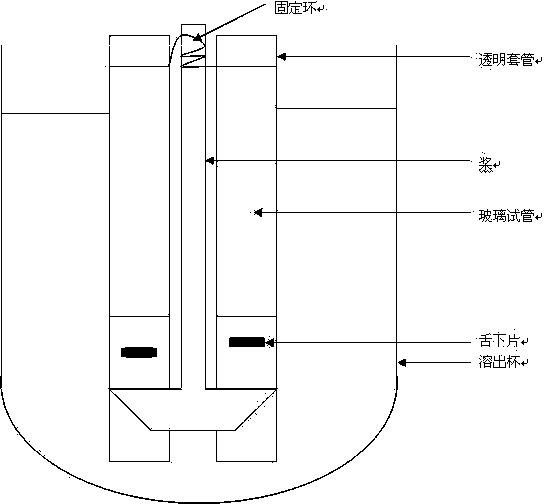
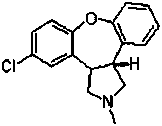
Asenapine composition and preparation method thereof
A composition and substance technology, applied in the field of asenapine composition, can solve the problems of large-scale freeze-drying equipment, long production cycle, fragility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prescription composition:
[0036] components content Asenapine Maleate 5 copies Anhydrous Directly Compressed Lactose 20 copies Spray Dried Mannitol 52.5 copies Camphor 17.5 servings PEG6000 5 copies
[0037] Preparation:
[0038] i) Asenapine maleate is passed through a 100-mesh sieve, and camphor is pulverized through a 60-mesh sieve for subsequent use;
[0039] ii) First mix the prescribed amount of camphor with PEG6000 evenly, and then mix with the remaining ingredients in the prescription;
[0040] iii) The mixed powder is directly compressed into tablets, and the hardness is maintained at 4-5kg / cm 2 about;
[0041] iv) Place the tablet at 80°C for heat treatment;
[0042] v) After 5 hours, take it out and weigh it to confirm that the camphor is completely removed.
Embodiment 2
[0044] Prescription composition:
[0045] components content Asenapine Maleate 5 copies Anhydrous Directly Compressed Lactose 20 copies Spray Dried Mannitol 52.5 copies Camphor 17.5 servings PEG6000 5 copies
[0046] Preparation:
[0047] i) Asenapine maleate is passed through a 100-mesh sieve, and camphor is pulverized through a 60-mesh sieve for subsequent use;
[0048] ii) mixing the prescribed amount of asenapine maleate, anhydrous direct compression lactose, spray-dried mannitol and camphor, and dry granulating;
[0049] iii) Add PEG6000 to the dry granules, mix well, and press into tablets;
[0050] iv) placing the tablet under the condition of 80° C., and heat treatment;
[0051] v) After 5 hours, take it out and weigh it to confirm that the camphor is completely removed.
Embodiment 3
[0053] Prescription composition:
[0054] components content Asenapine Maleate 10 copies Anhydrous Directly Compressed Lactose 17.5 servings Spray Dried Mannitol 52.5 copies Camphor 20 copies PEG6000 5 copies
[0055] Preparation:
[0056] i) Asenapine maleate is passed through a 100-mesh sieve, and camphor is pulverized through a 60-mesh sieve for subsequent use;
[0057] ii) First mix the prescribed amount of camphor with PEG6000 evenly, and then mix with the remaining ingredients in the prescription;
[0058] iii) The mixed powder is directly compressed into tablets, and the hardness is maintained at 4-5kg / cm 2 about;
[0059] iv) Place the tablet at 80°C for heat treatment;
[0060] v) After 5 hours, take it out and weigh it to confirm that the camphor is completely removed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com