A kind of method for determining degradation impurities in orlistat capsules
A technology for orlistat and impurities, applied in the field of analytical chemistry, can solve the problems of inability to effectively separate and degrade impurities, and achieve the effects of high accuracy, strong specificity, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] Preparation of disruption solution:
[0044] ①. Orlistat stock solution: take an appropriate amount of orlistat capsule content powder, add acetonitrile-water mixed solution with a volume ratio of 4:1 to make a 0.5 mg / mL solution, and obtain it.
[0045] ②. Hydrochloric acid methanol destruction solution: Take an appropriate amount of orlistat capsule content powder, which is equivalent to 50 mg of orlistat, put it in a 100mL measuring bottle, add a mixed solution of methanol-water volume ratio 4:1, ultrasonically shake, Completely dissolve orlistat, add 5mL of 0.1mol / L hydrochloric acid, let stand at room temperature for 1 hour, add 5mL of 0.1mol / L sodium hydroxide solution for neutralization, and mix the solvent with a mixed solution of methanol-water volume ratio 4:1 Dilute to the mark, that is.
[0046] ③. Hydrochloric acid ethanol destruction solution: Take an appropriate amount of orlistat capsule content powder, which is equivalent to 50mg orlistat, put it in a ...
Embodiment 1
[0053] Embodiment 1: System suitability test
[0054] Instruments and experimental conditions:
[0055] High performance liquid chromatography: Shimadzu LC-16;
[0056] Chromatographic column: Cortecs C 18 (Waters, 150×4.6mm, 2.7μm);
[0057] Mobile phase A is 0.025% by volume phosphoric acid aqueous solution, and mobile phase B is acetonitrile; linear gradient elution according to Table 2.
[0058]
[0059]
[0060] The flow rate is 1.3mL / min;
[0061] Column temperature 35°C;
[0062] Detection wavelength 195nm;
[0063] The injection volume was 20 μl.
[0064] Experimental steps:
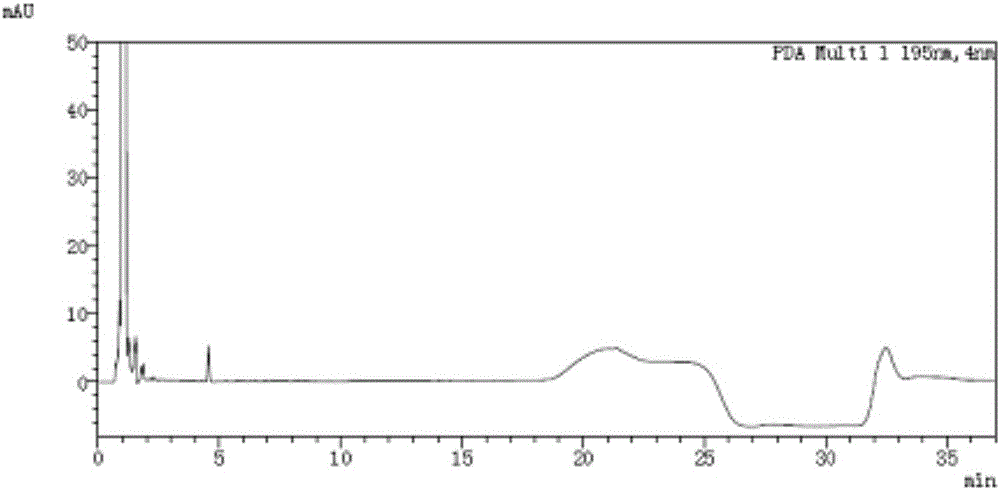
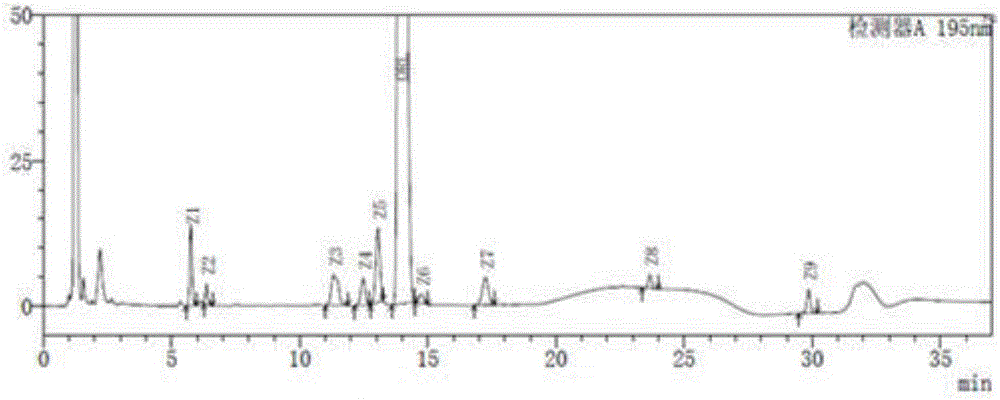
[0065] Take acetonitrile-water volume ratio is 4: 1 mixed solution and impurity mixed reference substance solution respectively, carry out high performance liquid chromatography analysis according to above-mentioned experimental conditions, record chromatogram, the result sees figure 1 , figure 2 , ORL in the figure represents orlistat. figure 2 The middle is the chromatogram co...
Embodiment 2
[0069] Embodiment 2: system suitability comparative test (USP37 method)
[0070] Instruments and experimental conditions:
[0071] High performance liquid chromatography: Shimadzu LC-16;
[0072] Chromatographic column: Nova-pak C18 (3.9mm×150mm, 4.0μm);
[0073] Mobile phase: acetonitrile-phosphoric acid-water mixed solution with a volume ratio of 88:0.05:12;
[0074] Flow rate: 1.0mL / min;
[0075] Column temperature: 35°C;
[0076] Wavelength: 195nm;
[0077] Injection volume: 20 μl.
[0078] Experimental steps:
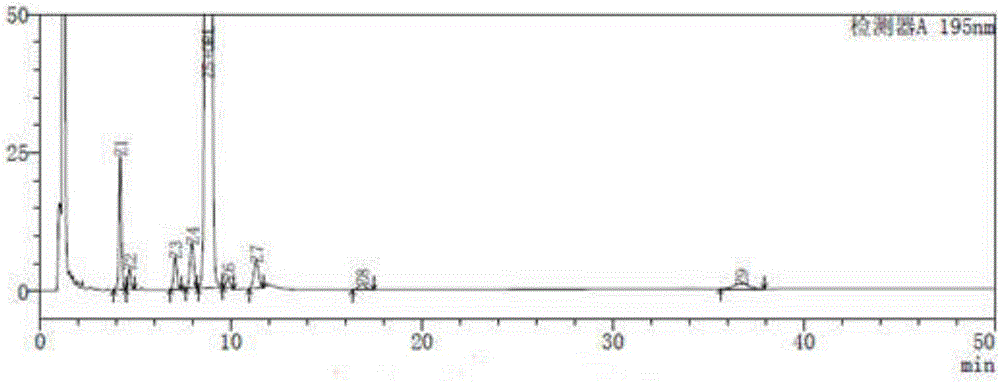
[0079] Get impurity mixed reference substance solution, carry out high performance liquid chromatography analysis according to above-mentioned experimental conditions, record chromatogram, the result sees image 3 And Table 4, it can be found that in the method of the USP 37th edition, orlistat is not separated from the ring opener methyl ester at all, and the peaks of each impurity are relatively close, which is not conducive to separation. Therefore, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com