to improve oral bioavailability, increase solubility and Effect of dissolution rate and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 12 mg of lurasidone hydrochloride and dissolve it in a mixed solvent of 3 mL of pH4 hydrochloric acid solution and 1 mL of ethanol as a good solvent; weigh 8 mg of poloxamer and 4 mg of Tween 80 and dissolve it in 40 mL of deionized water as a poor solvent; The good solvent was dripped into the poor solvent at a dropping rate of 16mL / min, and the stirring rate was controlled at 200r / min. After the dropping, the stirring was continued for 1.5h to obtain lurasidone hydrochloride nanocrystals. Detected with a laser particle size analyzer, the average particle diameter is 579.8nm, and the polydispersity index is 0.34.
Embodiment 2
[0031] Weigh 20 mg of lurasidone hydrochloride and dissolve it in a mixed solvent of 16 mL of pH4 hydrochloric acid solution and 4 mL of propylene glycol as a good solvent; weigh 5 mg of methylvitamin and dissolve it in 160 mL of deionized water as a poor solvent; Drop into the poor solvent at a dropping speed of 3 mL / min, control the stirring speed at 400 r / min, and continue stirring for 1 h after the dropping to obtain lurasidone hydrochloride nanocrystals. Detected with a laser particle size analyzer, the average particle diameter is 238.3nm, and the polydispersity index is 0.22.
Embodiment 3
[0033] Weigh 10 mg of lurasidone hydrochloride and dissolve it in a mixed solvent of 3 mL of pH4 hydrochloric acid solution and 1 mL of propylene glycol as a good solvent; weigh 10 mg of polyethylene glycol and dissolve it in 300 mL of deionized water as a poor solvent; Drop into the poor solvent at a dropping speed of 5 mL / min, control the stirring speed at 1200 r / min, and continue stirring for 3 h after the dropping to obtain lurasidone hydrochloride nanocrystals. Detected with a laser particle size analyzer, the average particle diameter is 376.2nm, and the polydispersity index is 0.26.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com