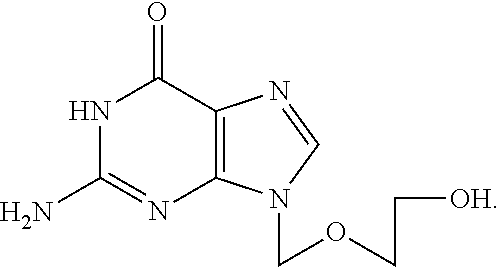
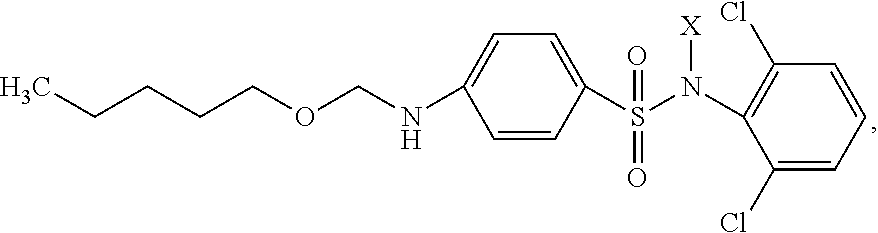
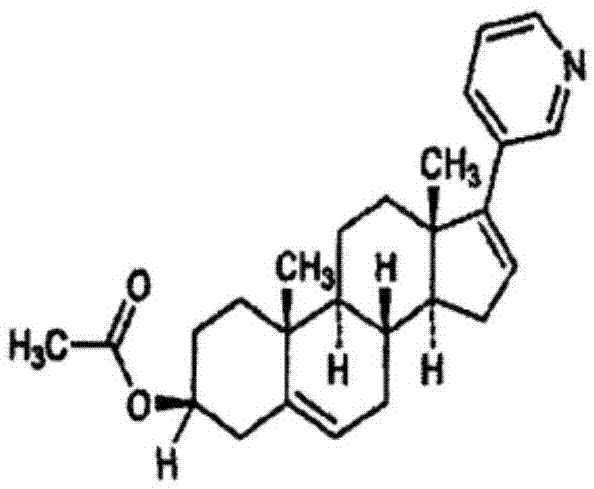
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
122 results about "Sublingual Tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Low dose sublingual tablets of opioid analgesics and preparation process
Owner:ETHYPHARM SA
Low dose tablets of opioid analgesics and preparation process
InactiveUS20070286900A1Improve liquidityImprove homogeneityBiocideNervous disorderAnalgesic agentsOpioid analgesics
Owner:ETHYPHARM SA
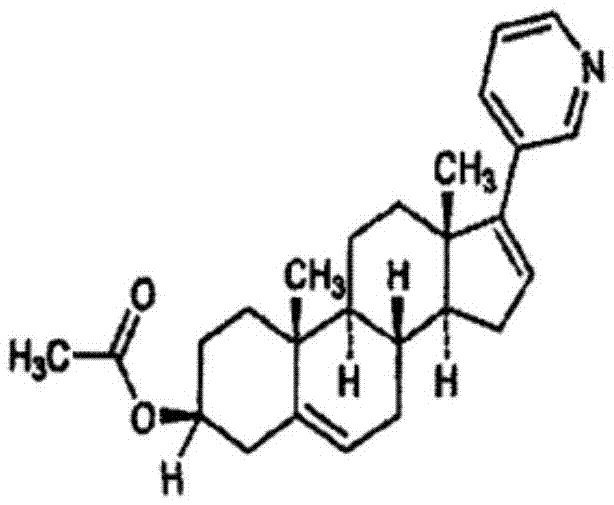
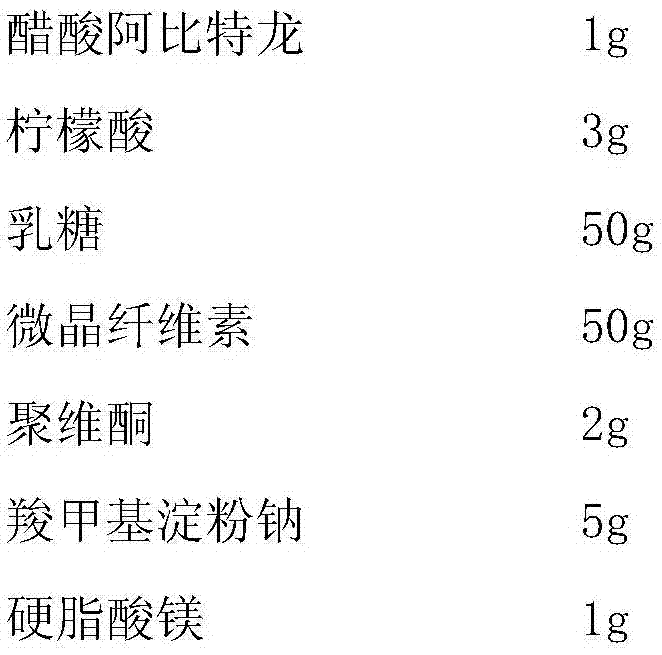
Abiraterone acetate sublingual tablet and preparation method thereof
ActiveCN106913539AGuaranteed curative effectAvoid stimulationOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventPolyethylene glycol
The present invention relates to a tablet, which uses water as a dissolution medium and has characteristics of rapid dissolution and good absorption. The preparation method comprises: dissolving abiraterone acetate and D-alpha-tocopheryl polyethylene glycol 1000 succinate in an organic solvent, carrying out pressure reducing drying to remove the organic solvent, mixing the obtained dried product, a filler and a disintegrant, adding a lubricant, mixing, and tableting to obtain the abiraterone acetate sublingual tablet. Compared to the abiraterone acetate sublingual tablet in the prior art, the abiraterone acetate sublingual tablet of the present invention has the following advantages that the abiraterone acetate sublingual tablet can be subjected to complete dissolution within 15 min so as to ensure the efficacy of the medicine, the complex micro-powder treatment does not required, and the bioavailability of the medicine is substantially improved.
Owner:SHANDONG NEWTIME PHARMA
Vitamin b12 compositions
InactiveUS20130131007A1Efficiently nor absorbsPromote absorptionBiocideDispersion deliveryDiseaseOrally disintegrating tablet
This disclosure provides compositions of vitamin B12, and methods of treatment or amelioration of a disease associated with vitamin B12 deficiency. The composition can take the form of a solid, semi-solid, gummy, or chewable lozenge. The composition can also take the form of a troche, a candy, a wafer, an orally disintegrating tablet, a sublingual tablet, a buccal tablet, a buccal patch, an oral dissolvable film, an aerosol or spray, a lip balm, and chewing gum.
Owner:BEBAAS
Medicinal composition
InactiveCN101926801AImprove stabilityEasy to storeSuppositories deliveryAntiviralsAdditive ingredientPharmaceutical formulation
The invention discloses a solid or liquid medicinal composition for non-gastrointestinal administration. The medicinal composition contains 1-deoxynojirimycin component and / or pharmaceutically acceptable salt thereof, and the 1-deoxynojirimycin component and / or the pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier or excipient are prepared into the medicinal composition for non-gastrointestinal administration together. The medicinal composition for non-gastrointestinal administration can be injection, pills, sublingual tablets, oral patches, buccal tablets, respiratory inhalation aerosol, powder for inhalation, suppository and capsules for rectums and transdermal preparations. The medicinal composition can be used for preventing and / or treating virus infection of mammals comprising human in a non-gastrointestinal administration mode, and the composition has good broad-spectrum antivirus effect and high safety.
Owner:辽宁科泰生物基因制药股份有限公司
Sildenafil citrate sublingual tablet and preparation method thereof
The invention discloses a sildenafil citrate sublingual tablet and a preparation method thereof. The sildenafil citrate sublingual tablet is prepared from 1 part by weight of sildenafil citrate and 4 to 8 parts by weight of pharmaceutical adjuvant , wherein the pharmaceutical adjuvant comprises 2 to 4 percent of disintegrant, 94 to 96 percent of diluter, 0.5 to 1.2 percent of lubricator, 0.3 to 1 percent of corrective and 0.1 to 0.2 percent of binder, which are based on the total weight of the pharmaceutical adjuvant. Test results show that the prepared sublingual tablet has shorter disintegration time, higher dissolving speed in vitro and higher body bioavailability than the conventional tablets.
Owner:张睿
Misoprostol medicine combination used in mouths
InactiveCN102872024AEasy to takeGreat tasteOrganic active ingredientsPill deliveryOrally disintegrating tabletWater drinking
The invention discloses a misoprostol medicine combination used in mouths. The misoprostol medicine combination can be used in mouths and is good in taste, can be dissolved, disintegrated and dispersed in the mouths without water drinking or can be disintegrated and dispersed by aid of mouth chewing and then be absorbed or swallowed in the mouths. The misoprostol medicine combination can be sublingual tablets, chewable tablets, mouth lozenges, mouth disintegrating tablets, mouth film agents and dropping pills, and preferably be the sublingual tablets, the chewable tablets, the mouth disintegrating tablets and the mouth lozenges. The medicine combination can be used for promoting uterine contraction and preventing and treating postpartum hemorrhage. The medicine combination is simple in dosing method, safe, efficient, capable of being accepted by people easily, simple in preparation process and suitable for industrial production.
Owner:天津市聚星康华医药科技有限公司
Sublingual tablet for anaesthesia and preparation method thereof
InactiveCN107137399AAvoid first pass effectDisintegrates quicklyOrganic active ingredientsAnaestheticsAdhesiveHepatic first pass effect
The invention discloses a sublingual tablet for anaesthesia. The preparation method of the sublingual tablet comprises the following steps: taking dexmedetomidinehydrochloride and ketamine hydrochloride as raw materials, adding a certain amount of a filling agent, a disintegrating agent, a corrigent, an adhesive and a lubricant, and performing pretreatment, mixing, granulatinganddrying, total blending, aluminum plastic inner packaging, outer packaging and the likeseparately. The sublingual tablet does not require building a special administrationchannel, is directly dosed through sublingual veins, avoids the liver first pass effect, takes effect quickly and is good in effect; particle angles of the sublingual tablet are all smaller than 35 DEG C duringpreparation, so that the particle mobility of the sublingual tablet is good, finished products is rapid to disintegrate and can be completelydisintegrated within 3 minutes, the weight difference of finished products is small, the increment of impurities is small during storage, the stability is good, the shelf life reaches 24 months, the preparation technology is simple and practical, and the sublingual tablet is worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
Olanzapine medicine absorbed through oral mucosa
InactiveCN102451162AEasy to industrializeIncrease productivityOrganic active ingredientsNervous disorderIrritationAdhesive
The invention relates to an olanzapine medicine absorbed through an oral mucosa. The medicine is composed of olanzapine taken as an effective medicinal component and auxiliary components acceptable in oral mucosa medicines, wherein, the auxiliary components comprise a disintegrant, a filling agent, a flavoring agent, an adhesive, a mucosa absorption enhancer, and a lubricant. Specifically, the medicine includes the following preparation forms employed currently: sublingual tablets, toroches, buccal tablets, buccal adhesive tablets, and buccal patches, etc. Able to be completely or mainly absorbed by a sublingual mucosa and / or a buccal mucosa, the medicine of the invention has the advantages of no irritation on the oral cavity, ability to substantially improve medicinal bioavailability and treatment effect, as well as convenient administration, and can bring patients good compliance.
Owner:重庆市力扬医药开发有限公司
Tegasevod maleate oral preparation and its preparation process-for curing intestinal irritability syndrome
InactiveCN1443535AQuality improvementQuality is easy to controlOrganic active ingredientsDigestive systemEffervescent tabletOral medicine
The present invention relates to an oral medicine preparation for curing intestinal irritability syndrome using constipatino as main sympton-tijaseluo maleate. It is made up by using tijaseluo maleate as active component and adding proper auxiliary material through a certain preparation process, and can be made into various oral dosage forms of tablet, oral disintegrant table, effervescent tablet, capsule, suspension gel and powder preparation, etc.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Butylphthalide sublingual tablet and preparation method thereof
The invention provides a butylphthalide sublingual preparation. The butylphthalid sublingual preparation contains butylphthalide, lauric acid polyethylene glycol glyceride, an excipient, an adsorbent and a lubricating agent. A butylphthalide solid dispersion prepared by taking lauric acid polyethylene glycol glyceride as a carrier can be quickly dissolved in vitro, and a sample is prepared by adopting a hot melting extrusion method, so that drug loading capacity is high and sample stability is good.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Respiratory syncytial virosome vaccine and preparation method thereof
The invention discloses a respiratory syncytial virosome vaccine, which mainly comprises a respiratory syncytial virosome, wherein the virosome comprises a complete respiratory syncytial virus (RSV) surface, and can stimulate good dual cell and body fluid immune response. The virosome protein antigens are prepared into formulations such as injection, nasal spray, sublingual tablet and transdermal agent by adding or not adding an adjuvant, can safely and effectively prevent RSV infection after being immunologically inoculated to different animals or people, and provide the ideal vaccine for the safe and effective immune prevention and control of the RSV infection of different age groups. Researches show that the respiratory syncytial virosome vaccine has a good immunological protection effect on the different age groups.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
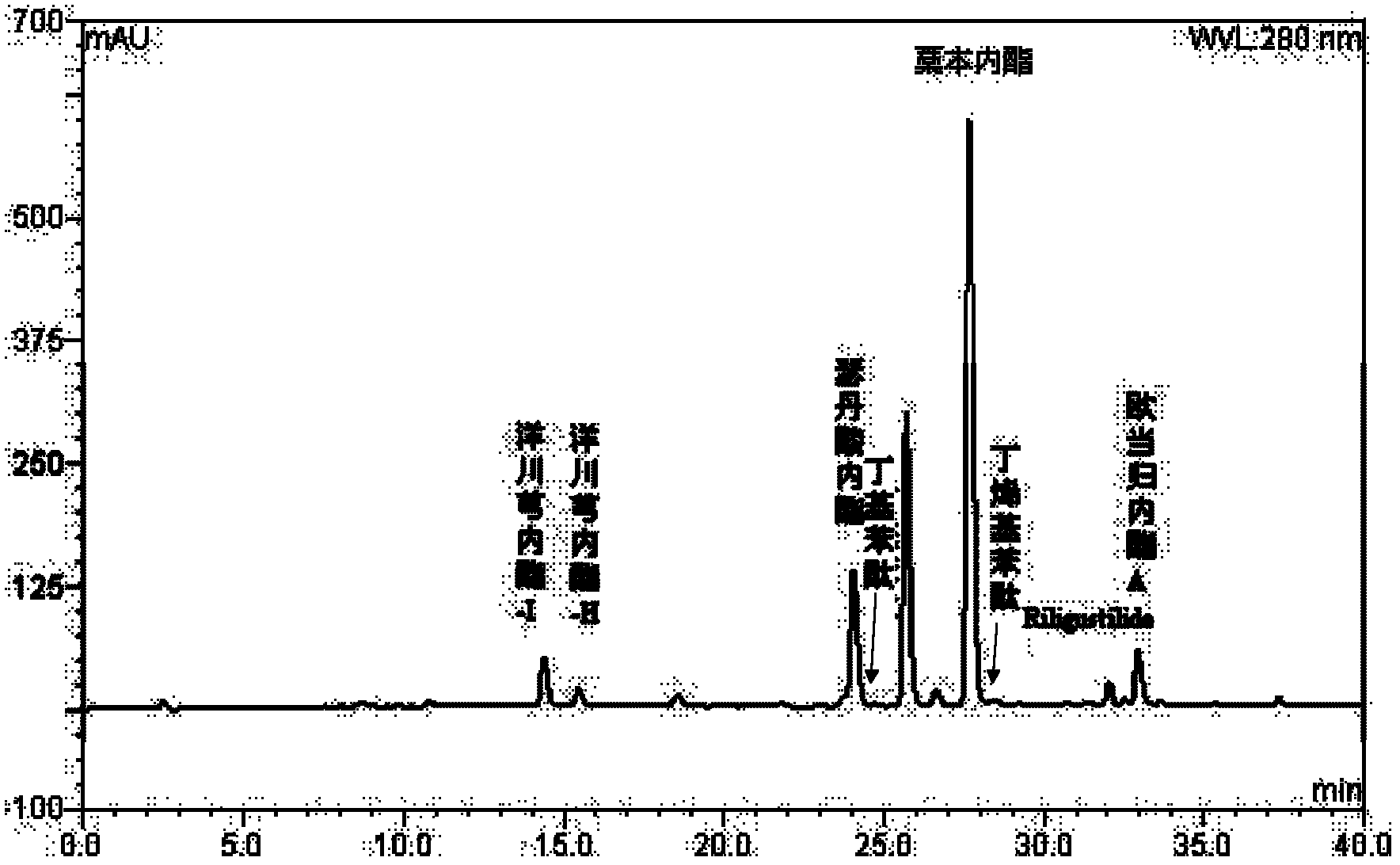
Preparation containing ligustilide type component for treating cardio-cerebrovascular disease and preparation method thereof
InactiveCN102631387APrevent escapeImprove bioavailabilityCardiovascular disorderPlant ingredientsDiseaseWater vapor
The invention discloses a preparation containing a ligustilide type component and a preparation method thereof. The ligustilide type component comprises the following components by weight percent: ligustilide 14%-42%, sedanolide 3%-20%, butylidenephthalide 0.1%-5%, butylphthalide 0.1%-3%, senkyunolide-H 0.2%-3%, senkyunolide-I 0.4%-5%, levistilide A 0.5%-1.5%, and riligustilide 0.4%-1.2%. The preparation method prevents the ligustilide type component from being destroyed by the wet distillation method and the solvent extraction method, and the quality of the ligustilide type component is more ensured without solvent residue. The preparation containing the ligustilide type component is safe and controllable on the effective part. Drop pills and sublingual tablets containing the ligustilide type component can avoid the defects of the oral preparation after administration such as liver first pass effect and gastrointestinal reaction, and an injection containing the ligustilide type component can also avoid the possible situations during using the injections such as acute poisoning reaction and allergic reaction. The preparation containing the ligustilide type component is safer and more effective, and has good economic benefit and social benefit.
Owner:TIANJIN UNIV
Zolmitriptan tongue tablet
InactiveCN101181267AAvoid first pass effectAvoid enzymatic digestionOrganic active ingredientsNervous disorderTreatment choicesSuperior vena caval
The invention provides a zolmitriptan sublingual tablet, which is characterized in that it contains the main drug zolmitriptan, a disintegrating agent and a filler. In every 1000 sublingual tablets, the content of zolmitriptan is 1 -50g, the content of disintegrating agent is 2-20g, the content of filler is 40-200g; it also contains 0-20g of corrective agent, 10-60g of binder and 0.5-5g of lubricant. The present invention utilizes the characteristics of non-keratinized sublingual mucosa, rich capillaries, and fast blood flow, aiming at the absorption of zolmitriptan ordinary tablets through the gastrointestinal tract, slow onset of action, first-pass effect, and low bioavailability. The deficiencies of advanced prior art, zolmitriptan is made into sublingual tablet, can make it absorb through sublingual mucosa, directly enter blood circulation through jugular vein and superior vena cava, take effect rapidly; Avoid oral administration The first-pass effect improves bioavailability and ensures curative effect. Moreover, water is not needed when taking the medicine, and it is placed under the tongue to contain it, so it is convenient to take and has a good taste. With the characteristics of rapid onset of action, convenient administration and high bioavailability, it can provide patients with a new treatment option and fill the gap in the market.
Owner:重庆医科大学医药研究所
Antivirus compound formulation, preparation process, quality control method and use thereof
The invention discloses a compound preparation which comprises two or more of extracts of baikal skullcap root glycosides, barren wort flavones and houttuynia cordata, and can be prepared into injections, capsules, tablets, dispersible tablets, sublingual tablets, granules, oral liquid preparations, drop pills and other pharmacologically allowable dose forms. The preparation can be used for treating pneumonia, respiratory tract infection, influenza, avian Influenza, SARS, asthma, and low body immunity.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
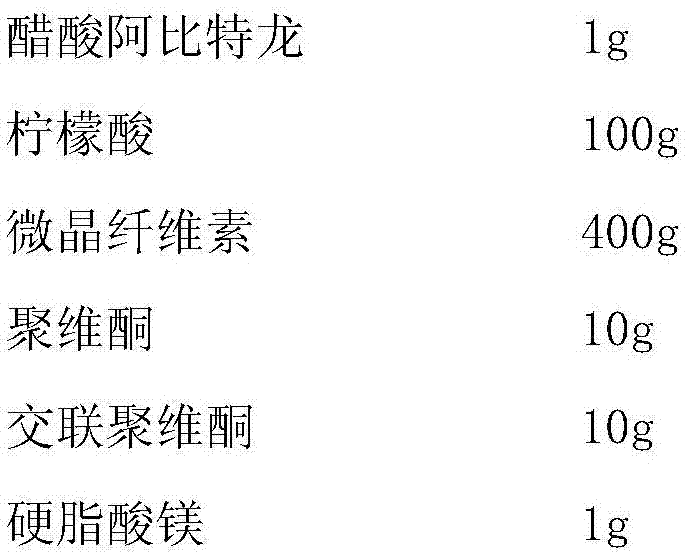
Abiraterone acetate sublingual tablet and preparation method thereof
ActiveCN106913537AGuaranteed curative effectAvoid stimulationOrganic active ingredientsPill deliveryDissolutionBioavailability
The present invention elates to an abiraterone acetate sublingual tablet and a preparation method thereof, and provides a tablet, which uses water as a dissolution medium, is not added with a surfactant, and has characteristics of rapid dissolution and good absorption. The preparation method comprises: carrying out heating melting on abiraterone acetate and a solid acid, granulating the mixed powder of a filler, a disintegrant and a binder, drying, adding a lubricant to the dried granules, and tableting to obtain the abiraterone acetate sublingual tablet. Compared to the abiraterone acetate sublingual tablet in the prior art, the abiraterone acetate sublingual tablet of the present invention has the following advantages that the abiraterone acetate sublingual tablet can be subjected to complete dissolution within 15 min so as to ensure the efficacy of the medicine, the additive is not required to be added, the complex micro-powder treatment does not required, and the bioavailability of the medicine is substantially improved.
Owner:SHANDONG NEWTIME PHARMA
Antivirus Chinese medicinal formulation, preparation process, quality control method and application thereof
The invention discloses a compound Chinese medicinal preparation which comprises baikal skullcap root, barren wort and houttuynia cordata, and can be prepared into injections, capsules, tablets, dispersible tablets, sublingual tablets, granules, oral liquid preparations, drop pills and other pharmacologically allowable dose forms. The preparation has the advantages of definite curative effects, better stability, lower toxic and side effects, The preparation can be used for treating pneumonia, respiratory tract infection, influenza, avian Influenza, SARS, asthma, and low body immunity.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
Sildenafil citrate sublingual tablet and its preparation method
InactiveCN101057850ARich blood vesselsImprove permeabilityPill deliverySexual disorderCITRATE ESTERMedicine
The invention discloses an acidum citricum hypoglossal tablet and the preparing method, relating to a tablet and the preparation method. The invention means to solve problems: current acidum citricum are all oral tablets and capsule, the content is large and increases liver injury, and it is easy to generate adverse effect. The comprised component and their weight proportion are as follows: acidum citricum 20 -30 units, manna sugar 45-65 units, lactin 65-105 units, sweetener 0. 5-1. 5 units, ethanol solution with concentration being 5%PVPK30 30 -40 units and dolomol 4-8 units. The preparation method comprises following steps: sifting sot material with nylon screen of 18-20 order to produce particular, drying at 55-65 Deg. C; sifting granular with screen of 14-16 order and placing for 5-7 hours for sheeting. The tablet is characterized by small content, increased biological utilization rate, low adverse effect rate, and the method is characterized by short and simple process.
Owner:HEILONGJIANG UNIV
Asenapine composition and preparation method thereof
The invention relates to a composition which contains asenapine maleate and is taken by sublingual or buccal administration and a preparation method of the compositionBy using the asenapine maleate as an active ingredient and using an easy-sublimating pore-foaming agent, sublingual tablets which are quickly dispersed and dissolved under the tongue in tens of seconds are prepared, thus the active ingredient can be absorbed quickly through sublingual veins so as to enter blood circulation directly. The composition takes effect quickly and is convenient to take. The invention also discloses a novel in vitro evaluation method.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Abuse potential low compound buprenorphin hydrochloride naloxone hydrochloride sublingual tablet
InactiveCN1943575AReduce Abuse PotentialAvoid abuseOrganic active ingredientsNervous disorderBuprenorphine HydrochlorideAlcohol
A buprenorphine hydrochloride / naloxone hydrochloride sublingual tablets with low misuse potential , said tablets contain medicinal contents of buprenorphine hydrochloride and naloxone hydrochloride, part by weight thereof is 2:1-6:1, 1) using alcohol to dissolve regulator corrigent and bond of PH of prescription amount for use; 2) grinding main medicine buprenorphine hydrochloride / naloxone hydrochloride, filler and lubricant and sifting out thereof; 3) mixing evenly the prescription amount of buprenorphine hydrochloride / naloxone hydrochloride, filler, bond and disintegrating agent, thereafter adding contents in step 1 to prepare into soft stuff, again making into pellet, drying, sorting out, adding lubricant again, mixing evenly and pressing into tablet.
Owner:岳振江
Sublingual tablet of natural hirudin
InactiveCN1840176AShort curative effectPeptide/protein ingredientsMetabolism disorderDiseaseMedicine
Disclosed is a natural hirudin lozenge, which comprises natural hirudin and water-soluble bulking agent, each tablet of contains natural hirudin 5-6000 international units. The lozenge has good effect in treating and preventing cardiovascular and cerebrovascular diseases such as hypertension, hyperlipoidemia, apoplexy and sequelae.
Owner:周维海
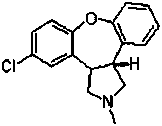
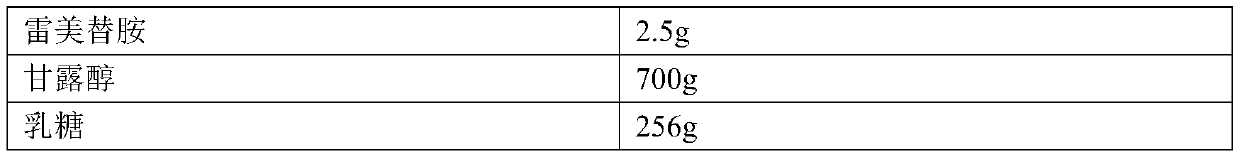
Ramelteon sublingual tablet and preparation method thereof
InactiveCN110433142AGreat tasteGrind evenlyOrganic active ingredientsNervous disorderCross-linkSucrose
The invention belongs to the field of pharmaceutical preparations, and relates to ramelteon sublingual tablets and a preparation method thereof. The sublingual tablet contains effective amounts of ramelteon, a filler, a disintegrant, a lubricant, and a flavoring agent, and the proportion of a main drug is 0.1 to 0.5%; the filler is selected from one or a combination of mannitol, lactose, sucrose and xylitol; the disintegrant is selected from one or the combination of crospovidone, cross linked sodium carboxymethyl cellulose, and low-substituted hydroxypropyl cellulose; and the lubricant is selected from one or a combination of magnesium stearate, aerosil, and sodium stearyl fumarate; and the flavoring agent is mint flavor. The invention also provides the preparation method of the ramelteonsublingual tablets, that is, ramelteon and the filler are ground and mixed by a ball mill, and mixed with the disintegrating agent, the lubricant, and the flavoring agent, and the materials are pressed to prepare tablets. The ramelteon sublingual tablet of the invention can avoid the first pass effect of the liver and improve the bioavailability.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Rizatriptan drug absorbed through mouth mucosa
InactiveCN103156817AEasy to takeAvoid first pass effectOrganic active ingredientsNervous disorderIrritationRizatriptan
Disclosed is a rizatriptan drug absorbed through mouth mucosa. Malic acid rizatriptan is an effective drug ingredient, and the rizatriptan drug absorbed through mouth mucosa is jointly composed of malic acid rizatriptan and auxiliary ingredients which are acceptable in a mouth mucosa drug, wherein the auxiliary ingredients comprise a disintegrating agent, a filling agent, a flavoring agent, a bonding agent, a mucosa sorbefacient and a lubricating agent. Preparation forms of specific drugs comprise sublingual tablets, lozenges, mouth and check lozenges, mouth adhering tablets and mouth pasting tablets and the like. The drug can be completely or mainly absorbed through mouth mucosa such as hypogloeeis mucosa and / or mouth and cheek mucosa and is non-irritant to a mouth. Bioavailability and the curative effect of the drug can be obviously improved, and the rizatriptan drug absorbed through mouth mucosa can be taken conveniently, so that a patient is enabled to take the drug obediently.
Owner:重庆市力扬医药开发有限公司
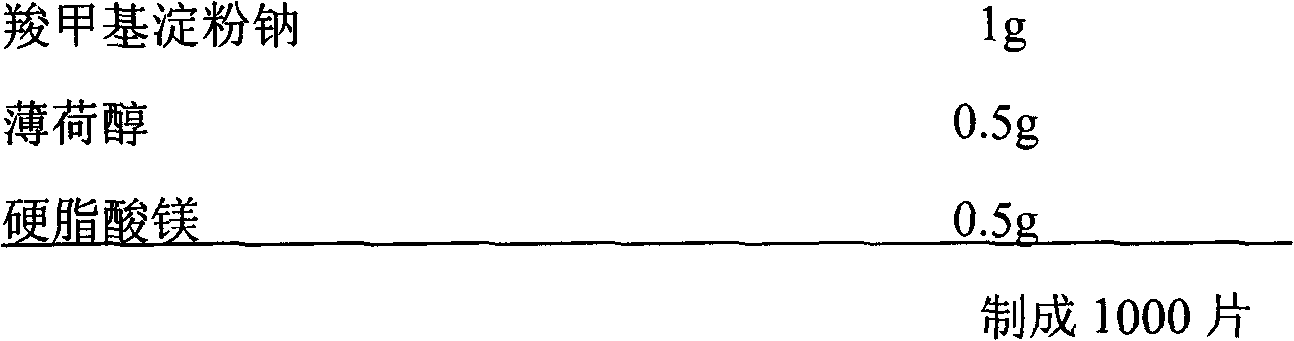
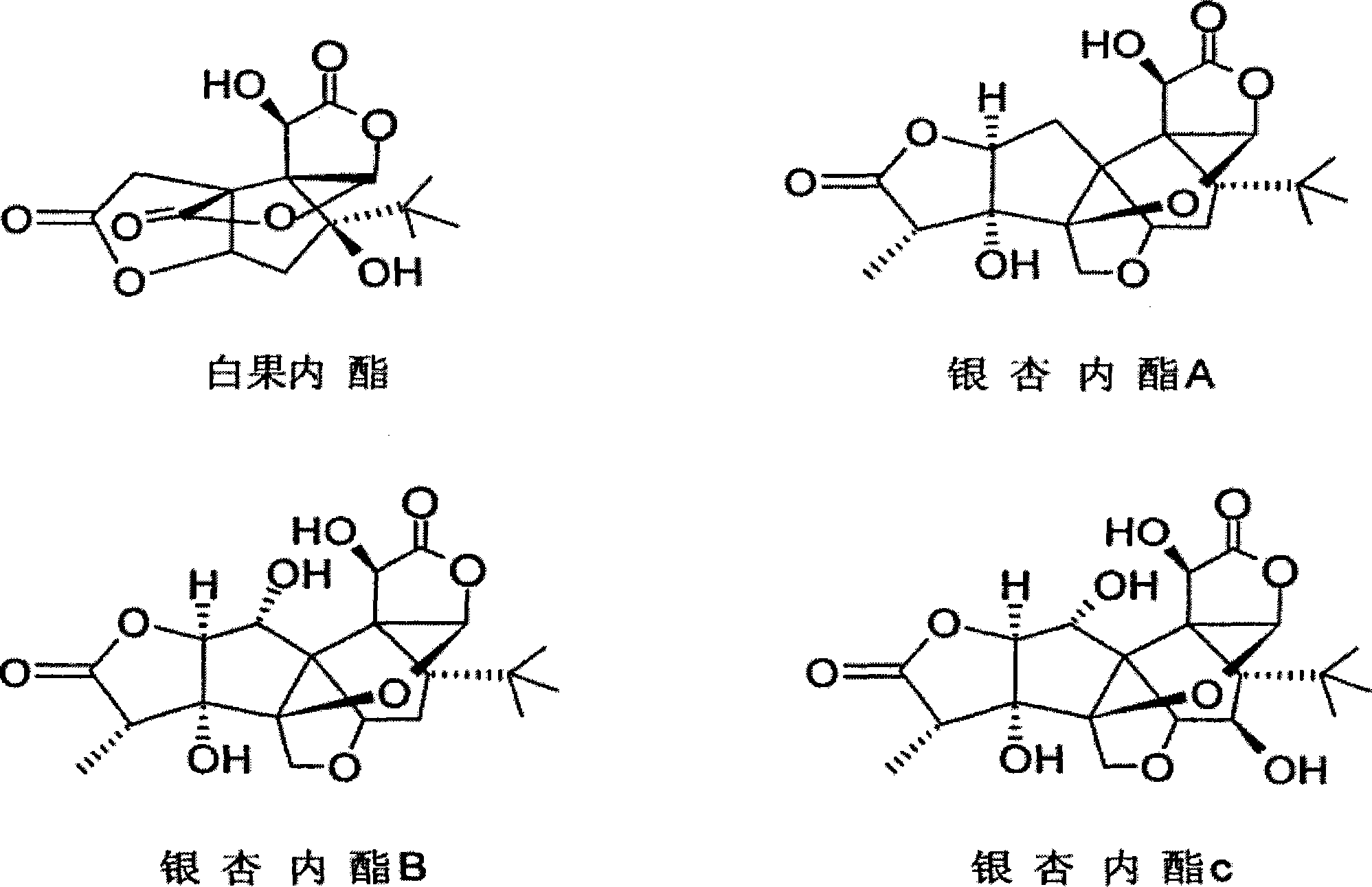
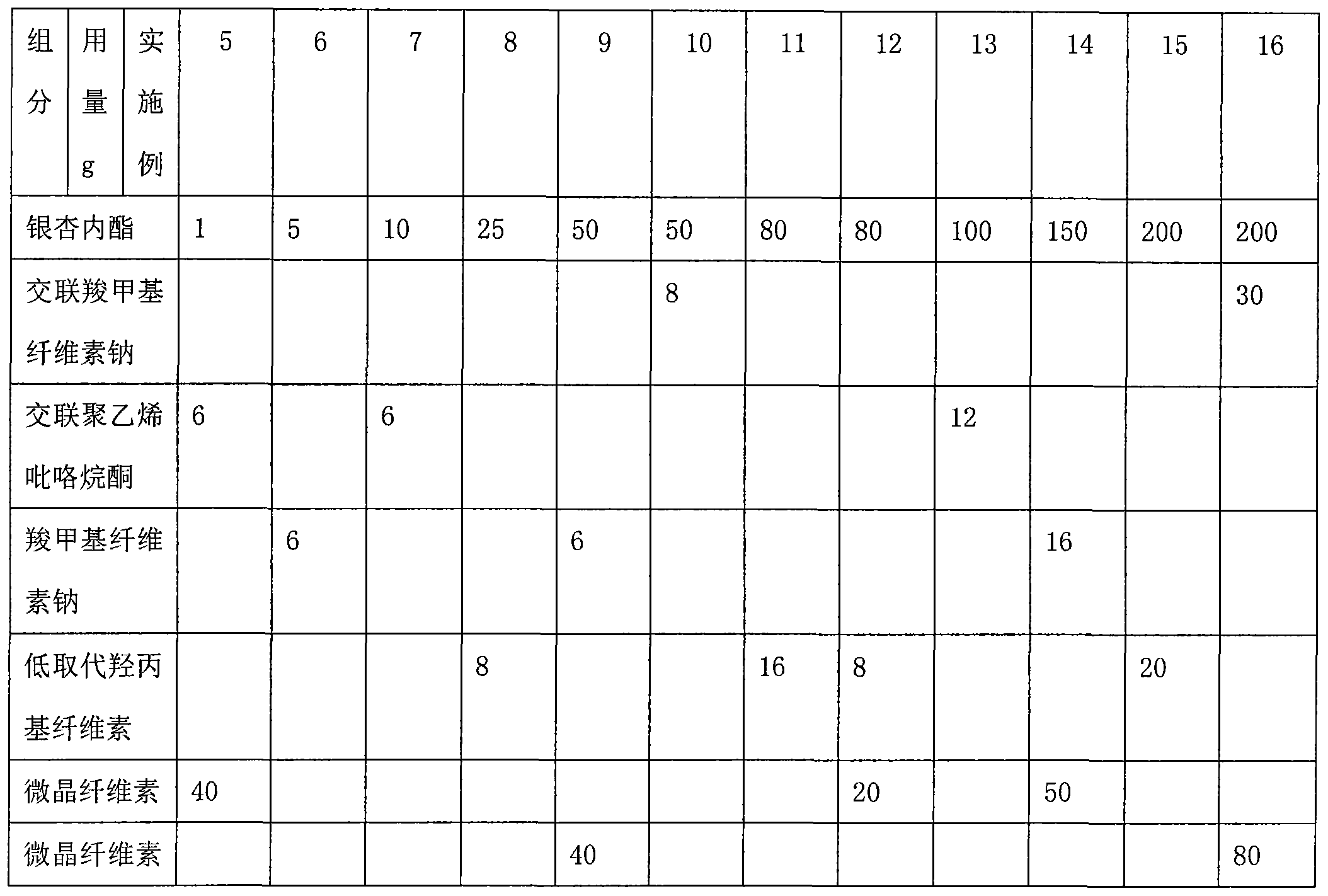
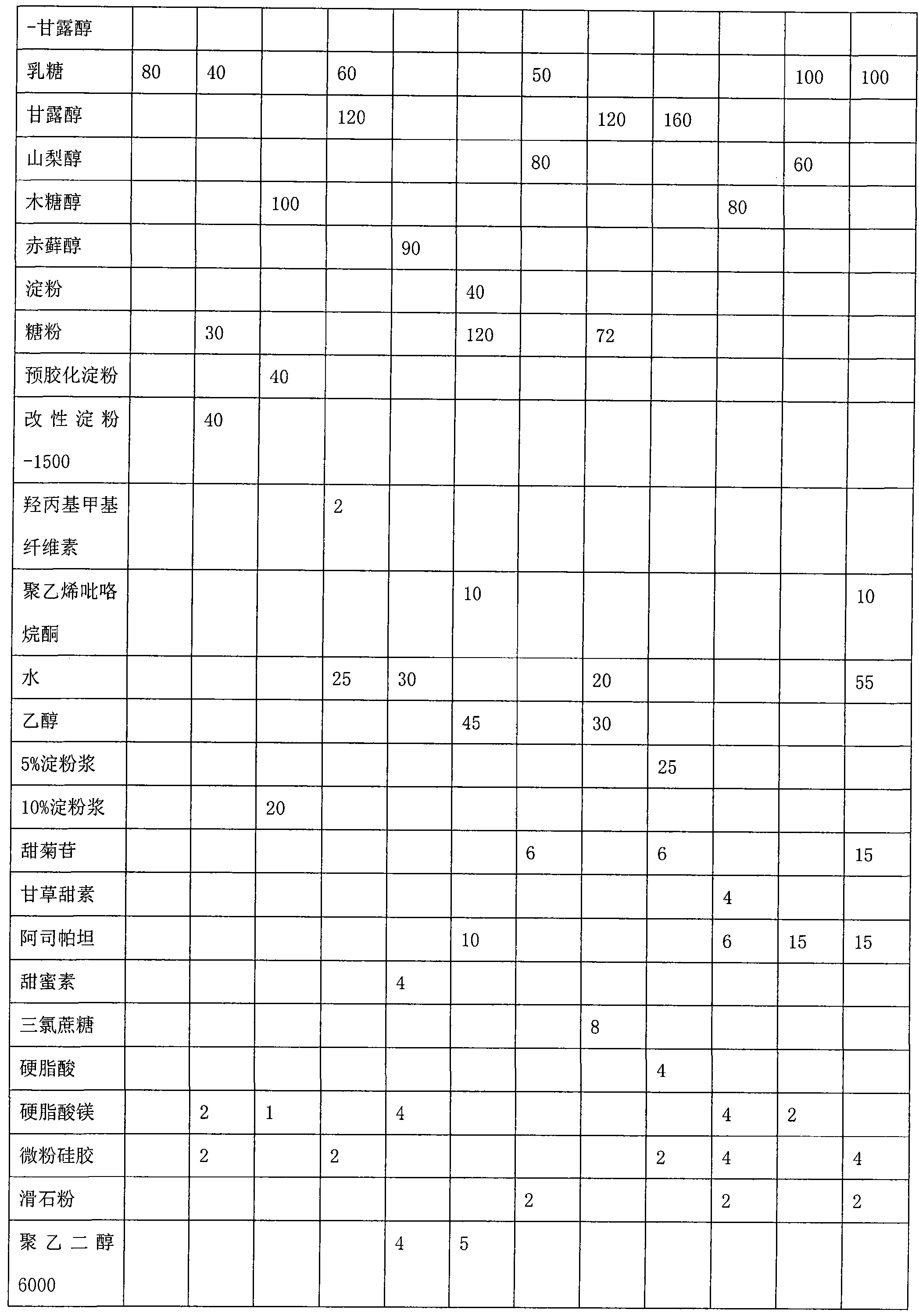
Ginkgolide sublingual tablet and preparation method thereof
InactiveCN103976967AImprove complianceRapid relief of migraine symptomsOrganic active ingredientsNervous disorderSuperior vena cavaBilobalides
The invention discloses a ginkgolide sublingual tablet, belonging to the technical field of a pharmaceutical composition, and a preparation method thereof. The sublingual tablet comprises the following materials in parts by weight: 1-200 parts of ginkgolide, 2-20 parts of a disintegrating agent and 40-200 parts of a filler, wherein the ginkgolide includes the following components in percentage by weight: 35-50% of ginkgolide A, 6-16% of ginkgolide B, 15-25% of ginkgolide C and 30-45% of bilobalide. The ginkgolide sublingual tablet disclosed by the invention is prepared by organically combining the disintegrating agent, the filler and the like; the sublingual tablet, which is located under the tongue when being taken, can disintegrate rapidly under the tongue; the sublingual tablet is absorbed through sublingual mucosa and directly participates in blood circulation through jugular vein and superior vena cava to become effective rapidly, so as to relieve migraine rapidly.
Owner:HUNAN UNIV OF SCI & ENG
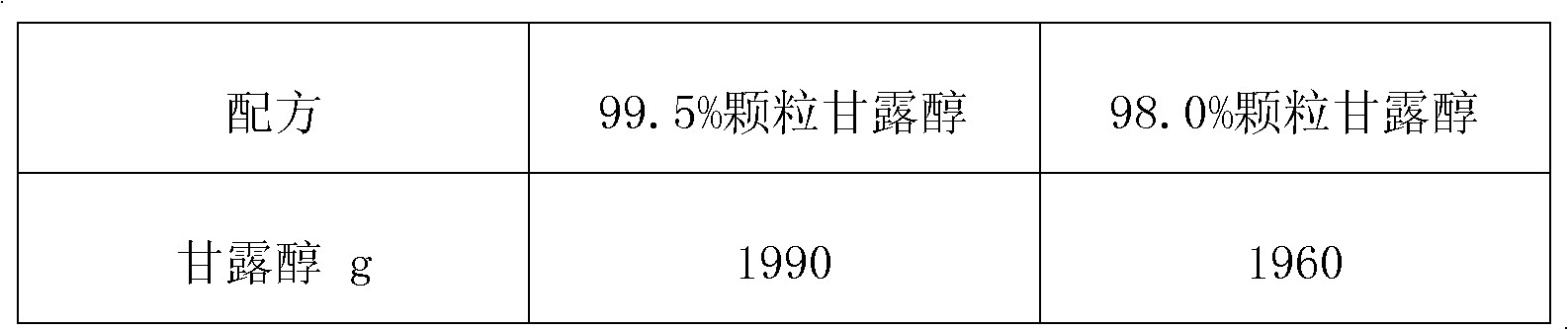
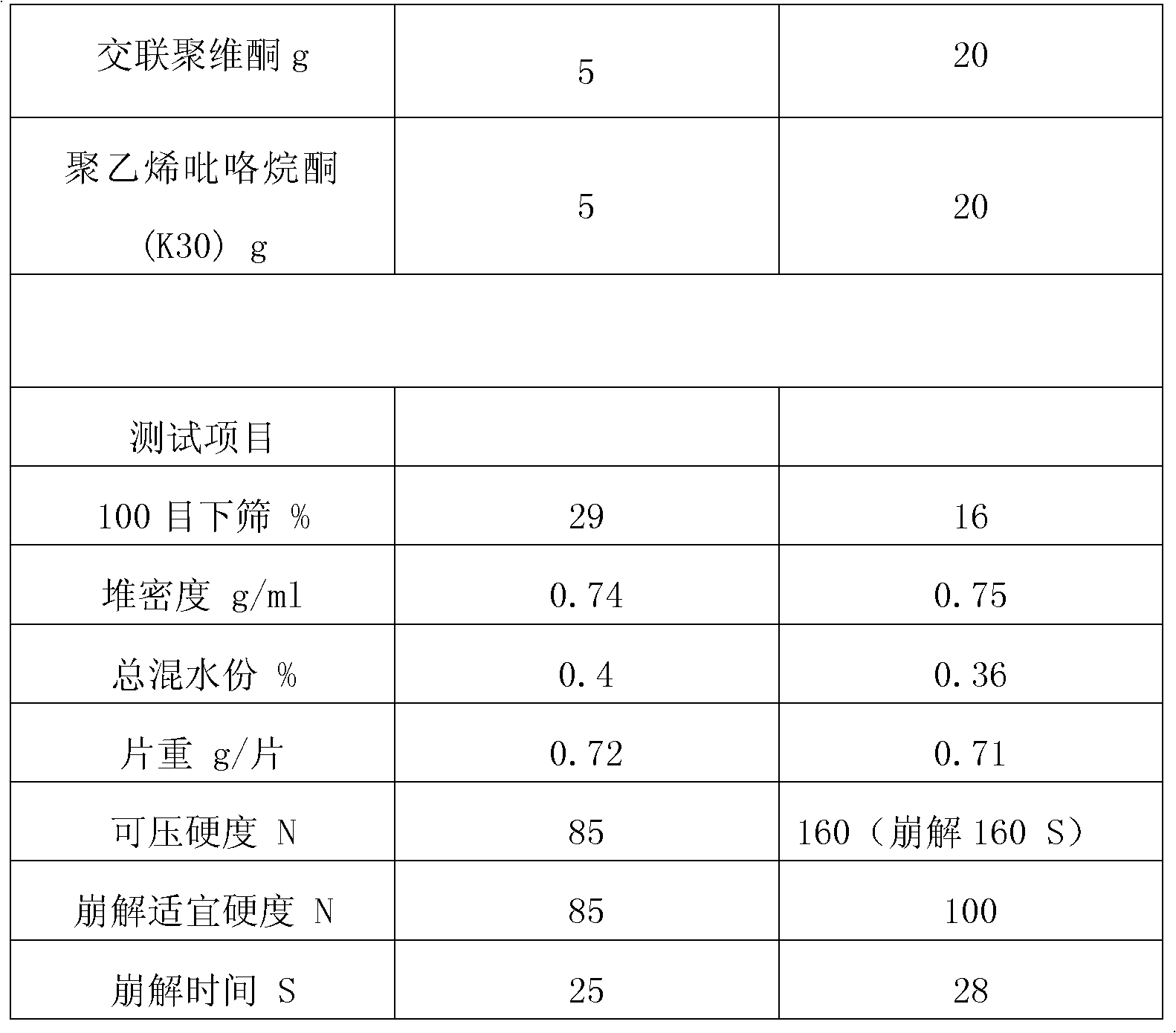
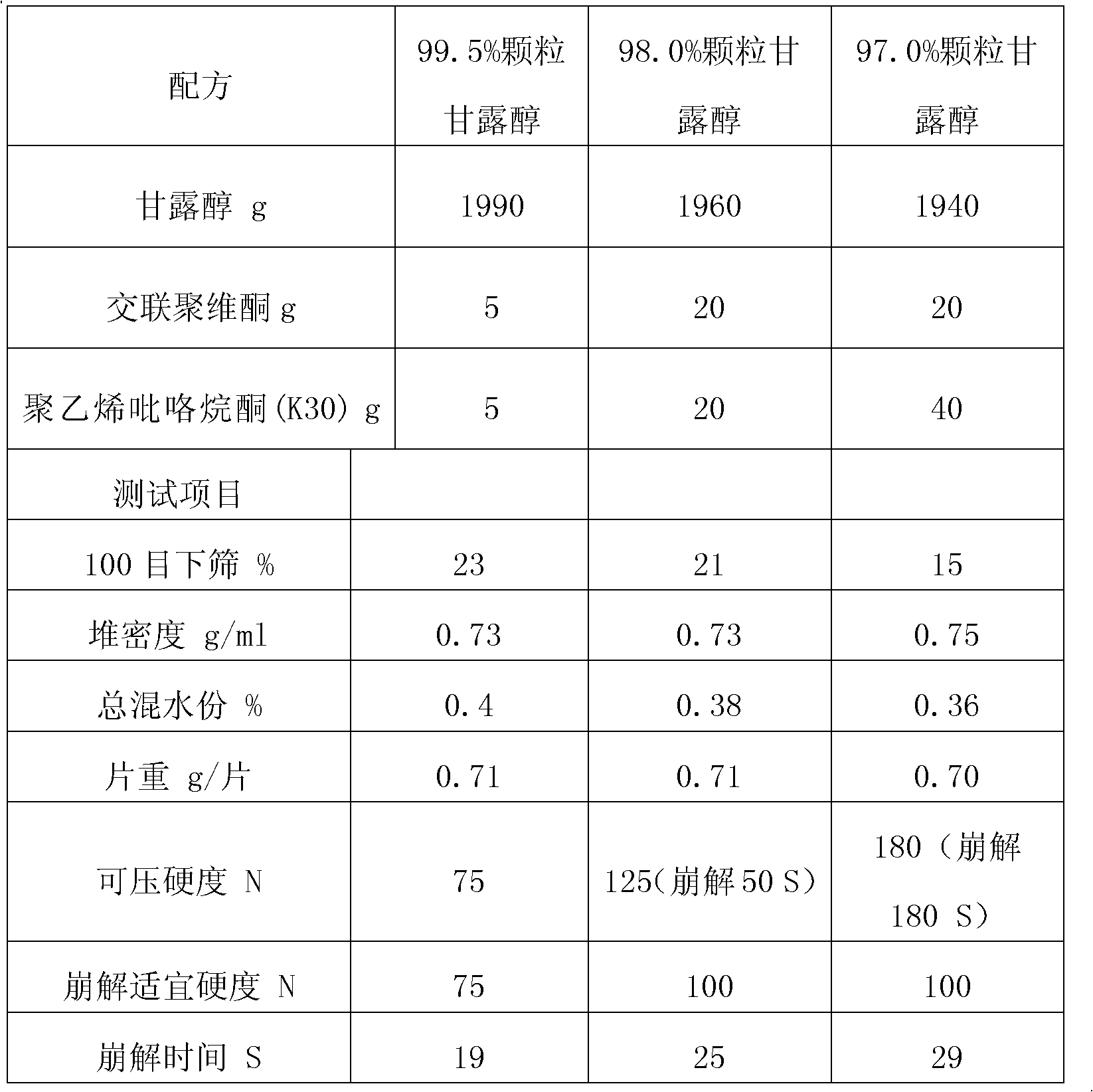
Preparation method for rapid-disintegrating directly-pressed particle mannitol preparation
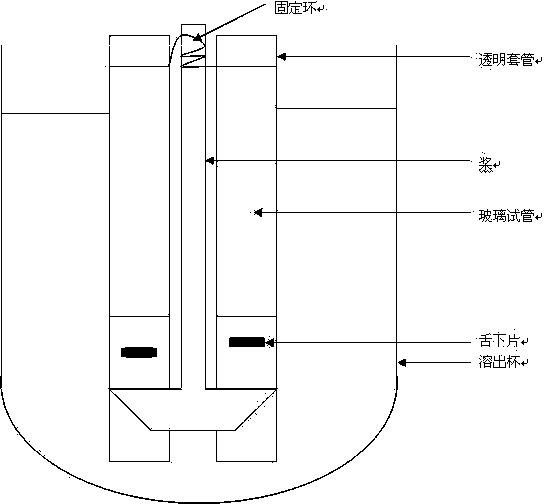
The invention relates to a preparation method for a rapid-disintegrating directly-pressed granular mannitol preparation. The preparation method comprises the following steps of: 1) weighing the following raw materials in percent by weight according to a particle mannitol formula: 0.2-5.0 percent of adhesive and 8-2 percent of purified water, pouring the adhesive into the purified water and preparing adhesive slurry for future use; 2) putting crushed or uncrushed mannitol and 0.2-5.0 percent of disintegrating agent in a high-speed agitating and granulating machine, starting up the high-speed agitating and granulating machine, adding the slurry and preparing high-quality soft materials; 3) transferring the product wet granules into a fluidized drying machine for drying, screening the dried materials and completely tidying the granules; and 4) mixing the screened and tidied materials with lubricating agent, pressing the mixture into tablets and conducting hardness and disintegration tests. By adopting the preparation method, widely applicable directly-pressed granules with different content and fineness can be produced, have a rapid disintegrating characteristic and satisfy the American standard that the disintegrating time of sublingual tablets is less than or equal to 30 seconds. Moreover, the preparation method has the advantages of easiness in operation, low production cost, simple technology and high product quality.
Owner:DSM JIANGSHAN PHARMACEUTICAL (JIANGSU) CO LTD
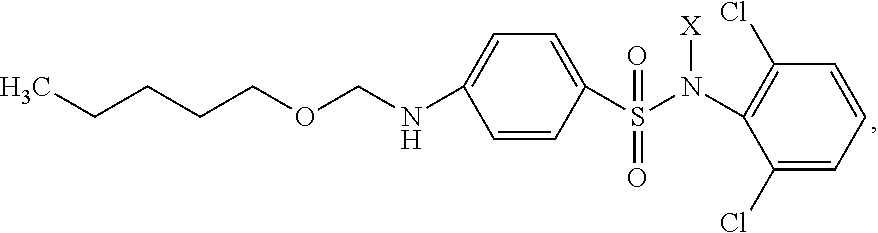
Drug with Activity against the Herpes Virus Family
The invention relates to medicine, and specifically to synthetic biologically active derivatives of carbopentoxysulfanilic acid. The novel substance comprises a (2,6-dichlorophenyl)amide salt of carbopentoxysulfanilic acid of general formula:where X is Na, K, NH4; the drug may be contained in tablets, including sublingual tablets, or in capsules, or in suppositories, or in drops, or in mixtures, or in ointments, creams or other forms for application to the skin and mucosae, or in an oral-buccal film, or in a spray, or in a liquid for parenteral administration, or in chewing gum. A preparation having pronounced activity against herpes viruses is thus produced.
Owner:TETS VIKTOR VENIAMINOVICH MR +1
Abiraterone acetate sublingual tablet and preparation method thereof
ActiveCN106913538AGuaranteed curative effectHigh dissolution rateOrganic active ingredientsPill deliveryOrganic solventFreeze-drying
The present invention relates to an abiraterone acetate sublingual tablet, wherein the preparation method comprises: dissolving abiraterone acetate, hydroxypropylcellulose and povidone in an organic solvent, adding a filler, uniformly dispersing, filling, carrying out freeze-drying, and packaging to form the abiraterone acetate sublingual tablet. Compared to the abiraterone acetate sublingual tablet in the prior art, the abiraterone acetate sublingual tablet of the present invention has the following advantages that the abiraterone acetate sublingual tablet can be subjected to complete dissolution within 10 min so as to ensure the efficacy of the medicine, the complex micro-powder treatment does not required, and the bioavailability of the medicine is substantially improved.
Owner:SHANDONG NEWTIME PHARMA
Desloratadine sublingual tablet and preparation method thereof
InactiveCN105963264ARich in capillariesFast blood flowOrganic active ingredientsPill deliveryAlcoholFlavouring agent
A kind of desloratadine sublingual tablet and its preparation method. It is a plain tablet or a film-coated tablet composed of a layer of film coating on the surface of the plain tablet. The plain tablet is dispersed by desloratadine. The body is used as the main material, mixed with fillers or flavoring-containing fillers, disintegrants, stabilizers and other pharmaceutically acceptable carriers as auxiliary materials and made into tablets; the desloratadine dispersion is : Desloratadine is prepared by dissolving it with ethanol and then adding it to a mixture of inclusion agent or inclusion agent, filler or inclusion agent, filler and disintegrant to make a soft material and then drying; the preparation method includes: a ) Preparation of desloratadine dispersion: Desloratadine is dissolved in ethanol and added to the mixture of inclusion agent, filler or inclusion agent, filler and disintegrant to make a soft material, and then Prepared by drying at a temperature of at least 50°C; b) Mix the obtained desloratadine dispersion with fillers or flavoring-containing fillers, disintegrants, stabilizers and other pharmaceutically acceptable carriers as excipients and press Made of slices.
Owner:ZHEJIANG KAIRUN PHARMA
Freeze-dried sublingual tablet containing hydrophobic active substances and preparation method thereof
InactiveCN105663065AQuick carryQuick refillOrganic active ingredientsPowder deliveryFreeze-dryingMolecular materials
The invention provides a freeze-dried sublingual tablet containing hydrophobic active substances, which is prepared from active substances and auxiliary materials and is characterized in that the auxiliary materials include medicine carriers and assistants, wherein the medicine carriers are natural polysaccharide high molecular materials which exist in water in nano sizes and have hydrophobic regions, and the active substances are hydrophobic active substances. The freeze-dried sublingual tablet containing hydrophobic active substances, provided by the invention, can effectively and quickly carry and supplement hydrophobic medicines, and the medicines can be carried uniformly, thereby being favorable for playing roles of the medicines; and meanwhile, the hydrophobic medicines are prepared into the freeze-dried sublingual tablets, and then the medicines can quickly pass through the sublingual region to enter the blood for circulation, thereby playing roles.
Owner:曾岚
Methods and compositions for the treatment of infertility using dilute hormone solutions
InactiveUS20050065136A1Alleviate and ameliorate and overcome infertilityUseful in treatmentOrganic active ingredientsBiocideTreatment infertilityProgesterones
A method and composition for the treatment of infertility is disclosed. The method relates to using progesterone dilutions, or any other steroid hormone, to treat infertility. The hormone dilution may be administered sublingually, by drops or sublingual tablet or, an intradermal route of administration may be chosen.
Owner:ROBY RUSSELL R
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com