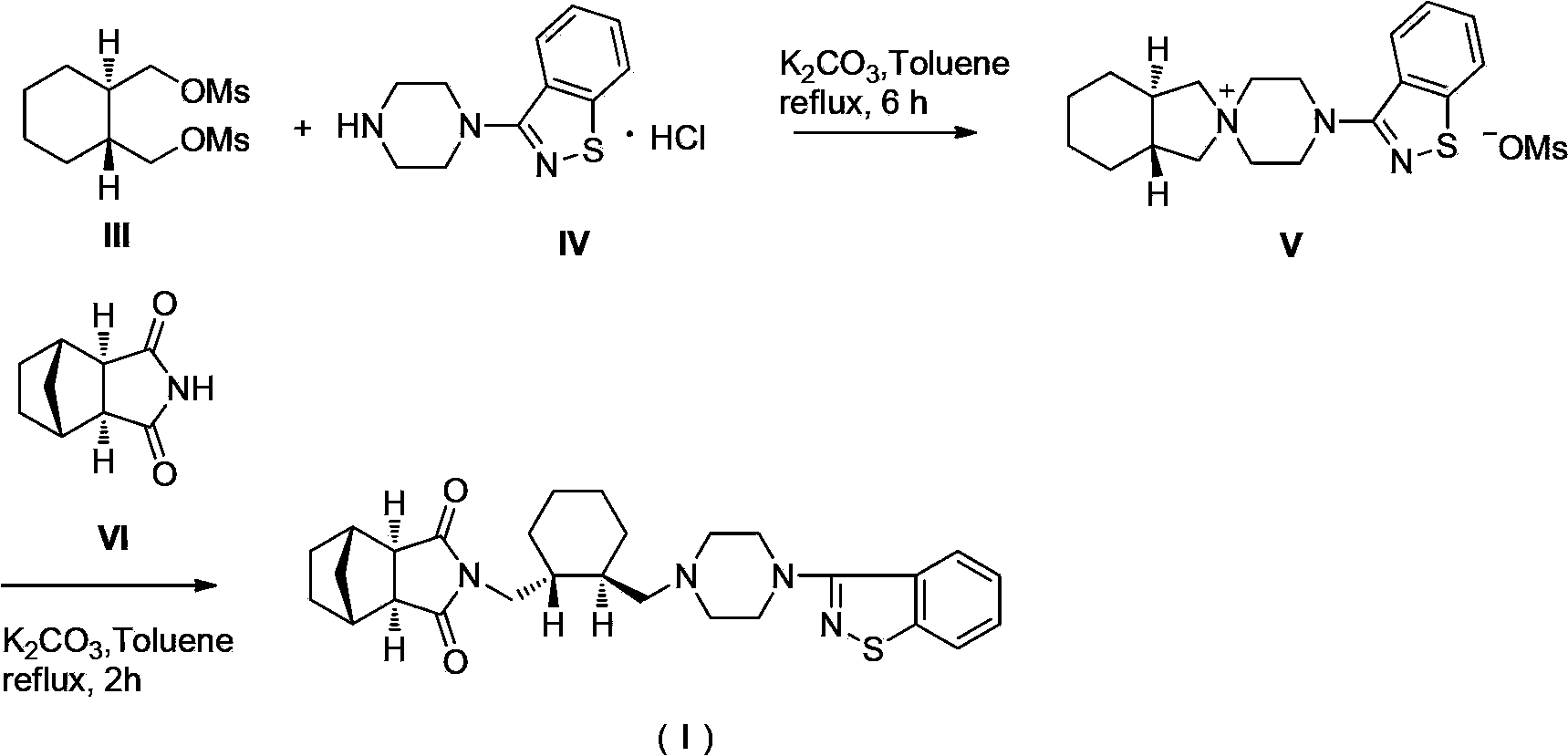
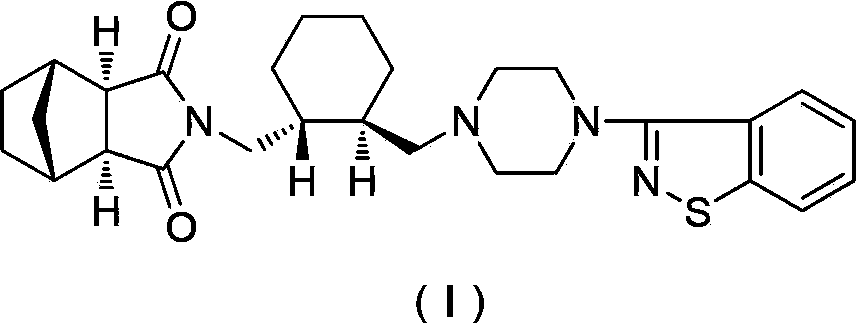
Preparation method of antipsychotic drug lurasidone
A diketone and compound technology, applied in the field of preparation of the antipsychotic drug lurasidone, can solve the problems of by-products, high solvent toxicity, and difficulty in industrial production, and achieve the effects of mild reaction conditions, simple operation, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following examples are intended to illustrate the present invention in detail, but not to limit the present invention.
[0018] 1. (1R,2R)-1,2-cyclohexanedimethanol dimesylate formula (III)
[0019]
[0020] Add 500.0g (1R,2R)-1,2-cyclohexanedimethanol to a 10L three-necked flask, add 1052.5g triethylamine and 835.0g methanesulfonyl chloride to 7500ml dichloromethane in sequence, and react for 8 hours after adding. Add 3750ml of purified water to the reaction system, stir and wash, separate the liquid and discard the water layer, wash the organic phase with dilute hydrochloric acid, then wash with saturated sodium bicarbonate in turn, wash with saturated brine, separate the liquid, and use anhydrous sodium sulfate for the organic phase dry. Anhydrous sodium sulfate was filtered off, and concentrated to dryness under reduced pressure. Diethyl ether was added thereto, filtered with suction, and the filter cake was air-dried at 60°C to obtain 889.4 g of off-white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com