Method for separating and detecting nebivolol hydrochloride impurity by liquid phase chromatography
A liquid chromatography and chiral chromatographic column technology is applied in the field of separation and determination of R-type and S-type optical isomers of nebivolol intermediate-4, which can solve problems such as undiscovered, achieve reduction of interference, Guaranteed solubility and quality controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instruments and Conditions
[0037] Shimadzu high performance liquid chromatography; column: Daicel OD-RH chiral column (150mm×4.6mm)
[0038] Mobile phase: (0.05mol / L potassium dihydrogen phosphate, 10% phosphoric acid to adjust pH to 3.0)-acetonitrile=85:15
[0039] Detection wavelength: 287nm; Column temperature: 25°C; Flow rate: 1.0ml / min; Injection volume: 10μl
[0040] Experimental steps:
[0041] Take (R,S)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid racemate 10mg, put it in a 20ml measuring bottle, add about 2ml of methanol to dissolve, and use a flow Phase diluted to the mark, shake well, as the test solution;
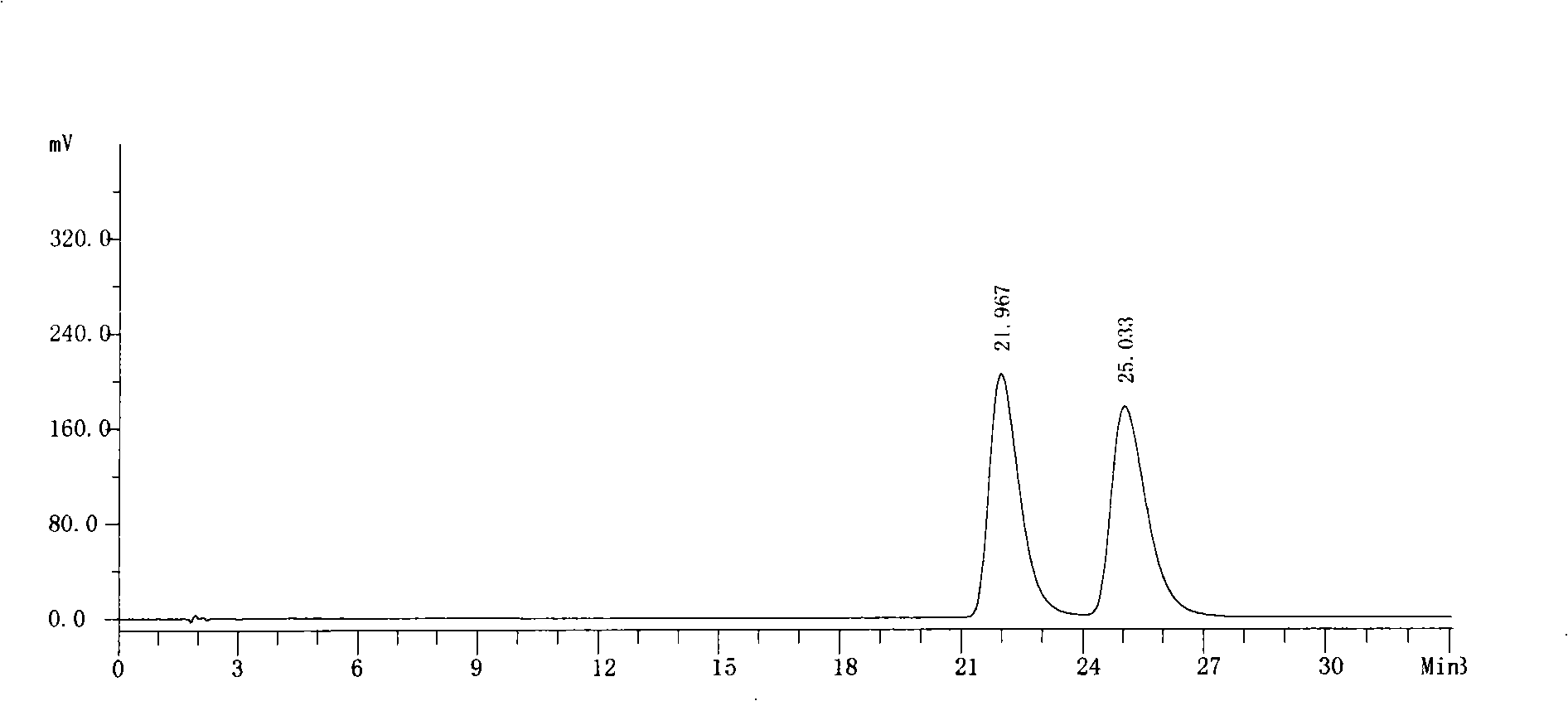
[0042] Get need testing solution, carry out high performance liquid chromatography analysis by above-mentioned condition, record chromatogram, the result sees figure 1 .
[0043] figure 1 The chromatographic peak with a retention time of 21.967 minutes is the chromatographic peak of S-6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid...
Embodiment 2
[0045] Instruments and Conditions
[0046] Shimadzu high performance liquid chromatography; column: Daicel OD-RH chiral column (150mm×4.6mm)
[0047] Mobile phase: (0.02mol / L potassium dihydrogen phosphate, 10% phosphoric acid to adjust pH to 3.8)-acetonitrile=80:20
[0048]Detection wavelength: 287nm; Column temperature: 25°C; Flow rate: 1.0ml / min; Injection volume: 10μl
[0049] Experimental steps:
[0050] Take (R,S)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid racemate 10mg, put it in a 20ml measuring bottle, add about 2ml of methanol to dissolve, and use a flow Phase diluted to the mark, shake well, as the test solution;
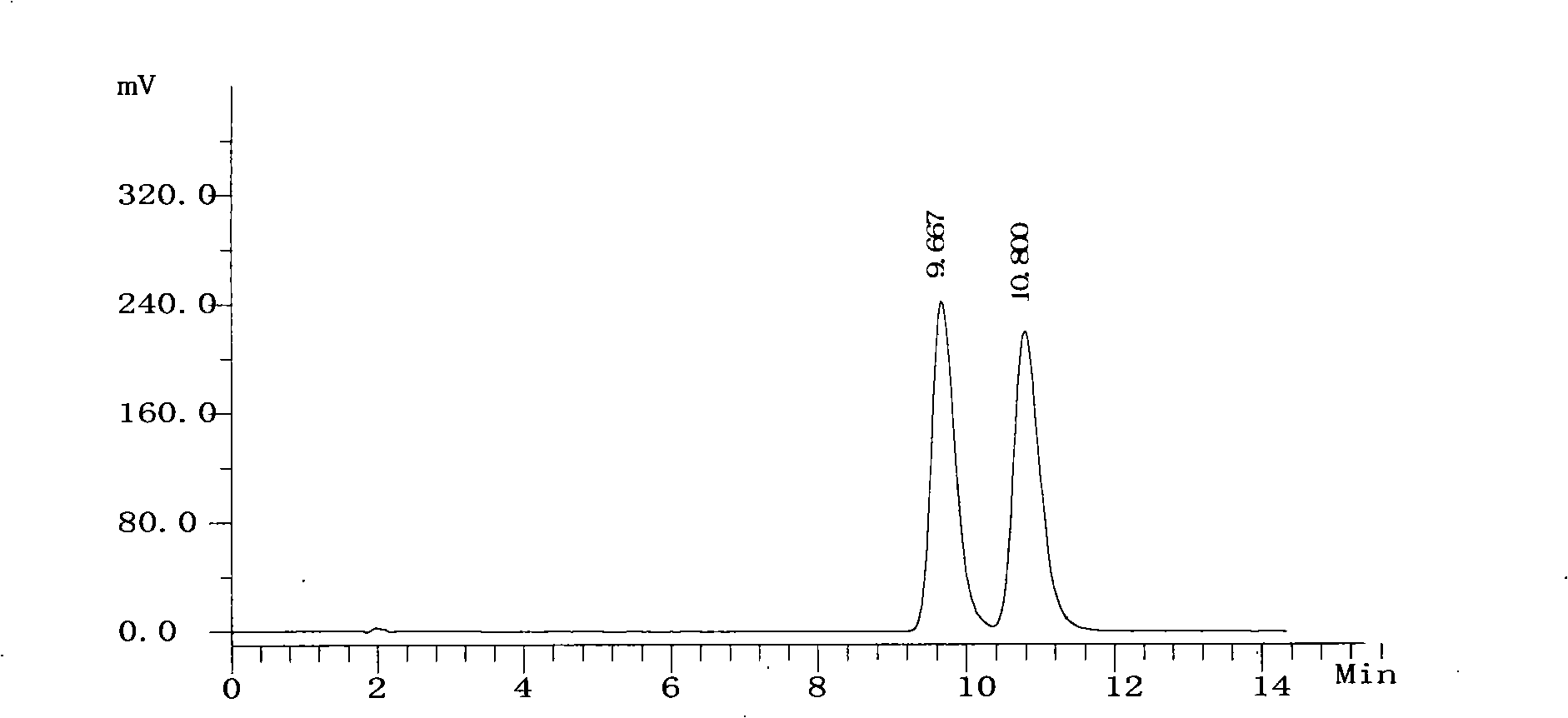
[0051] Get need testing solution, carry out high performance liquid chromatography analysis by above-mentioned condition, record chromatogram, the result sees figure 2 . figure 2 The chromatographic peak whose retention time is 9.667 minutes is the chromatographic peak of S-6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid, and the c...
Embodiment 3
[0053] Instruments and Conditions
[0054] Shimadzu high performance liquid chromatography; column: Daicel OD-RH chiral column (150mm×4.6mm)
[0055] Mobile phase: (0.05mol / L sodium acetate, glacial acetic acid to adjust pH to 2.5)-acetonitrile=75:25
[0056] Detection wavelength: 287nm; Column temperature: 25°C; Flow rate: 1.0ml / min; Injection volume: 10μl
[0057] Experimental steps:
[0058] Take (R,S)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid racemate 10mg, put it in a 20ml measuring bottle, add about 2ml of methanol to dissolve, and use a flow Phase diluted to the mark, shake well, as the test solution;
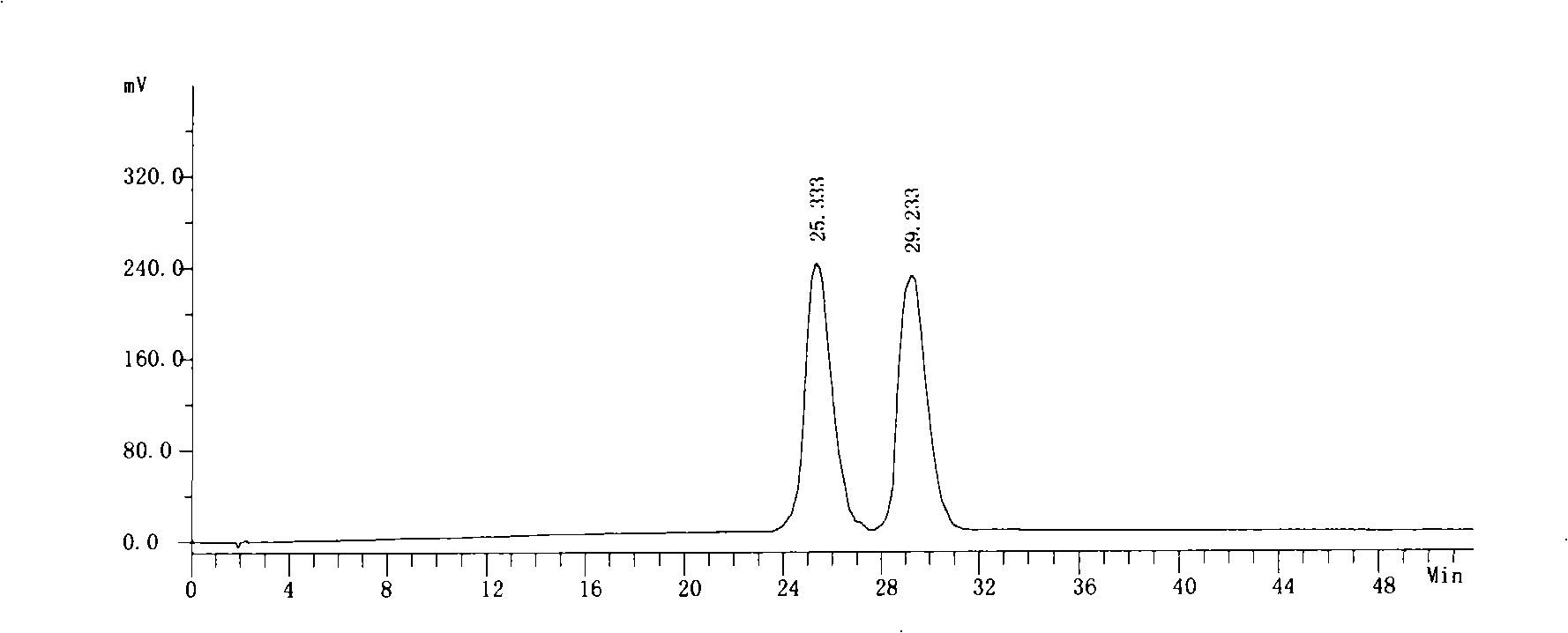
[0059] Get need testing solution, carry out high performance liquid chromatography analysis by above-mentioned condition, record chromatogram, the result sees image 3 . image 3 The chromatographic peak whose retention time is 25.333 minutes is the chromatographic peak of S-6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid, and the chromatographic p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com