A method for separating and measuring process impurities in bilastine and its preparations
A technology for process impurities and preparations, applied in the field of analytical chemistry, can solve problems such as time-consuming and achieve the effect of controllable quality and shortening of separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
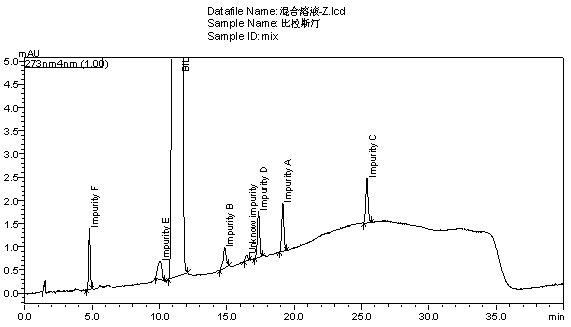
[0046] The chromatogram of embodiment 1 bilastine and 6 kinds of process impurities
[0047] Preparation of impurity A-F reference substance solution: Take about 40 mg of impurity A-F reference substance respectively, put them in a 25ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well to obtain impurity A-F stock solution. Precisely pipette 0.5ml of each of the above solutions, put them in a 50ml measuring bottle, add diluent to dilute to the mark, and shake well to obtain a reference substance mixture with a concentration of impurities A-F of 20 μg / ml.
[0048] Preparation of the mixed solution: Weigh about 40mg of the test product, put it in a 25ml measuring bottle, precisely pipette 2.5ml each of the impurity A-F reference substance solution, put it in the same measuring bottle as above, add diluent to dissolve and dilute to the mark, shake well, Instantly.
[0049] The diluent and the mixed solution were respectively injected according to the ...
Embodiment 2
[0057] Example 2 The impact of bilastine tablet preparation excipients on the determination of bilastine
[0058] Take an appropriate amount of bilastine tablets (including 40mg bilastine), put it in a 25ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well, filter, and take the filtrate as the test solution.
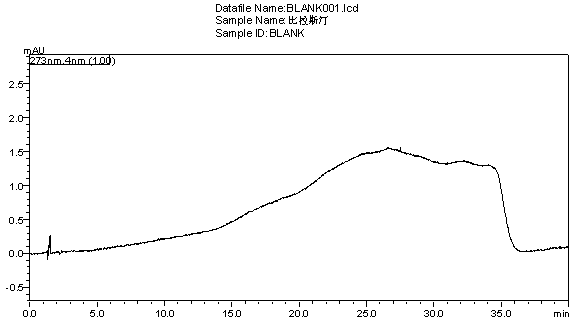
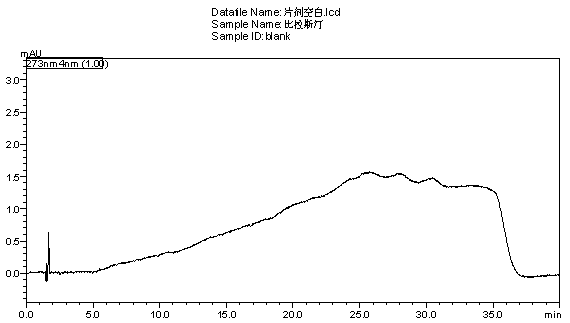
[0059] Get need testing solution, sample injection by chromatographic conditions in embodiment 1, record chromatogram, and carry out blank adjuvant test in the same way, the results are shown in image 3 , Figure 4 .
[0060] Conclusion: blank excipients do not interfere with the determination of this product, indicating that the method of the present invention can be used for the quality detection of bilastine tablets.
Embodiment 3
[0061] Example 3 Determination of Bilastine Oxidative Degradation Products
[0062] Take 40mg of bilastine, weigh it accurately, put it in a 25ml measuring bottle, add 2.0ml of 3% hydrogen peroxide, bathe in water at 30°C for 1.5h, take it out, cool it, dissolve it with a diluent and dilute to the mark, shake it up, and measure it as a degradation product with solution.
[0063] Get the above-mentioned degradation product measurement solution, sample injection by the chromatographic conditions in Example 1, record the chromatogram, do blank test simultaneously, record the chromatogram, as Figure 5 As shown, the impurity content was calculated according to the area normalization method. The test results are shown in Table 3.
[0064] Table 3 Determination results of oxidative degradation test
[0065]
[0066] Conclusion: Under the condition of oxidative degradation, impurity E was produced in 30℃ water bath for 1.5h, the level was 2.16%, and the original impurity had no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com