A pharmaceutical analysis method for efficiently determining gangliosides GM1 and impurities thereof
A ganglioside and high performance liquid chromatography technology is applied in the field of drug analysis for the efficient determination of ganglioside GM1 single component and related substances, and can solve the problems of ineffective separation, weakened detection sensitivity of impurities, underestimation of impurities and the like, Achieve the effect of being beneficial to product quality control, excellent separation selectivity, and good reference peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The mensuration of embodiment 1 ganglioside GM1
[0054] Instruments and Conditions
[0055] High performance liquid chromatography: Waters e2695-2489
[0056] Chromatographic column: C18 (Waters, 4.6×150mm, 3.5μm)
[0057] Mobile phase A: 0.01mol / L potassium dihydrogen phosphate solution-acetonitrile (30:70), adjust pH=7.1 with triethylamine.
[0058] Mobile Phase B: Acetonitrile
[0059] The gradient elution procedure was used as follows:
[0060]
[0061] Flow rate: 1.0ml / min, detection wavelength: 205nm, injection volume: 10μl, column temperature: 40°C.
[0062] Experimental steps:
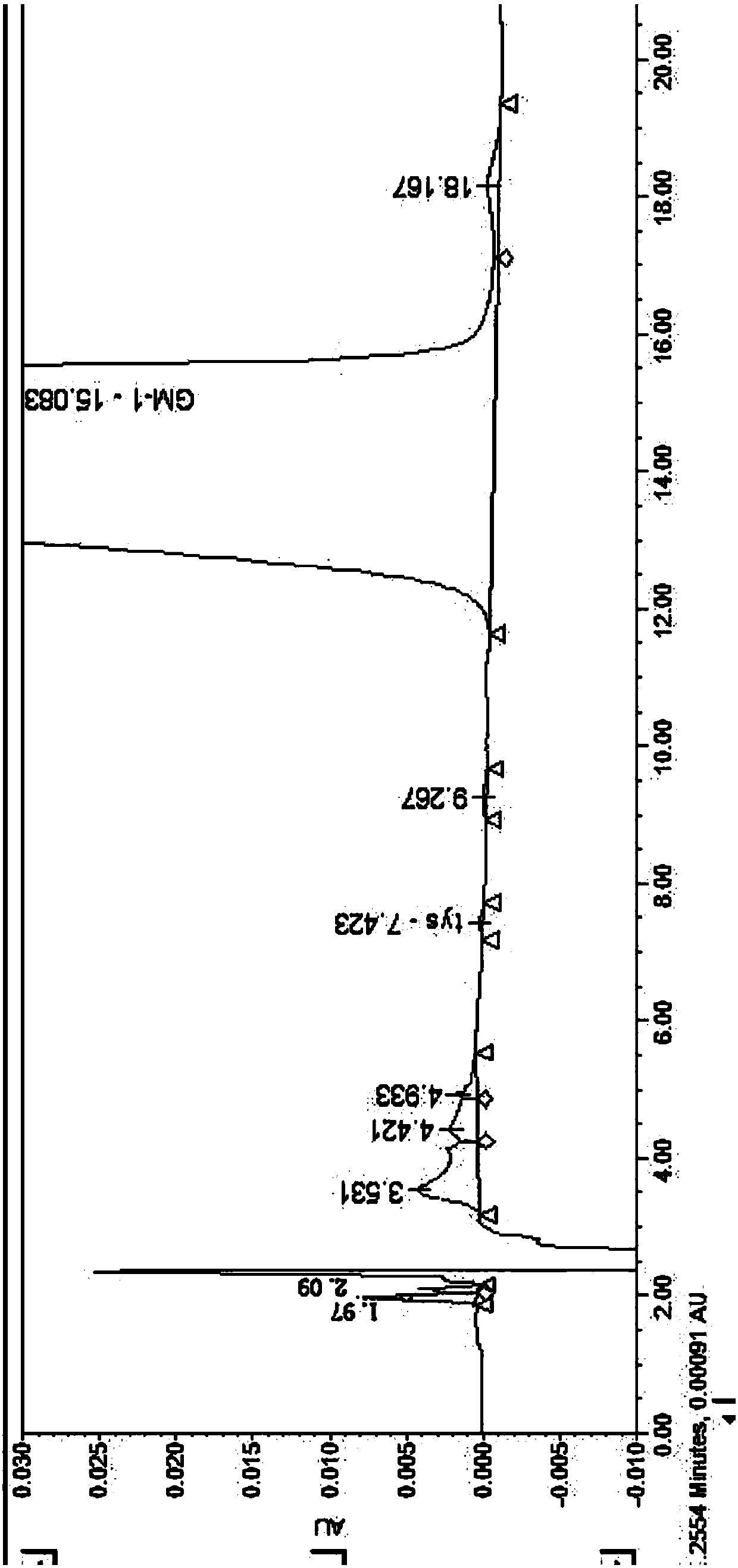
[0063] Take an appropriate amount of ganglioside GM1 and its related substances, dissolve the sample with an aqueous solution, and prepare a sample solution containing about 1.0 mg / ml of ganglioside GM1. Carry out HPLC analysis by above-mentioned test condition, record chromatogram, its HPLC collection of illustrative plates is as follows figure 2 shown. The content was calc...
Embodiment 2
[0065] Determination of embodiment 2 ganglioside GM1
[0066] Instruments and Conditions
[0067] High performance liquid chromatography: Agilent 1260-VWD
[0068] Chromatographic column: C18 (Waters, 4.6×150mm, 3.5μm)
[0069] Mobile phase A: 0.01mol / L potassium dihydrogen phosphate solution-acetonitrile (32:68), adjust pH=7.1 with triethylamine.
[0070] Mobile Phase B: Acetonitrile
[0071] The gradient elution procedure was used as follows:
[0072]
[0073] Flow rate: 1.2ml / min, detection wavelength: 205nm, injection volume: 10μl, column temperature: 40°C.
[0074] Experimental steps:
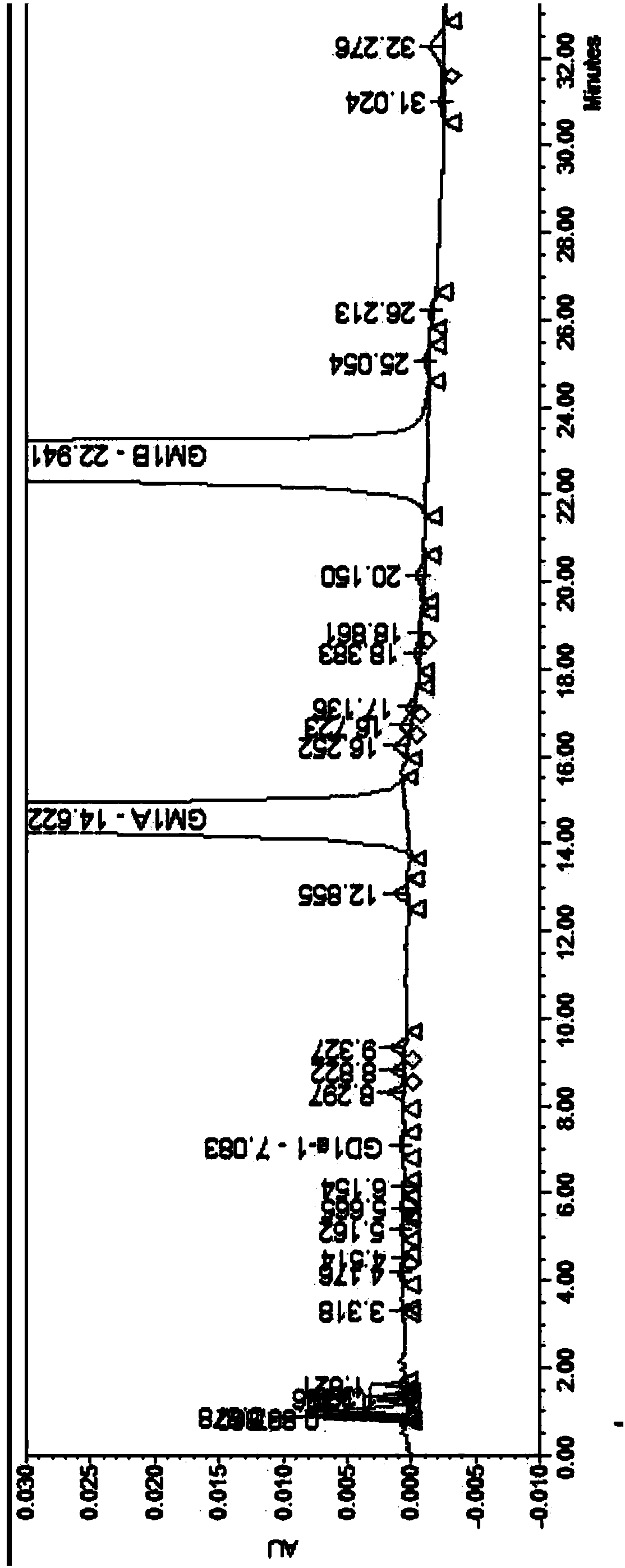
[0075] Take an appropriate amount of ganglioside GM1 and its related substances, dissolve the sample with an aqueous solution, and prepare a sample solution containing about 5 mg / ml of ganglioside GM1. Carry out high performance liquid chromatography analysis according to above-mentioned gradient condition, record chromatogram, two components of ganglioside GM1 and its related sub...
Embodiment 3
[0076] Determination of embodiment 3 ganglioside GM1
[0077] Instruments and Conditions
[0078] High performance liquid chromatography: Waters e2695-2489
[0079] Chromatographic column: C18 (Symmetry RP18, 4.6×150mm, 3.5μm)
[0080] Mobile phase A: 0.012mol / L potassium dihydrogen phosphate solution-acetonitrile (35:65), adjust pH=7.5 with triethylamine.
[0081] Mobile Phase B: Acetonitrile
[0082] The gradient elution procedure was used as follows:
[0083]
[0084] Flow rate: 1.2ml / min, detection wavelength: 205nm, injection volume: 10μl, column temperature: 40°C.
[0085] Experimental steps:
[0086] Take an appropriate amount of ganglioside GM1 and its related substances, dissolve the sample with an aqueous solution, and prepare a sample solution containing about 1.2 mg / ml of ganglioside GM1. Carry out high-performance liquid chromatography analysis according to above-mentioned gradient condition, record chromatogram, the result sees attached Figure 4 . The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com