Method for separation and determination of linezolid raw material x3 and its process impurity x2
A technology of linezolid and process impurities, which is applied in the field of analytical chemistry to achieve the effects of high accuracy, effective control of impurities and shortening of separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
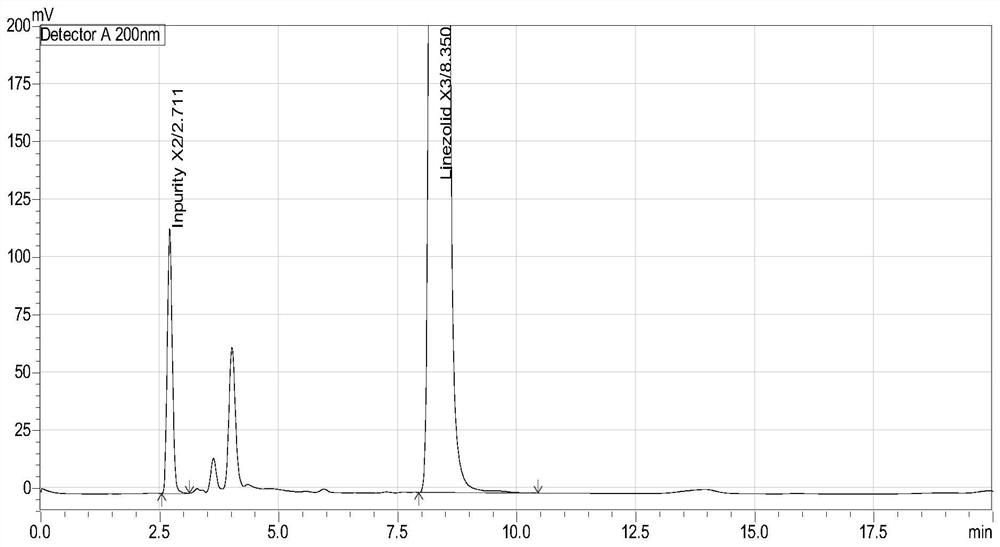
[0043] The chromatogram of embodiment 1 (S)-1-acetamido-2-acetoxy-3-chloropropane (X3) and process impurity (X2)
[0044] Mobile phase: 20% by volume acetonitrile in water.
[0045] Impurity X2 stock solution: Take 240.44mg of impurity X, weigh it accurately, put it in a 20ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and you get it.
[0046] Preparation of mixed solution: Take 20.27mg of the test product X31, weigh it accurately, put it in a 20ml measuring bottle, add 0.3ml of impurity X2 stock solution, add diluent to dissolve and dilute to the mark, and shake well.
[0047] The diluent and the mixed solution were respectively injected according to the above-mentioned chromatographic conditions, and the chromatogram was recorded. The measurement results are shown in Table 1. see results figure 1 .
[0048] Table 1 test results
[0049]
[0050] Conclusion: the blank diluent does not interfere with the determination of the sample; the...
Embodiment 2
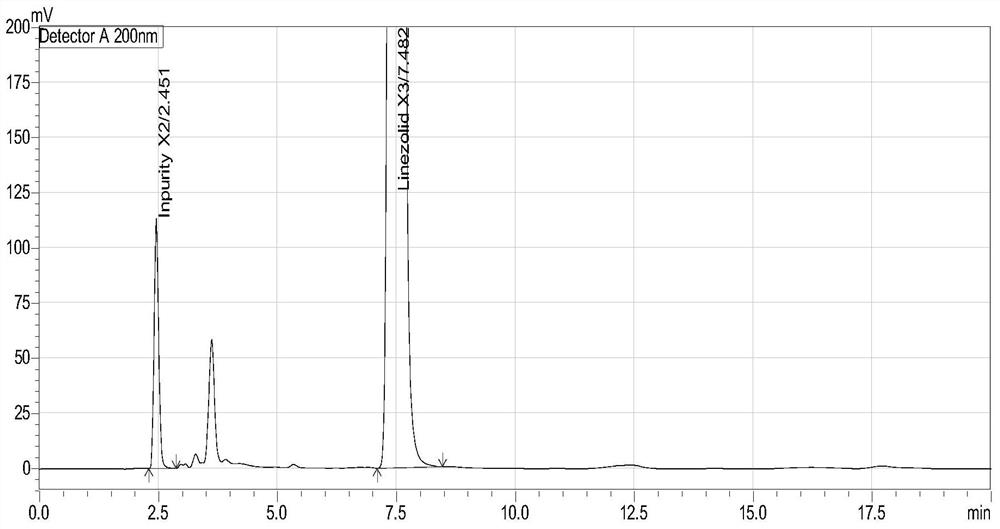
[0051] The chromatogram of embodiment 2 (S)-1-acetamido-2-acetoxy-3-chloropropane (X3) and process impurity (X2)
[0052] Trifluoroacetic acid aqueous solution: take 0.2ml of trifluoroacetic acid and add it to 1000ml of water;
[0053] Trifluoroacetic acid acetonitrile solution: take 0.2ml trifluoroacetic acid and add it to 1000ml acetonitrile;
[0054] Mobile phase: The volume ratio of trifluoroacetic acid aqueous solution to trifluoroacetic acid acetonitrile solution is 80:20.
[0055] Impurity X2 stock solution: take 240.05 mg of impurity X, weigh it accurately, put it in a 20ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and you get it.
[0056] Preparation of mixed solution: Take 20.16 mg of the test product X3, weigh it accurately, put it in a 20ml measuring bottle, add 0.3ml of impurity X2 stock solution, add diluent to dissolve and dilute to the mark, and shake well.
[0057] The diluent and the mixed solution were respectively injec...
Embodiment 3
[0061] Embodiment 3 different mobile relative to the separation situation of X3 and impurity X2
[0062] Trifluoroacetic acid aqueous solution: take 0.2ml of trifluoroacetic acid and add it to 1000ml of water;
[0063] Trifluoroacetic acid in acetonitrile solution: Take 0.2ml of trifluoroacetic acid and add it to 1000ml of acetonitrile.
[0064] Carry out the preparation of mobile phase ratio A, B, C as shown in Table 3.
[0065] Table 3 The ratio of mobile phase
[0066] Aqueous solution of trifluoroacetic acid (V) Trifluoroacetic acid acetonitrile solution (V) A 90 10 B 80 20 C 70 30
[0067] Impurity X2 stock solution: take about 40mg of impurity X2, accurately weigh it, put it in a 20ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and you get it.
[0068] Preparation of the mixed solution: Take about 120mg of the test product X3, accurately weigh it, put it in a 20ml measuring bottle, add 0.3ml of impu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com