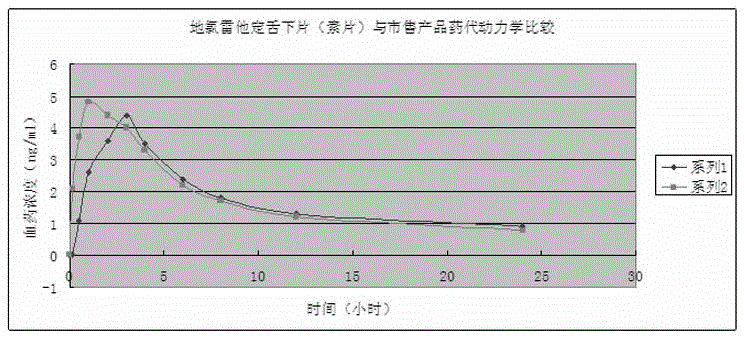
Desloratadine sublingual tablet and preparation method thereof
A technology of desloratadine and loratadine, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of gastric emptying time limitation of onset time and other problems , to achieve the effects of improving bioavailability, fast blood flow, and avoiding the first-pass effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] A method for preparing desloratadine sublingual tablets as described above, the preparation method includes:
[0053] a) Preparation of desloratadine dispersion: After desloratadine is dissolved in ethanol, it is added to the inclusion agent or inclusion agent, filler or inclusion agent, filler, disintegrant mixture to make a soft material, and Prepared by drying at a temperature of at least 50°C;
[0054] b) Mixing the obtained desloratadine dispersion with fillers or flavoring-containing fillers, disintegrants, stabilizers and other pharmaceutically acceptable carriers as auxiliary materials to prepare tablets.
[0055] In the step a) of the present invention, the preparation of desloratadine dispersion is: desloratadine is dissolved in 5-10ml ethanol and then added to sulfobutyl-β-cyclodextrin as an inclusion agent, micro The crystalline cellulose is used as a filler and croscarmellose sodium is used as a disintegrant to make a soft material in a homogeneous mixture, which...
Embodiment 1
[0067] Composition of plain tablets
[0068]
[0069] Preparation method:
[0070] (1) Preparation of desloratadine dispersion: PEG2000, microcrystalline cellulose, and croscarmellose sodium are mixed uniformly. Deloratadine is dissolved in 5-10ml of ethanol and then added to the above mixture to make a soft material, which is dried at 60°C to obtain a desloratadine dispersion.
[0071] (2) Mix the desloratadine dispersion with the above-mentioned auxiliary materials uniformly. Tablet.
Embodiment 2
[0073] Composition of plain tablets
[0074]
[0075]
[0076] Preparation method:
[0077] (1) Preparation of desloratadine dispersion: PEG4000, microcrystalline cellulose, and croscarmellose sodium are mixed uniformly. Deloratadine is dissolved in 5-10ml of ethanol and then added to the above mixture to make a soft material, which is dried at 60°C to obtain a desloratadine dispersion.
[0078] (2) Mix the desloratadine dispersion with the above-mentioned auxiliary materials uniformly. Tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com