Method for separation and determination of Acitretin and its impurities
A technology of acitretin and impurities, which is applied in the field of separation and determination of acitretin and its impurities, can solve the problems of effective separation of the main peak, weak polarity, and ineffective separation, and achieve quality control, good sensitivity and high accuracy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
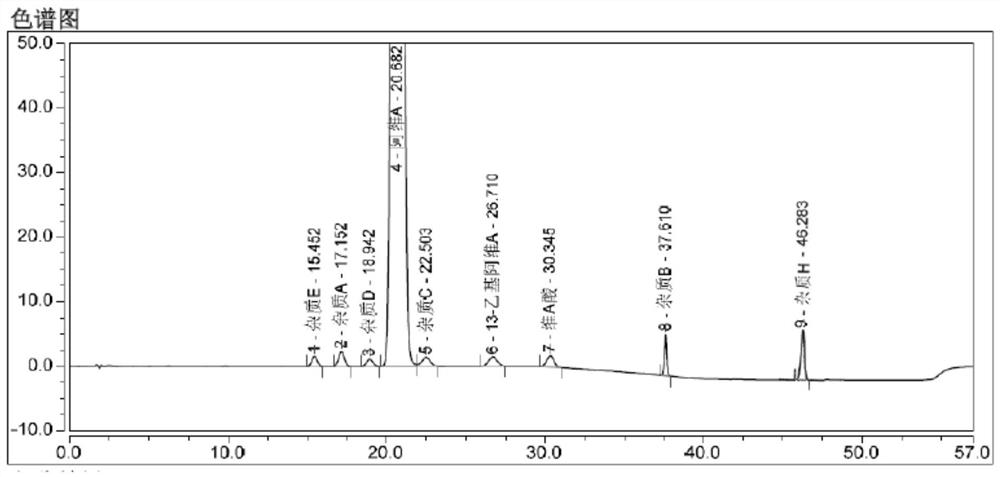
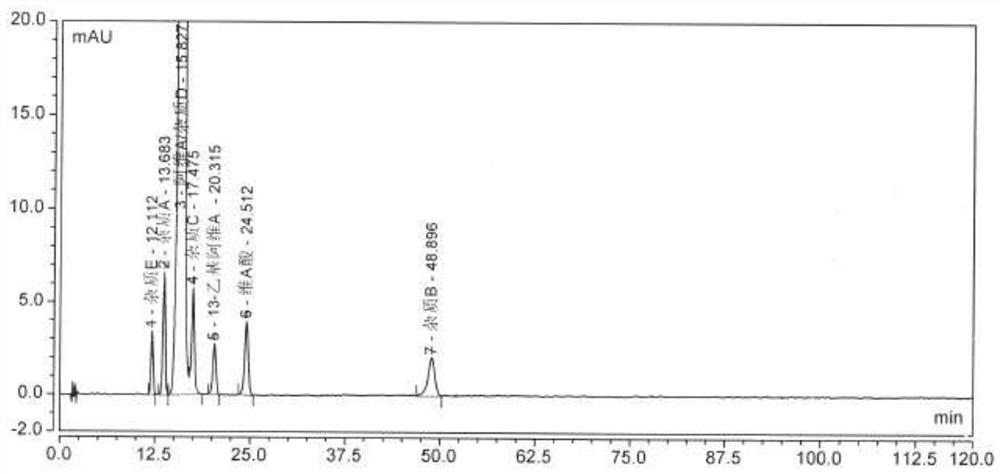
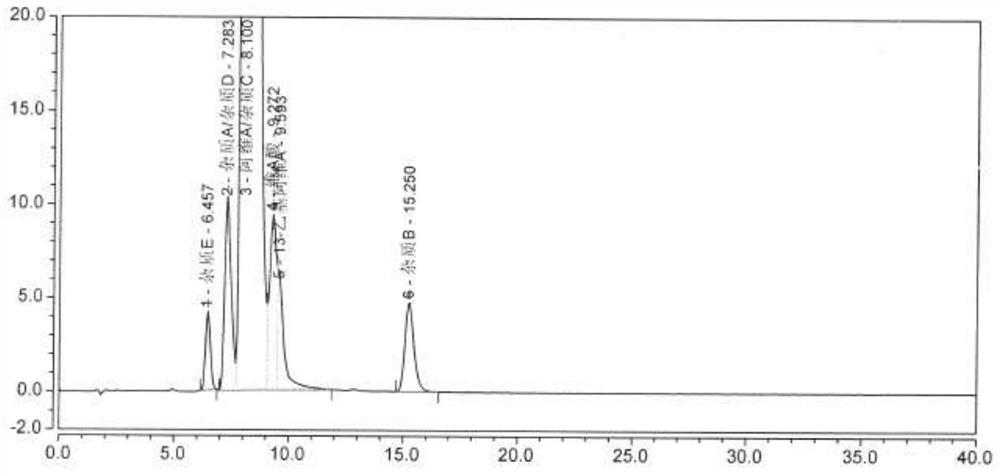
[0046] Select a chromatograph with a model of Shimadzu LC-20A, a chromatographic column model of Alltima C18 (250 × 4.6 mm, 5 μm), mobile phase A is 0.5% acetic acid water; mobile phase B is methanol; take the sample solution of step (1) 10μl was injected into the liquid chromatograph, the flow rate of the mobile phase was set to 1.5ml / min, the detection wavelength was 360nm, the temperature of the column oven was 25°C, and the data shown in Table 1 was followed by linear gradient elution to complete the sample solution. separation and measurement, when the sample separation and measurement results are as follows figure 1 shown.
[0047] Table 1 Volumes of Mobile Phase A and Mobile Phase B for Linear Gradient Elution
[0048]
Embodiment 2
[0049] Example 2 Limit of Quantitation and Limit of Detection
[0050] Quantitative limit solution: Precisely weigh each impurity reference substance, prepare a solution of a certain concentration, and dilute step by step to obtain a quantitative limit solution, as shown in Table 1.
[0051] Detection limit solution: Precisely pipette 5ml of quantitation limit solution, put it in a 10ml volumetric flask, add diluent to dilute to the mark, and shake well to obtain detection limit solution, as shown in Table 2.
[0052] test methods:
[0053] The above-mentioned quantitative limit solution was continuously injected for 3 times, and the detection limit solution was continuously injected for 2 times, and the ratio of the peak height of the main peak to the noise (signal-to-noise ratio) was calculated. Record the chromatogram, and the test results are shown in Table 1 and Table 2.
[0054]
[0055]
[0056] Table 2 Quantitative limit of Acitretin and various impurities
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com