Method for separating and detecting optical isomer of brivaracetam starting material
A technique for optical isomers and gas chromatography, which is applied in the field of separation and determination of brivaracetam starting material and its optical isomers by gas chromatography, and can solve the problems of affecting the purity and quality of drugs, incomplete removal of optical impurities, etc. , to achieve the effect of ensuring purity and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instruments and Conditions
[0037] Chromatograph: Agilent 6890 N gas chromatograph;
[0038] Detector: hydrogen flame ionization detector;
[0039] Chromatographic column: CYCLOSIL-B capillary column (Agilent, 30m×0.25mm*0.25μm)
[0040] Injection port temperature: 240°C;
[0041] Detector temperature: 280°C;
[0042] Carrier gas (nitrogen) flow rate: 1.0ml / min;
[0043] Split ratio: 20:1;
[0044] Injection volume: 1μl
[0045] Oven heating program:
[0046] Heating rate (℃ / min) temperature (°C) Hold time (min) / 60 0 20 160 0 5 180 3 5 220 8
[0047] Experimental procedure
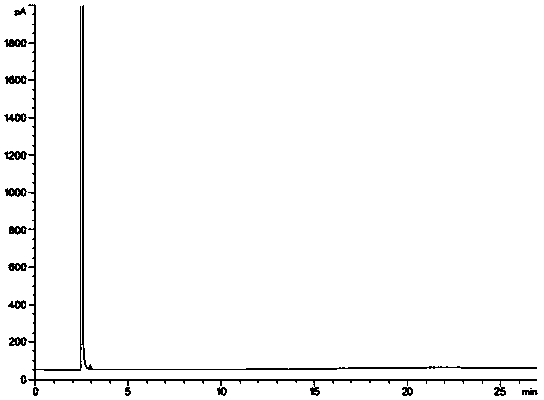
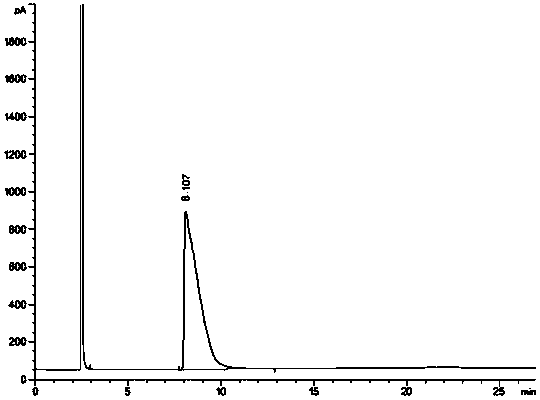
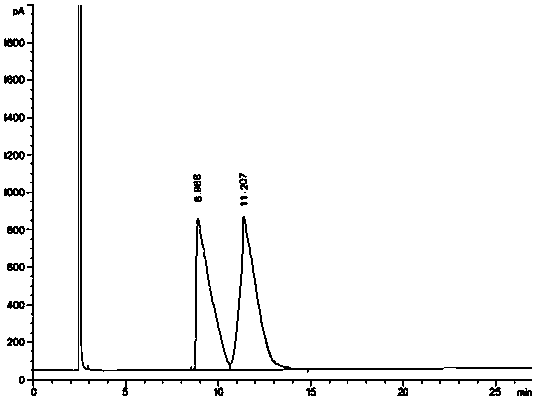
[0048] Take an appropriate amount of the racemate of the starting material of brivaracetam, dissolve it with a solvent, and prepare a system suitability solution containing 1.0 mg / ml of the starting material of brivaracetam and its optical isomer; An appropriate amount of the starting material is dissolved in a solvent, prepared into a test soluti...
Embodiment 2
[0050] Chromatograph: Agilent 6890 N gas chromatograph;
[0051] Detector: hydrogen flame ionization detector;
[0052] Chromatographic column: CYCLOSIL-B capillary column (Agilent, 30m×0.25mm*0.25μm)
[0053] Injection port temperature: 240°C;
[0054] Detector temperature: 280°C;
[0055] Carrier gas (nitrogen) flow rate: 0.8ml / min;
[0056] Split ratio: 20:1;
[0057] Injection volume: 1μl
[0058] Oven heating program:
[0059] Heating rate (℃ / min) temperature (°C) Hold time (min) / 60 0 20 160 0 5 180 3 5 220 8
[0060] Experimental procedure
[0061]Take an appropriate amount of the racemate of the starting material of brivaracetam, dissolve it with a solvent, and prepare a system suitability solution containing 1.0 mg / ml of the starting material of brivaracetam and its optical isomers, inject it into a gas chromatograph, Analyze according to the above chromatographic conditions and record the chromatograms. see attached ...
Embodiment 3
[0063] Chromatograph: Agilent 6890 N gas chromatograph;
[0064] Detector: hydrogen flame ionization detector;
[0065] Chromatographic column: CYCLOSIL-B capillary column (Agilent, 30m×0.25mm*0.25μm)
[0066] Injection port temperature: 230°C;
[0067] Detector temperature: 250°C;
[0068] Carrier gas (nitrogen) flow rate: 1.0ml / min;
[0069] Split ratio: 20:1;
[0070] Injection volume: 1μl
[0071] Oven heating program:
[0072] Heating rate (℃ / min) temperature (°C) Hold time (min) / 60 0 20 160 0 5 180 3 5 220 8
[0073] Experimental procedure
[0074] Take an appropriate amount of the racemate of the starting material of brivaracetam, dissolve it with a solvent, and prepare a system suitability solution containing 1.0 mg / ml of the starting material of brivaracetam and its optical isomers, inject it into a gas chromatograph, Analyze according to the above chromatographic conditions and record the chromatograms. see attached...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com