Method for separating and determining diflucortolone as well as 6 beta diflucortolone and 16 beta diflucortolone thereof
A difluorometasone, stationary phase technology, applied in the field of analytical chemistry, to achieve the effect of good separation, excellent separation performance and durability, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
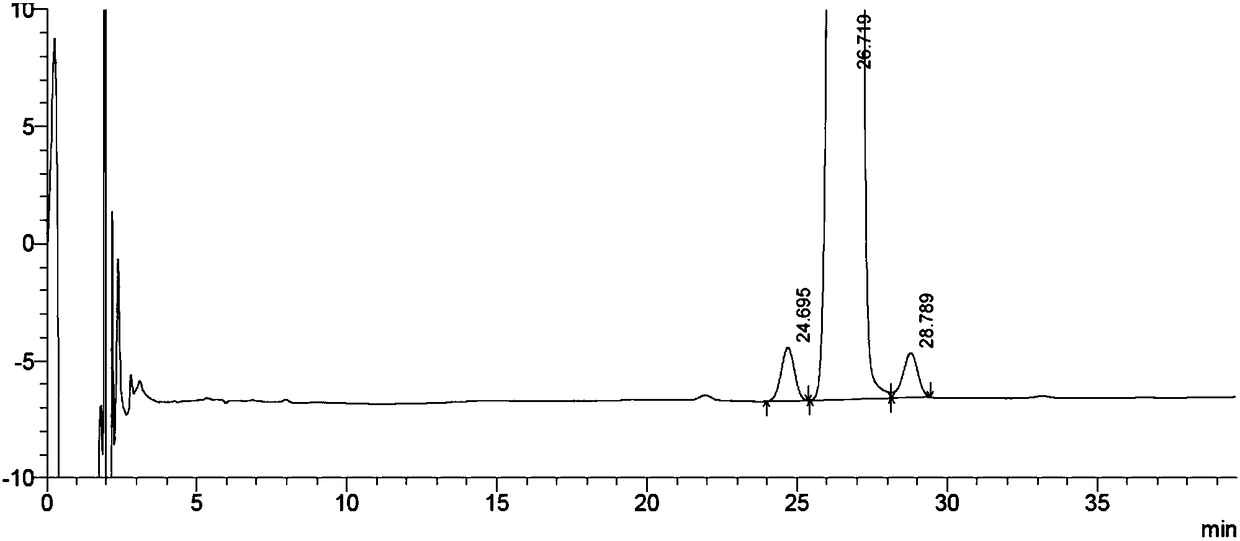
[0073] Example 1 Separation and Determination of Diflumethasone and 6β Diflumethasone and 16β Diflumethasone
[0074] The mobile phase that embodiment adopts and diluent are as follows:
[0075] mobile phase:
[0076] Mobile phase A: Inorganic salt buffer system Buffer salt: Weigh 3.7g of potassium hexafluorophosphate (0.02mol / L potassium hexafluorophosphate) into a 1000ml volumetric flask, add water to dissolve and dilute to the mark, adjust the pH value to 3.0± with phosphoric acid 0.1;
[0077] Mobile phase B: Methanol;
[0078] The volume ratio of mobile phase A and mobile phase B is 50:50.
[0079] Diluent: 50% methanol (methanol V: water V = 50:50).
[0080] 1) Sample dilution
[0081] 6β-diflumetasone: Take about 10 mg of 6β-diflumetasone reference substance, put it in a 100ml measuring bottle, add diluent to dissolve and dilute to the mark, and shake well to obtain 6β-diflumetasone stock solution.
[0082] 16β-diflumetasone: take about 10 mg of 16β-diflumetasone ...
Embodiment 2
[0088] Example 2 Basic research on detection limit and quantitative limit of diflumethasone, 6β diflumethasone and 16β diflumethasone by chromatographic system
[0089] Quantitation limit solution: Accurately weigh diflumethasone, 6β diflumethasone and 16β diflumethasone reference substances, make a solution with a certain concentration, and dilute step by step to obtain a quantification limit solution.
[0090] Detection limit solution: Precisely pipette 7.0ml of the limit of quantification solution, put it in a 20ml measuring bottle, add diluent to dilute to the mark, and shake well to obtain the limit of detection solution.
[0091] test methods:
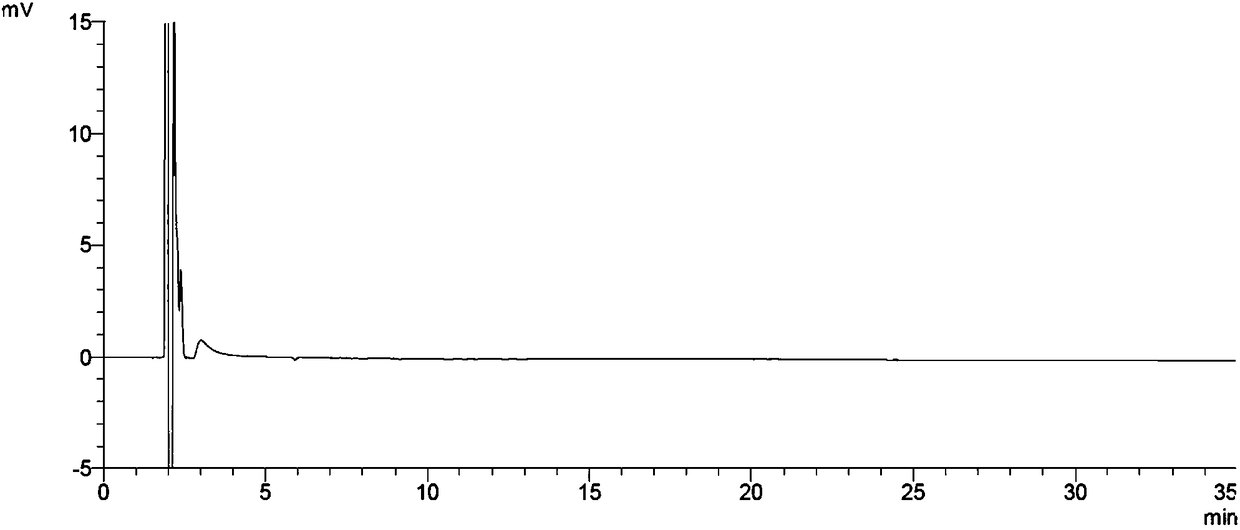
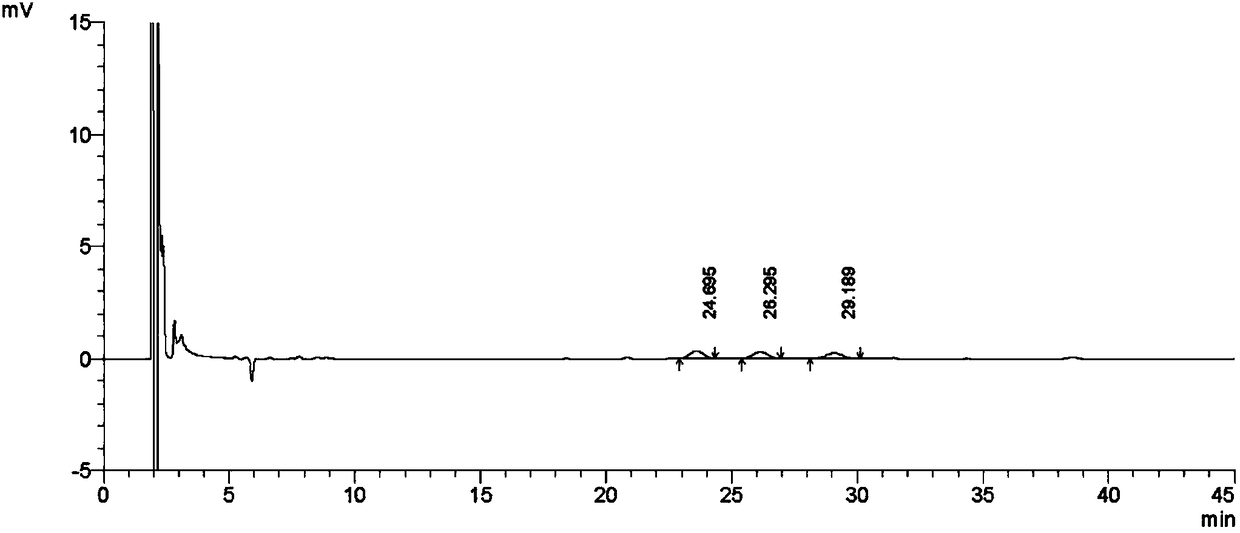
[0092] The above quantification limit solution was continuously injected 3 times, and the detection limit solution was continuously injected 2 times, and the ratio of the main peak height to noise (signal-to-noise ratio) was calculated. Record the chromatogram ( image 3 ), the test results are shown in Table 2 and Table 3.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com