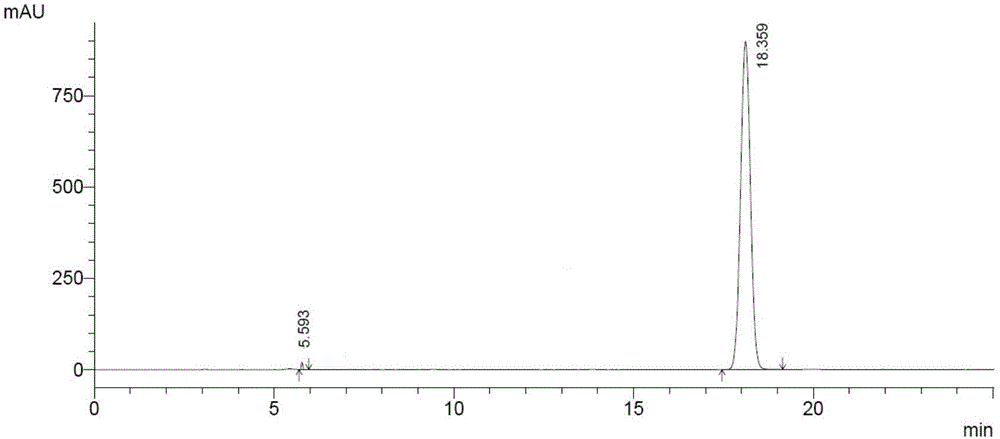
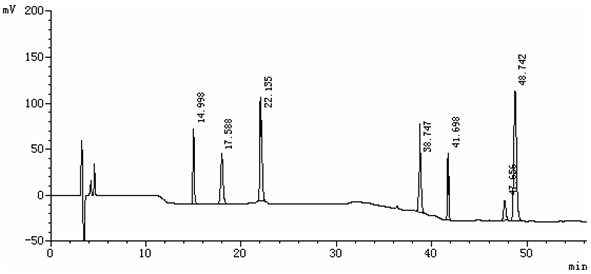
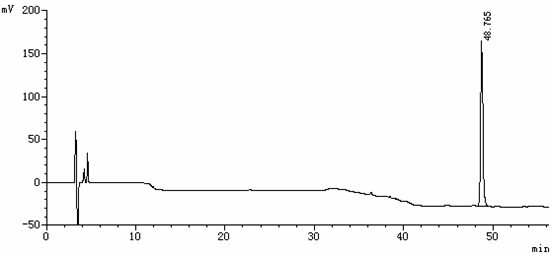
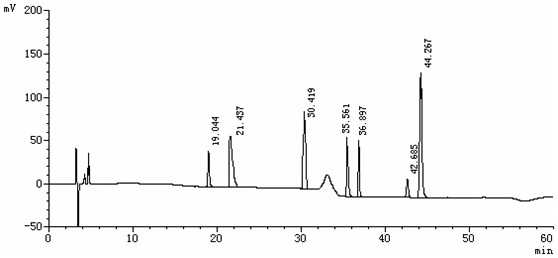
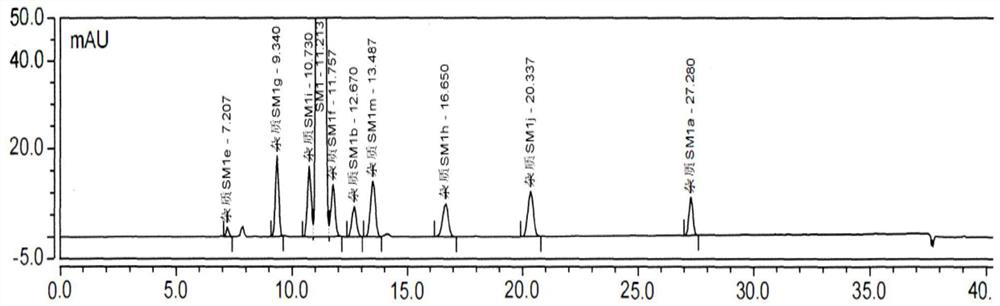
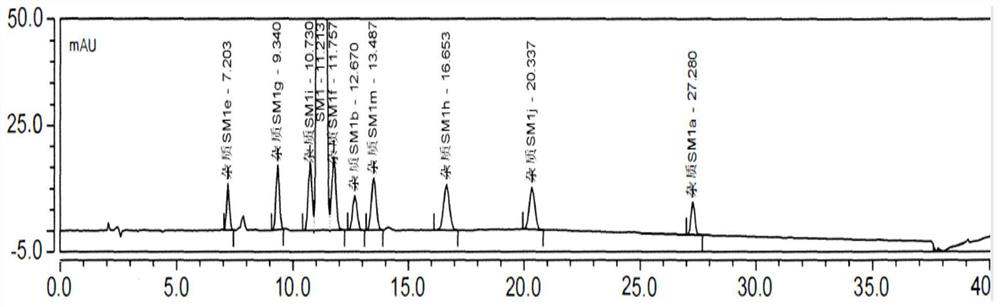
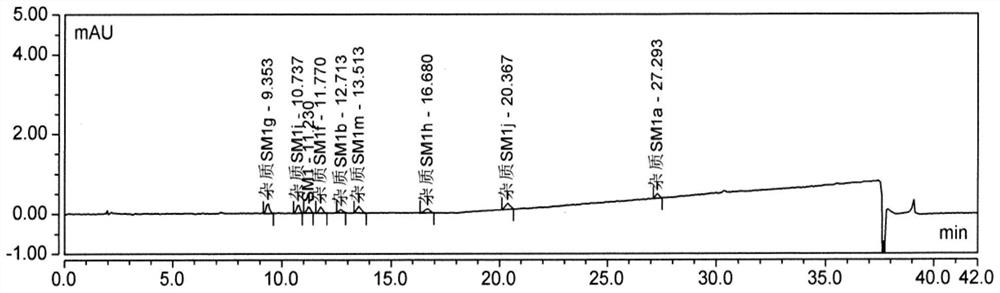
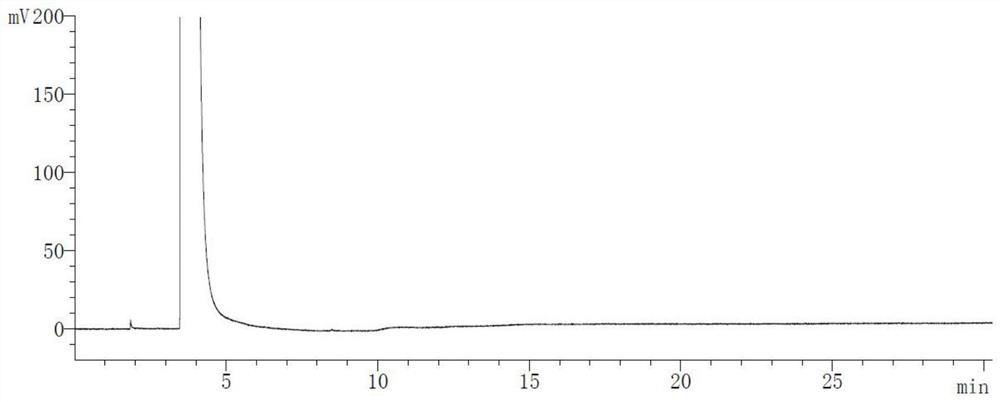
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43results about How to "Solving Separation Assay Problems" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
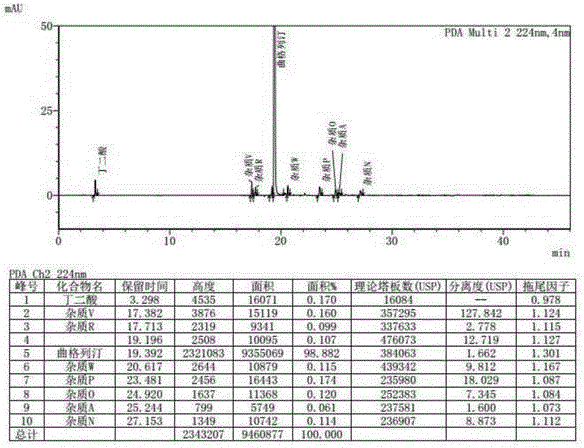
Method for determining related substances in Trelagliptin tablets
ActiveCN105738517AEfficient separationQuality is easy to controlComponent separationOrganic solventTrelagliptin
The invention provides a method for determining related substances in Trelagliptin tablets, and relates to the field of analytical chemistry. The method comprises the steps that a high-performance liquid chromatography method is adopted, a sample solution is injected into a high-performance liquid chromatographic instrument, determination of the related substances in the Trelagliptin tablets is completed, and the chromatographic conditions are that a chromatographic column takes silica gel of which the surface is provided with electric hybrid particles as a filler, a mobile phase A is an acidic aqueous solution, a mobile phase B is an organic solvent, the sum of the volume percent of the mobile phase A and the volume percent of the mobile phase B maintains 100% all the time, and linear gradient elution is conducted. According to the method for determining the related substances in the Trelagliptin tablets, an Xselect C18 chromatographic column is adopted, optimization is conducted on the mobile phase gradient elution program, and the related substances in the Trelagliptin tablets can be effectively separated. The separation determination problem of the related substances in the Trelagliptin tablets is solved, and therefore it is guaranteed that the quality of the Trelagliptin tablets is controllable.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Method for separating and measuring related impurities in acrivastine and preparation thereof
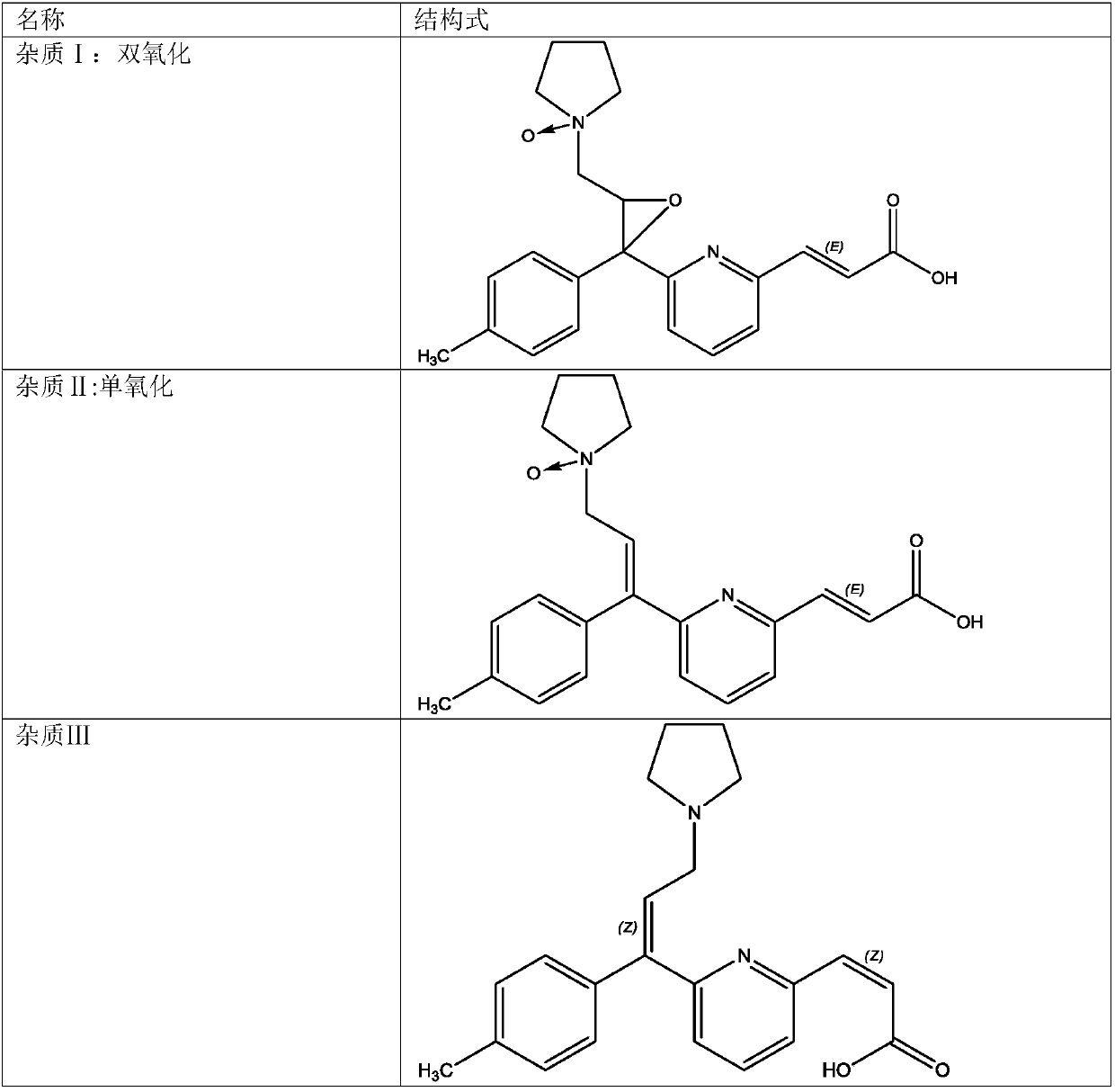
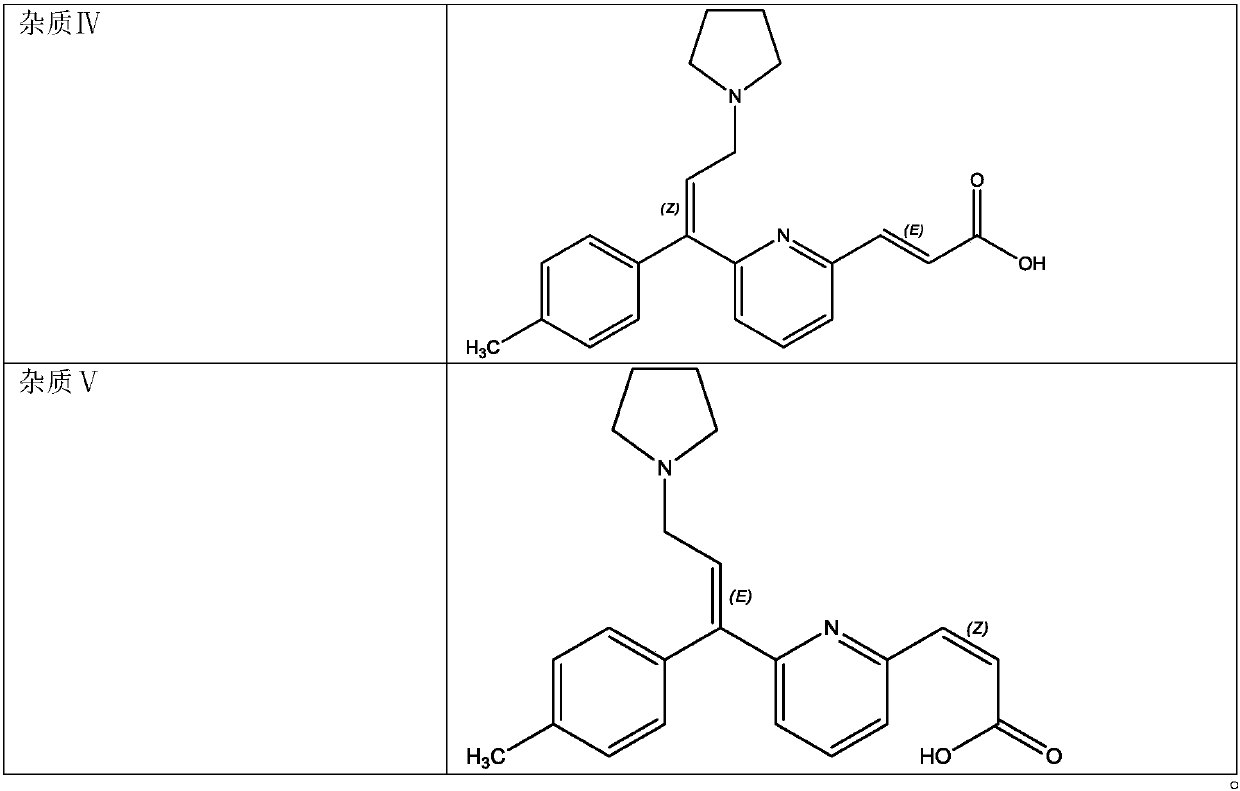
ActiveCN107831230AShort separation timeExcellent separation performance and durabilityComponent separationSolid phasesChemistry
The invention belongs to the field of analytical chemistry, and specifically relates to a method for separating and measuring related impurities in acrivastine and a preparation thereof. According tothe method, octadecyl silane bonded silica gel is adopted as a solid phase, and a mobile phase consisting of aqueous solutions of acetonitrile, tetrahydrofuran and triethylamine is adopted for elutionfor solid-liquid separation; the related impurities include one or more of a double oxidation impurity I, a single oxidation impurity II, Z-type III, E-type IV and E-isomer V. According to the methodprovided by the invention, the related impurities in the acrivastine and the preparation thereof are separated and detected by high performance liquid chromatography, and can be totally separated anddetected within 18 minutes; the method has the advantages of high separation degree, strong specificity, high sensitivity, easiness in operation, simpleness and quickness, so as to guarantee that thequality of the acrivastine and the preparation thereof is controllable, and finally determines the safety and effectiveness of a product, and is of great significance to quality control on the acrivastine.
Owner:CHONGQING HUAPONT PHARMA
Method for testing related substances of levocetirizine hydrochloride intermediate
The invention belongs to the field of analytical chemistry, and discloses a method for separately testing a levocetirizine hydrochloride intermediate and related substances of the levocetirizine hydrochloride intermediate by liquid chromatography. The method comprises the following steps: a chromatographic column taking octadecylsilane chemically bonded silica as a filler uses a certain proportion of buffer salt solution-organic phase as a mobile phase to quantificationally test the content of the levocetirizine hydrochloride intermediate and the content of the related substances of levocetirizine hydrochloride intermediate, so that the quality of the levocetirizine hydrochloride intermediate is effectively controlled, and the controllable quality of levocetirizine hydrochloride is ensured. The method is high in specificity and accuracy, and simple and convenient to operate.
Owner:AVENTIS PHARMA HAINAN
Separating measuring method for otioxetine hydrobromide end product related substances
InactiveCN109521102AEfficient separationSolving Separation Assay ProblemsComponent separationHydrobromideSilanes
The invention belongs to the field of analytical chemistry and discloses a method of separating and measuring otioxetine hydrobromide and related substances thereof by liquid chromatography. The method can measure the contents of otioxetine hydrobromide and related substances thereof quantitively by taking an octyl alkyl silane bonded silica gel as a chromatographic column of filler and a buffer salt solution-organic phase in a certain proportion as a moving phase, so that the quality of the otioxetine hydrobromide is controlled effectively. The method is high in specificity, high in accuracyand simple to operate.
Owner:万全万特制药(厦门)有限公司
Method for separation and determination of vortioxetine hydrobromide intermediate related substances by liquid chromatography
InactiveCN106568862AEfficient separationQuality is easy to controlComponent separationBenzeneHydrobromide
The invention belongs to the field of analytical chemistry, discloses a method for separation and determination of vortioxetine hydrobromide intermediate 1-bromo-2-iodo-benzene related substances by liquid chromatography. The method takes a chromatographic column with octadecylsilane bonded silica gel as a packing, takes a certain ratio of water-organic phase as a mobile phase, and can be used for quantitative determination of the contents of a vortioxetine hydrobromide intermediate and the related substances thereof, so as to effectively control the quality of the vortioxetine hydrobromide intermediate and ensure controllable quality of a vinpocetine hydrobromide final product. The method has the advantages of strong specificity, high accuracy and convenience in operation.
Owner:万全万特制药江苏有限公司
Liquid phase analysis method of maleic acid asenapine and impurities thereof
InactiveCN104297366AEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilanes
The invention belongs to the field of analytical chemistry and discloses a method for separating and determining maleic acid asenapine and impurities thereof by a liquid chromatography. The method adopts a chromatographic column taking octyl silane bonded silica gel as a filler, buffer salt solution-organic phase with certain proportion is used as a moving phase, the contents of the maleic acid asenapine and the impurities thereof can be quantitatively determined, and the quality of the maleic acid asenapine can be effectively controlled. The method has strong specificity, high accuracy and convenience in operation.
Owner:AVENTIS PHARMA HAINAN
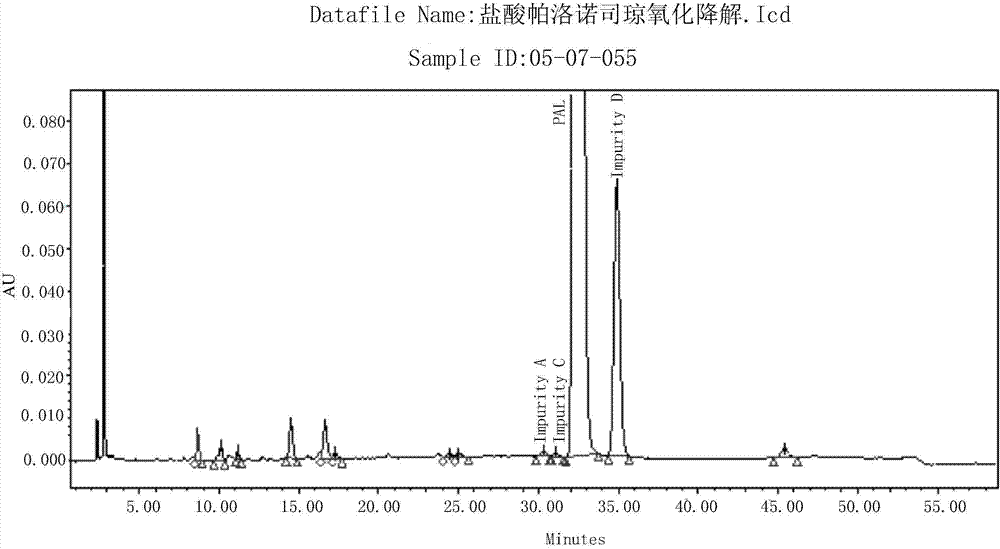
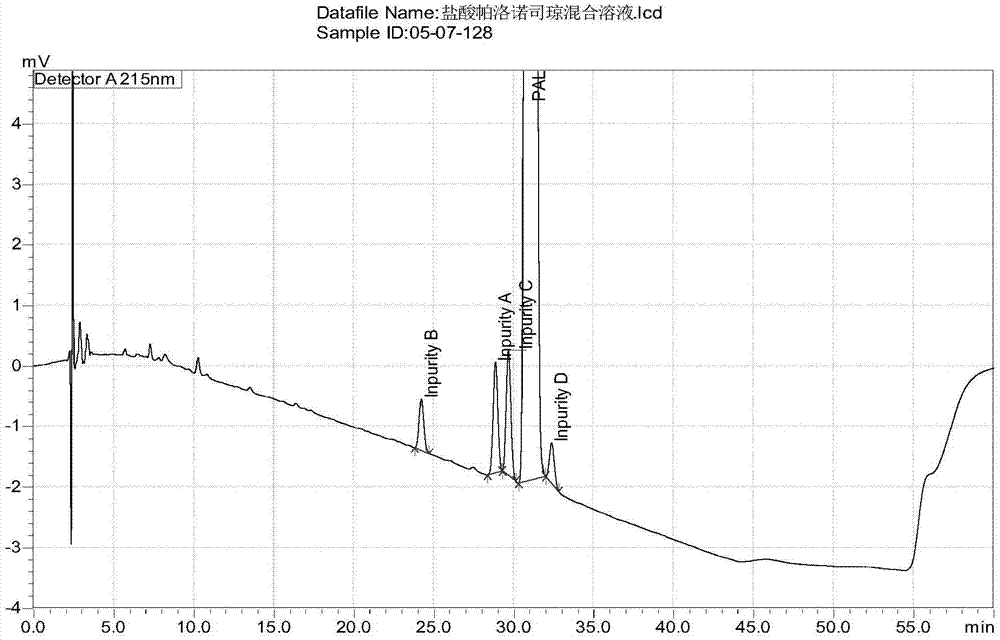
Method for separating and detection palonosetron hydrochloride and impurity
ActiveCN104764840AQuality is easy to controlSolving Separation Assay ProblemsComponent separationInorganic saltsOrganic solvent
The invention belongs to the field of analytical chemistry, relates to a method for the simultaneous determination of palonosetron hydrochloride and four impurities. In particular, a high performance liquid chromatography method is employed for the simultaneous separation and determination of palonosetron hydrochloride and four process impurities A-D (including a degradation product). The column used in the method employs octadecyl silane bonded silica gel as a filler, and the method employs an inorganic salt buffer system supplemented with a certain proportion of an ion pair reagent and an organic solvent as the mobile phases for gradient elution. The method can simultaneously fully separate the process impurities and degradation products of palonosetron hydrochloride, has the advanategs of simpleness, feasibility, good reproducibility and strong specificity, and can effectively determinw palonosetron hydrochloride bulk drugs and medicine and the content of the relevant materials in the preparation.
Owner:CHONGQING HUAPONT PHARMA
Method for separating and measuring lurasidone hydrochloride intermediate related substances through liquid chromatography
InactiveCN105891392AGuaranteed purityLess side effectsComponent separationChromatography columnPiperazine
The invention belongs to the field of analytical chemistry and discloses a method for separating and detecting lurasidone hydrochloride intermediate 3-(1-piperazinyl)-1,2-benzisothiazole and related substances through liquid chromatography. According to the method, octyl silane bonded silica gel serves as a chromatographic column of filler, ion-pairing reagent-buffer salt solution-organic phase in a certain proportion serves as a mobile phase, and the content of the lurasidone hydrochloride intermediate and the related substances of the intermediate can be measured, so that purity of reactants in the production process of lurasidone hydrochloride is effectively controlled, side reactions and impurities are reduced, the yield of the product is increased, and the quality of the product is improved. The method is high in specificity, high in accuracy and easy and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
A pharmaceutical analysis method for efficiently determining gangliosides GM1 and impurities thereof
ActiveCN109725068AStrong separation abilitySuperior selectivityComponent separationGangliosideChemistry
The invention discloses a pharmaceutical analysis method for efficiently determining gangliosides GM1 and impurities thereof, and belongs to the technical field of pharmaceutical analysis. According to the method, a chromatographic column taking octadecylsilane chemically bonded silica as a filler is used as a stationary phase, and a mixed solution of a buffer salt solution and an organic phase isused as a mobile phase, so that the gangliosides GM1 related substances and content can be effectively separated and determined.
Owner:QILU PHARMA
Method for separating and determining oxiracetam and midbody of oxiracetam by utilizing liquid chromatography
ActiveCN104483400AEfficient separationSolving Separation Assay ProblemsComponent separationSilica gelOctadecyl silane-bonded silica
The invention belongs to the field of analytical chemistry, and discloses a method for separating and determining oxiracetam and a midbody of the oxiracetam by utilizing liquid chromatography. The method is characterized in that octadecyl silane bonded silica gel is used as a chromatographic column of filler, an organic phase with a given ratio of ion to reagent is adopted as a mobile phase, the content of each midbody in the oxiracetam can be quantitatively determined, so that the quality of the oxiracetam can be effectively controlled. According to the method, the exclusiveness is strong, the accuracy is high, and the operation is simple and convenient.
Owner:万全万特制药(厦门)有限公司
Method for separating and measuring relevant substance of lurasidone hydrochloride intermediate by using gas chromatographic technique
ActiveCN106525996ARapid and effective separation assaySolving Separation Assay ProblemsComponent separationGas phaseVapor phase chromatography
The invention belongs to the field of analytical chemistry, and the invention discloses a method for separating and detecting a relevant substance of a lurasidone hydrochloride intermediate 3-(1-piperazinyl)-1,2-benzisothiazole by using a gas chromatographic technique. A methylpolysilicone medium polar capillary chromatographic column and a hydrogen flame ionization detector are adopted by the method; the content of the relevant substance of the lurasidone hydrochloride intermediate can be quantitatively measured; thus, the purity of a reactant in the process of synthesizing the lurasidone hydrochloride is effectively controlled; the occurrences of side reactions and the generation of impurities are reduced; the yield of a product is improved. The method provided by the invention is quick in separation and detection, high in specificity and high in accuracy, and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Method for separating and determining related substances of lurasidone hydrochloride intermediate by using gas chromatography
ActiveCN105911155ARapid and effective separation assaySolving Separation Assay ProblemsComponent separationKetoneSide reaction
The invention discloses a method for separating and determining a lurasidone hydrochloride intermediate (1R,2R)-1,2-cyclohexanedimethanol and related substances thereof by using gas chromatography. The method employs a polysiloxane nonpolar capillary chromatographic column and a hydrogen flame ionization detector, can quantitatively determine the contents of the lurasidone hydrochloride intermediate and the related substances thereof, so purity of reactants in the process of synthesis of lurasidone hydrochloride is effectively controlled, occurrence of side reactions and generation of impurities are reduced, and product yield is improved. The method provided by the invention is fast in separation and detection, good in specificity, high in accuracy, and easy and simple to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
A method for determining related substances in trexagliptin tablets
ActiveCN105738517BEfficient separationQuality is easy to controlComponent separationTrelagliptinGradient elution
The invention provides a method for determining related substances in Trelagliptin tablets, and relates to the field of analytical chemistry. The method comprises the steps that a high-performance liquid chromatography method is adopted, a sample solution is injected into a high-performance liquid chromatographic instrument, determination of the related substances in the Trelagliptin tablets is completed, and the chromatographic conditions are that a chromatographic column takes silica gel of which the surface is provided with electric hybrid particles as a filler, a mobile phase A is an acidic aqueous solution, a mobile phase B is an organic solvent, the sum of the volume percent of the mobile phase A and the volume percent of the mobile phase B maintains 100% all the time, and linear gradient elution is conducted. According to the method for determining the related substances in the Trelagliptin tablets, an Xselect C18 chromatographic column is adopted, optimization is conducted on the mobile phase gradient elution program, and the related substances in the Trelagliptin tablets can be effectively separated. The separation determination problem of the related substances in the Trelagliptin tablets is solved, and therefore it is guaranteed that the quality of the Trelagliptin tablets is controllable.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Method for separating and determining memantine starting material and related substances by using gas chromatography
InactiveCN113125574ARapid and effective separation assaySolving Separation Assay ProblemsComponent separationMemantine HydrochlorideVapor phase chromatography
The invention belongs to the field of analytical chemistry, and discloses a method for separating and determining a memantine hydrochloride starting material 1, 3-Dimethylaminamantine and related substances thereof by using a gas chromatography. The method adopts a capillary chromatographic column filled with 100% dimethyl polysiloxane anda hydrogen flame ionization detector, and canquantitatively determine the content of the memantine hydrochloride starting material and the related substances thereof, so that the purity of reactants in the process of synthesizing memantine hydrochloride is effectively controlled, side reactions and impurities are reduced, and the product yield is improved. The method is high in specificity, high in accuracy and simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Method for separating etoricoxib and related substances thereof by using high performance liquid chromatography
ActiveCN104614467AEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilanes
The invention belongs to the field of analytical chemistry and discloses a method for separating and determining etoricoxib and related substances thereof by using liquid chromatography. The method can be used for quantitatively determining the contents of etoricoxib and related substances thereof by using phenyl silane bonded silica gel as a chromatographic column of fillers and using a certain proportion of buffer salt solutions-organic phases as mobile phases, thus effectively controlling the quality of etoricoxib. The method has strong specificity and high precision and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Method for measuring impurity in carboxymethyl chitosan by liquid chromatography
InactiveCN101498697AEffective separation assaySolving Separation Assay ProblemsComponent separationUltraviolet detectorsChromatography column
The invention relates to a method for separating and measuring the impurity-diglycol acid (natrium) (DGA) in carboxymethyl chitosan by a liquid chromatography method. A high-efficiency liquid chromatography method for separating and measuring the impurity DGA in carboxymethyl chitosan products adopts a chromatographic column taking C18 as stuffing, takes mixed solutions prepared by buffer salts with the PH value being 1.5-3.5 and organic phases according to a certain mixture ratio as a flowing phase, and performs detection and quantization by an ultraviolet detector. The method can effectively separate and measure the impurity DGA in the carboxymethyl chitosan and solves the problem of separation and measurement of the impurity DGA in the carboxymethyl chitosan, thereby ensuring the controllable quality of the carboxymethyl chitosan.
Owner:OCEAN UNIV OF CHINA
Method for separating and detecting nebivolol hydrochloride impurity by liquid phase chromatography
ActiveCN101328161AEffective separation assayGuaranteed SolubilityOther chemical processesSolid sorbent liquid separationCelluloseFormate
The invention discloses a method for measuring nebivolol hydrochloride impurities by a liquid phase chromatography method. Cellulose three(3, 5-dimethylphenyl amino formate is taken as a chiral chromatographic column of a filling material, and a mixed solution of a buffer salt solution and an organic phase according to certain ratio is taken as a mobile phase. The method can rapidly and accurately separate and measure the two optical isomers.
Owner:AVENTIS PHARMA HAINAN
Method for separating and detecting optical isomer of brivaracetam starting material
InactiveCN110873763ARapid and effective separation assayQuality is easy to controlComponent separationQuantitative determinationPhysical chemistry
The invention belongs to the field of analytical chemistry. The invention discloses a method for rapidly separating and detecting the optical purity of a brivaracetam starting material (R)-4-propyl-dihydrofuran-2-one by using gas chromatography. According to the method, a cyclodextrin chiral capillary chromatographic column and a hydrogen flame ionization detector are adopted, so that the opticalpurity and the isomer content of the brivaracetam starting material can be quantitatively determined, and the stability of the optical isomer of the brivaracetam starting material is indicated. The method disclosed by the invention is strong in specificity, high in accuracy, good in durability and simple and rapid to operate.
Owner:万全万特制药(厦门)有限公司
Method for determining cvortioxetine intermediate and its isomers by using liquid chromatography
InactiveCN110873760AEfficient separationSolving Separation Assay ProblemsComponent separationFluid phasePhysical chemistry
The invention belongs to the field of analytical chemistry. The invention discloses an analysis method for separating and determining a vortioxetine hydrobromide intermediate and its isomers by usingliquid chromatography. The method uses a chromatographic column filled with phenyl bonded silica gel as a filler and a certain proportion of a buffer salt solution-organic phase as a mobile phase, andcan quantitatively determinethe content of the vortioxetine hydrobromide intermediate and isomers thereof so that the mass of a vortioxetine hydrobromide starting material is effectively controlled,side reactions are reduced, and therefore it is guaranteed that the quality of a vortioxetine hydrobromide final product is controllable. The method is strong in specificity, high in accuracy and simple and convenient to operate.
Owner:万全万特制药(厦门)有限公司
Method for separating and determining diflucortolone as well as 6 beta diflucortolone and 16 beta diflucortolone thereof
ActiveCN108226340AEasy to separateIncreased durabilityComponent separationStationary phasePotassium hexafluorophosphate
The invention belongs to the field of analytical chemistry, and concretely relates to a method for separating and determining diflucortolone as well as 6 beta diflucortolone and 16 beta diflucortolonethereof. A stationary phase adopted by the method is an octylsilanebonded silica gel. The method comprises the concrete steps of injecting a diflucortolone sample solution into a chromatographic instrument, adopting a mixed solution of a potassium hexafluorophosphatebuffer salt system and an organic solvent as a moving phase for eluting, and detecting in a detector. The method can be used for separating and content-determining of the diflucortolone as well as the 6 betadiflucortolone and the 16 beta diflucortolone thereof at the same time, achieves better effects on tailing factors and theoretical pedal number, has excellent separating property and durability, and is simple, convenient, feasible, good in reproducibility, efficient, fast, and high in specificity.
Owner:CHONGQING HUAPONT PHARMA
Method for separation and determination of Acitretin and its impurities
Owner:CHONGQING HUABANGSHENGKAI PHARM
A method for determining related substances in trexagliptin succinate raw material
ActiveCN105699547BQuality is easy to controlEfficient separationComponent separationSuccinic acidGradient elution
The invention discloses a method for measuring related substances in succinic acid Trelagliptin raw materials and relates to the field of analytic chemistry. According to the method, high performance liquid chromatography is adopted, a sample solution is injected into a high performance liquid chromatograph, and the related substances in the succinic acid Trelagliptin raw materials are measured; according to chromatographic conditions, a 100-5C18 chromatographic column of the Kromasil company is adopted as a chromatographic column, a flowing phase A and a flowing phase B are both a mixed solution of an acid aqueous solution and an organic solvent, the sum of the volume percentage of the flowing phase A and the volume percentage of the flowing phase B is kept 100% all the time, and linear gradient elution is performed. According to the method, the 100-5C18 chromatographic column of the Kromasil company is adopted, the gradient elution program of the flowing phases is optimized, and the related substances in the succinic acid Trelagliptin raw materials can be effectively separated. The method solves the separation and measurement problem of the related substances in the succinic acid Trelagliptin raw materials, and therefore it is guaranteed that the mass of the succinic acid Trelagliptin raw materials is controllable.
Owner:中山万远新药研发有限公司
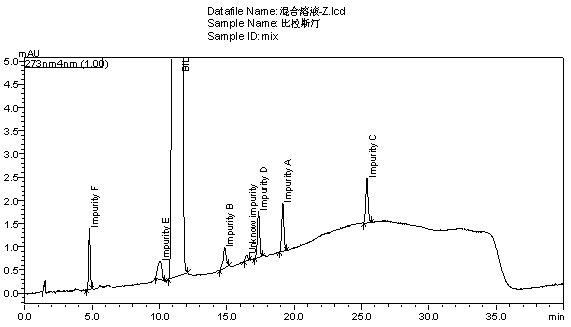
A method for separating and measuring process impurities in bilastine and its preparations
ActiveCN105319288BQuality is easy to controlSolving Separation Assay ProblemsComponent separationInorganic saltsOrganic solvent
The invention belongs to the field of analytical chemistry, relates to a method for separating and measuring bilastine and technical impurities in a preparation of bilastine, and specifically relates to a method for separating and measuring bilastine and six technical impurities A-F (including a degradation product) in the preparation of bilastine by adopting high performance liquid chromatography. An adopted chromatographic column takes octylsilane bonded silicone as a filler, and adopts an inorganic salt buffer system with an ion-pairing agent added and an organic solvent, wherein the inorganic salt buffer system and the organic solvent is according to a certain ratio, as a moving phase for gradient elution. According to the method, the technical impurities A-F of bilastine and the degradation product can be totally separated. The method is simple to operate and good in reproducibility. The content of a raw medicine of bilastine and relevant materials in the preparation can be effectively measured, and the method is good in specificity.
Owner:CHONGQING HUAPONT PHARMA
A method for separating etoricoxib and its related substances by high performance liquid chromatography
ActiveCN104614467BEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilanes
The invention belongs to the field of analytical chemistry and discloses a method for separating and determining etoricoxib and related substances thereof by using liquid chromatography. The method can be used for quantitatively determining the contents of etoricoxib and related substances thereof by using phenyl silane bonded silica gel as a chromatographic column of fillers and using a certain proportion of buffer salt solutions-organic phases as mobile phases, thus effectively controlling the quality of etoricoxib. The method has strong specificity and high precision and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
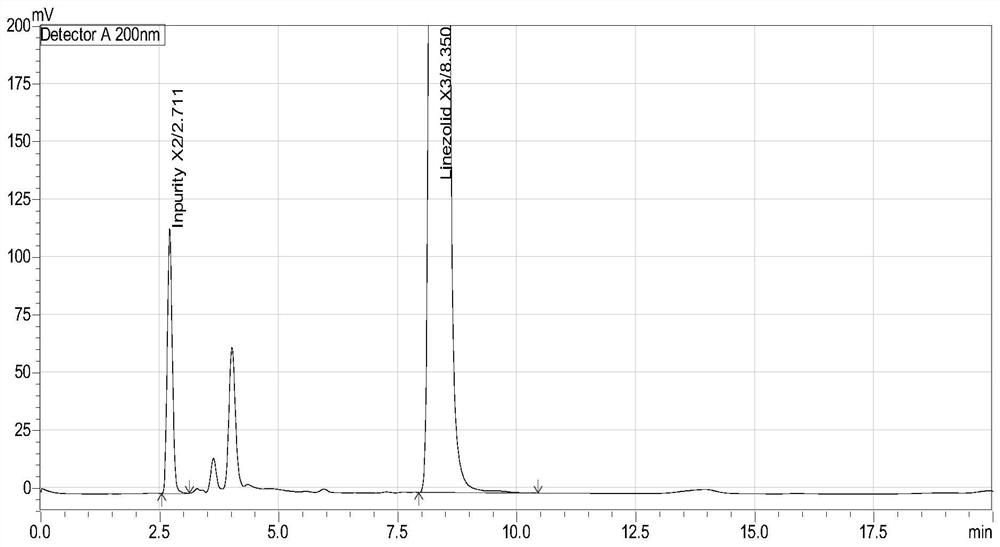
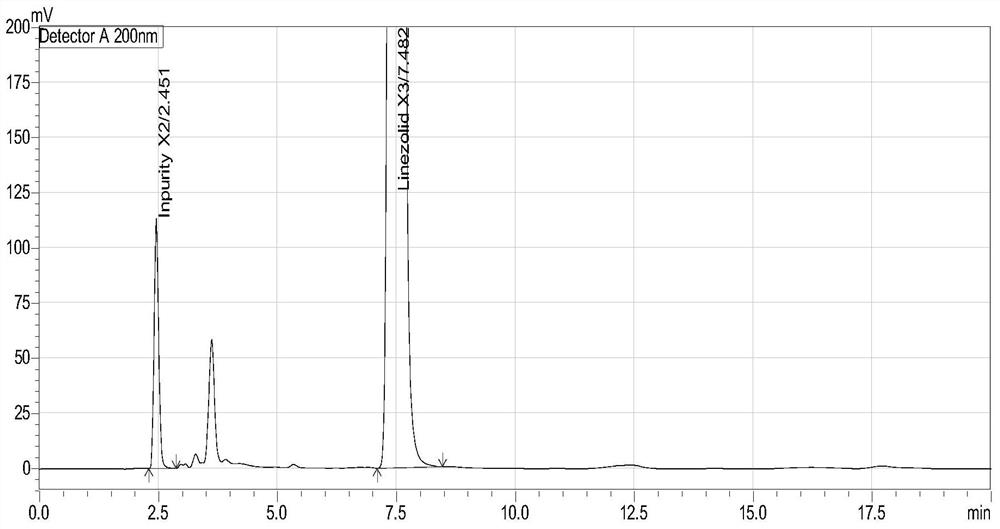
Method for separation and determination of linezolid raw material x3 and its process impurity x2
ActiveCN106146332BEffective controlEfficient separationComponent separationCarboxylic acid amide separation/purificationSilanesSilica gel
The invention belongs to the field of analytical chemistry and relates to a method for separating and determining a linezolid raw material (S)-1-acetamido-2-acetoxyl-3-chloropropane X3 and its process impurities X2, in particular to a method for separating and determining a linezolid raw material X3 and its process impurities X2 by adopting a high performance liquid chromatography. A chromatographic column adopted in the method uses octadecyl silane bonded silica gel as a filler, water added with an ion-pairing agent and acetonitrile which are in certain proportion are adopted as mobile phases. The method can completely separate the linezolid raw material (S)-1-acetamido-2-acetoxyl-3-chloropropane X3 and the its process impurities X2, is simple, convenient, feasible, good in reproducibility and strong in specificity and can effectively determine the related substance content in the linezolid raw material (S)-1-acetamido-2-acetoxyl-3-chloropropane X3.
Owner:CHONGQING HUABANGSHENGKAI PHARM
A kind of method for determining degradation impurities in orlistat capsules
ActiveCN105738506BEfficient detectionSolving Separation Assay ProblemsComponent separationSolid sphereGradient elution
The invention discloses a method for measuring degraded impurities in orlistat capsules and relates to the field of analytical chemistry.According to the method, a high performance liquid chromatographic method is adopted, an impurity mixed reference substance solution and a sample solution are injected in a high performance liquid chromatograph, and measurement of the degraded impurities in the orlistat capsules is completed.According to the chromatographic conditions, silica gel solid sphere particles are used as filler of a chromatographic column; a mobile phase A is an acidic water solution, a mobile phase B is an organic solvent, the sum of the percent by volume of the mobile phase A and the percent by volume of the mobile phase B is kept 100% all the time, and linear gradient elution is carried out.According to the method, the Cortecs C18 (Waters, 150 mm*4.6 mm, 2.7 micrometers) chromatographic column is adopted, and by optimizing the mobile phase gradient elution procedure, all the degraded impurities and process impurities hard to separate nearby a main peak can be separated under the same chromatographic conditions, and the degraded impurities in the orlistat capsules can be effectively detected.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
A method for separating and measuring diflumethasone and its 6β diflumethasone and 16β diflumethasone
ActiveCN108226340BEasy to separateIncreased durabilityComponent separationPotassium hexafluorophosphateStationary phase
The invention belongs to the field of analytical chemistry, and concretely relates to a method for separating and determining diflucortolone as well as 6 beta diflucortolone and 16 beta diflucortolonethereof. A stationary phase adopted by the method is an octylsilanebonded silica gel. The method comprises the concrete steps of injecting a diflucortolone sample solution into a chromatographic instrument, adopting a mixed solution of a potassium hexafluorophosphatebuffer salt system and an organic solvent as a moving phase for eluting, and detecting in a detector. The method can be used for separating and content-determining of the diflucortolone as well as the 6 betadiflucortolone and the 16 beta diflucortolone thereof at the same time, achieves better effects on tailing factors and theoretical pedal number, has excellent separating property and durability, and is simple, convenient, feasible, good in reproducibility, efficient, fast, and high in specificity.
Owner:CHONGQING HUAPONT PHARMA
Method for separating and detecting gatecarboxylic acid ethyl ester and/or related impurities by HPLC (High Performance Liquid Chromatography) method
PendingCN114113355ASolving Separation Assay ProblemsHigh sensitivityComponent separationBulk chemical productionChromatographic columnChemistry
The invention relates to the technical field of pharmaceutical analysis, in particular to a method for analyzing ethyl gatetanecarboxylate and related impurities by using high performance liquid chromatography. A chromatographic column adopted by the method takes octadecyl bonded silica gel as a filling agent, a buffer salt solution and an organic solvent are adopted for gradient elution, the flow velocity is 0.9-1.1 ml / min, and the column temperature is 18-22 DEG C; the detection wavelength is 220 nm or 250 nm; the method can effectively separate and accurately quantify seven known impurities possibly existing in the starting material ethyl gatetanecarboxylate of moxifloxacin hydrochloride within 42 minutes, the separation effect of the other two known impurities possibly existing in the starting material and the seven known impurities is good, and the method has no interference on impurity identification and quantitative detection. The problem of separation and determination of known impurities in ethyl gatetanecarboxylate, which cannot be solved in the prior art, is provided; the analysis method is high in sensitivity; the specificity is high; the reproducibility is good; the operation is simple and feasible.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Method for separating and determining compound A and imidazole in amisulpride by gas chromatography
PendingCN113514562ARapid and effective separation assaySolving Separation Assay ProblemsComponent separationOrganic chemistry methodsChromatographic separationPolyethylene glycol
The invention belongs to the field of analytical chemistry, and discloses a method for separating and determining a compound A[(2RS)-1-ethyl pyrrolidinone-2-yl-methylamine] and imidazole in an amisulpride final product {4-amino-N-[[(2RS)1-ethyl pyrrolidinone-2-yl] methyl]-5-(ethyl sulfonyl)-2-methoxybenzamide} by using a gas chromatographic method. According to the method, a capillary chromatographic column filled with polyethylene glycol and a hydrogen flame ionization detector are adopted, and the content of impurities A[(2RS)-1-ethyl pyrrolidone-2-yl-methylamine] and imidazole in a final product of amisulpride can be quantitatively determined, so that the purity of reactants in the process of synthesizing amisulpride is effectively controlled, side reactions and impurities are reduced, and the product yield is improved. The method is high in specificity, high in accuracy and simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
A kind of method for separating midanacin and related substances by high performance liquid chromatography
ActiveCN104614468BEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilanes
The invention belongs to the field of analytical chemistry and discloses a method for separating and determining imidafenacin and related substances thereof by using liquid chromatography. The method can be used for quantitatively determining the contents of imidafenacin and related substances thereof by using phenyl silane bonded silica gel as a chromatographic column of fillers and a certain proportion of buffer salt solutions-organic phases as mobile phases, thus effectively controlling the quality of imidafenacin. The method has strong specificity and high precision and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com