Method for separating and detecting gatecarboxylic acid ethyl ester and/or related impurities by HPLC (High Performance Liquid Chromatography) method
A technology for the addition of ethyl carboxylate and ethyl carboxylate, which is used in material separation, measurement devices, analysis materials, etc., to achieve the effects of strong specificity, good reproducibility and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Take an appropriate amount of this product, accurately weighed, add diluent [water-acetonitrile (1:1)] to dissolve and quantitatively dilute to make a solution containing about 1mg in every 1ml, as the test solution (put it into the control immediately after preparation) 5°C sample tray); accurately measure 5.0ml, put it in a 50ml measuring bottle, dilute to the mark with diluent, shake well, then precisely measure 2.0ml, put it in a 100ml measuring bottle, dilute to the mark with diluent, Shake well as a control solution. Use octadecyl bonded silica gel as filler (4.6mm×250mm, 5μm); use buffer salt (weigh 2.0g potassium hexafluorophosphate, add 1000ml water to dissolve, add 3.0ml triethylamine, adjust pH 3.0 with phosphoric acid) Be mobile phase A, with acetonitrile as mobile phase B, carry out linear gradient elution according to Table 1, detection wavelength is respectively 250nm and 220nm (the measurement wavelength of impurity MOXH-SM1e is 220nm, and the detection ...
Embodiment 2
[0074] Exclusive
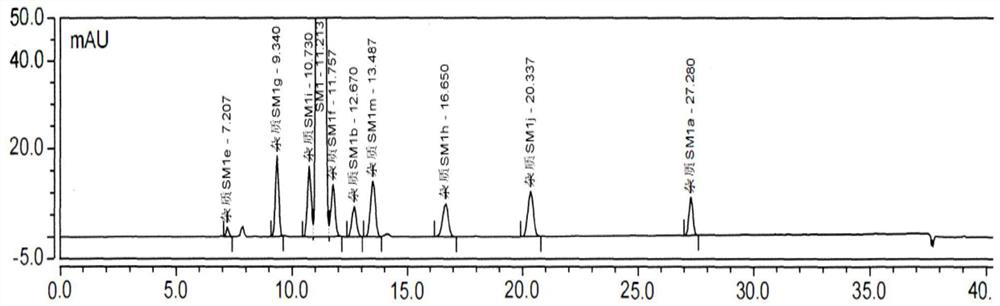
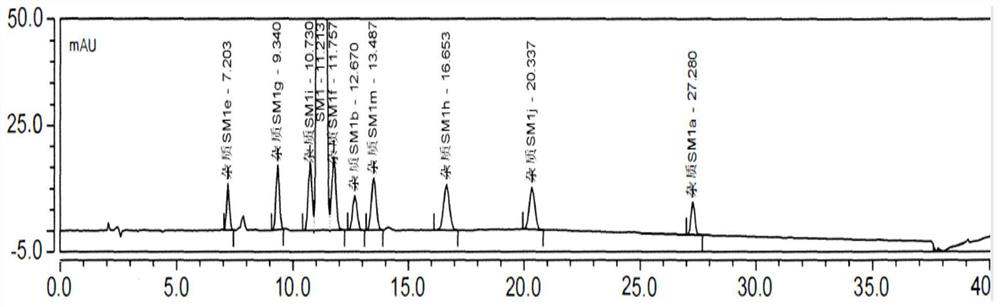
[0075] Impurities that may exist in SM1: Impurity MOXH-SM1a, Impurity MOXH-SM1b, Impurity MOXH-SM1e, Impurity MOXH-SM1m, Impurity MOXH-SM1g, Impurity MOXH-SM1h, Impurity MOXH-SM1j, Impurity MOXH-SM1f, Impurity MOXH- SM1i has a total of 9 impurities. In this method, a total of 7 impurities of impurity MOXH-SM1a, impurity MOXH-SM1b, impurity MOXH-SM1e, impurity MOXH-SM1m, impurity MOXH-SM1g, impurity MOXH-SM1h, and impurity MOXH-SM1j will be carried out. Research. Take 10 μl each of the blank solution, each impurity positioning solution, the test solution, and the mixed solution respectively, inject samples in sequence, and record the chromatogram. The measurement results are shown in Table 2-4, attached Figure 1-2 .
[0076] Table 2 Specific 250nm HPLC chromatogram integration results
[0077]
[0078] Table 3 Specific 220nm HPLC chromatogram integration results
[0079]
[0080]
[0081] Table 4 specificity test results
[0082]
[0083] Co...
Embodiment 3
[0085] detection limit
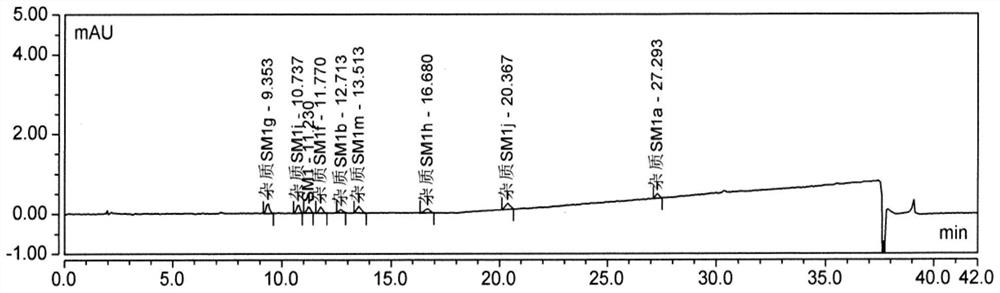
[0086] The detection limit solution was taken for three consecutive injections, and the ratio of the main peak height to noise (signal-to-noise ratio) was calculated. The test results are shown in Table 5-7, attached Figure 3-4 .
[0087] Table 5 Detection limit 250nm HPLC chromatogram
[0088]
[0089] Table 6 Detection limit 220nm HPLC chromatogram integration result
[0090]
[0091] Table 7 Detection limit determination result
[0092]
[0093]
[0094] Conclusion: The detection limit concentration of impurity MOXH-SM1e is 0.069 μg / ml, which is expressed as 0.007% by the concentration in the sample, and the average signal-to-noise ratio is 3.8; the detection limit concentration of impurity MOXH-SM1g is 0.067 μg / ml, which is expressed by the concentration in the sample Expressed as 0.007%, the average signal-to-noise ratio is 11.2; the detection limit concentration of impurity MOXH-SM1b is 0.073 μg / ml, expressed as 0.007% in the con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com