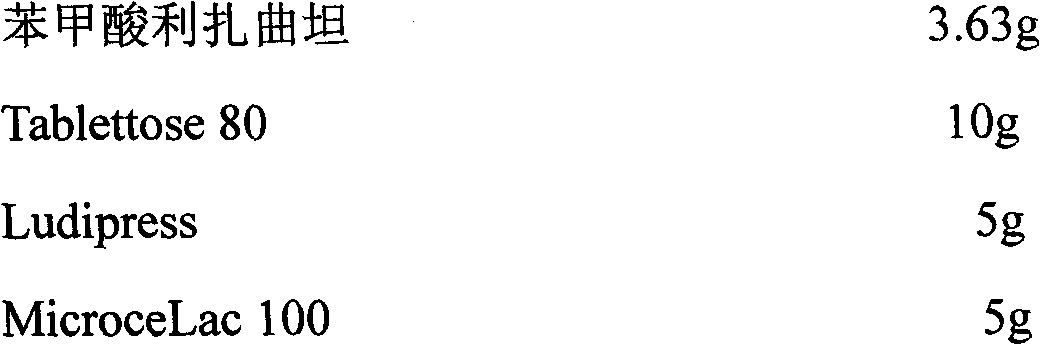Rizatriptan drug absorbed through mouth mucosa
A technology of oral mucosa and rizatriptan, which is applied in the field of rizatriptan medicine, can solve the problem of affecting the efficacy of the drug and patient compliance, which is only 13-28%, and achieves the avoidance of the first-pass effect, rapid onset of action, and ease of use. convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
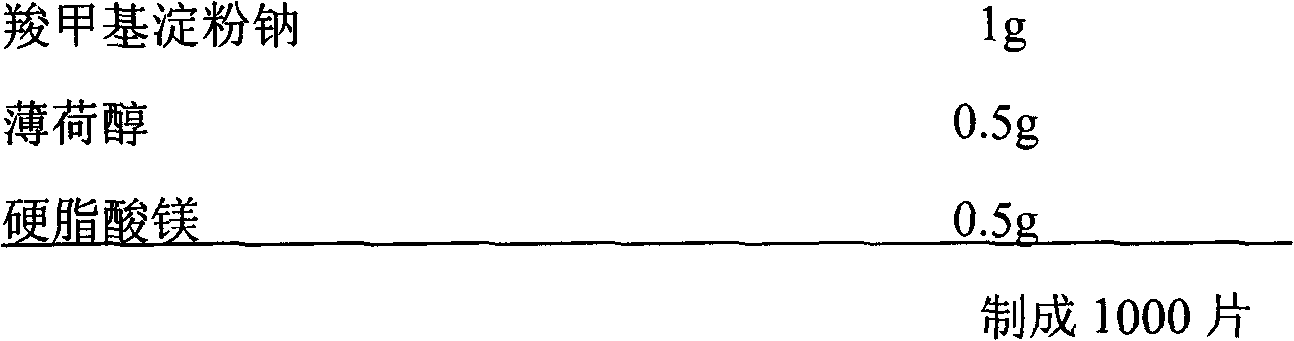
[0022] Embodiment 1 sublingual tablet
[0023] composition:
[0024]
[0025]
[0026] Preparation method: crush the raw material of rizatriptan benzoate, Tablettose 80, Ludipress and other auxiliary components through a 100-mesh sieve, mix evenly, and directly compress the tablet.
Embodiment 2
[0027] Embodiment 2 sublingual tablet
[0028] composition:
[0029]
[0030] Preparation method: Mix the prescription amount of rizatriptan benzoate with auxiliary ingredients such as pregelatinized starch and microcrystalline cellulose, add 0.4% xanthan gum aqueous solution, granulate, dry, granulate, add external cross-linked polymer Vitamin ketone and talcum powder are mixed evenly and compressed into tablets.
Embodiment 3
[0031] Embodiment 3 sublingual tablet
[0032] composition:
[0033]
[0034] Preparation method: The raw material of rizatriptan benzoate and auxiliary ingredients such as MicroceLac 100 and microcrystalline cellulose are pulverized through a 100-mesh sieve, mixed evenly, and directly compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com