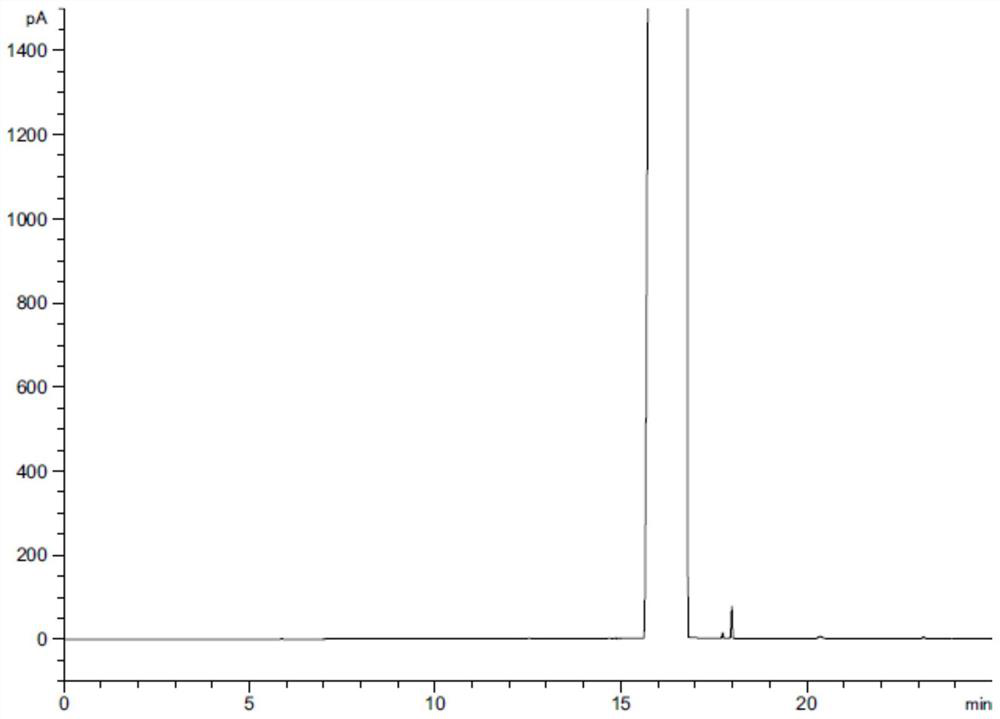
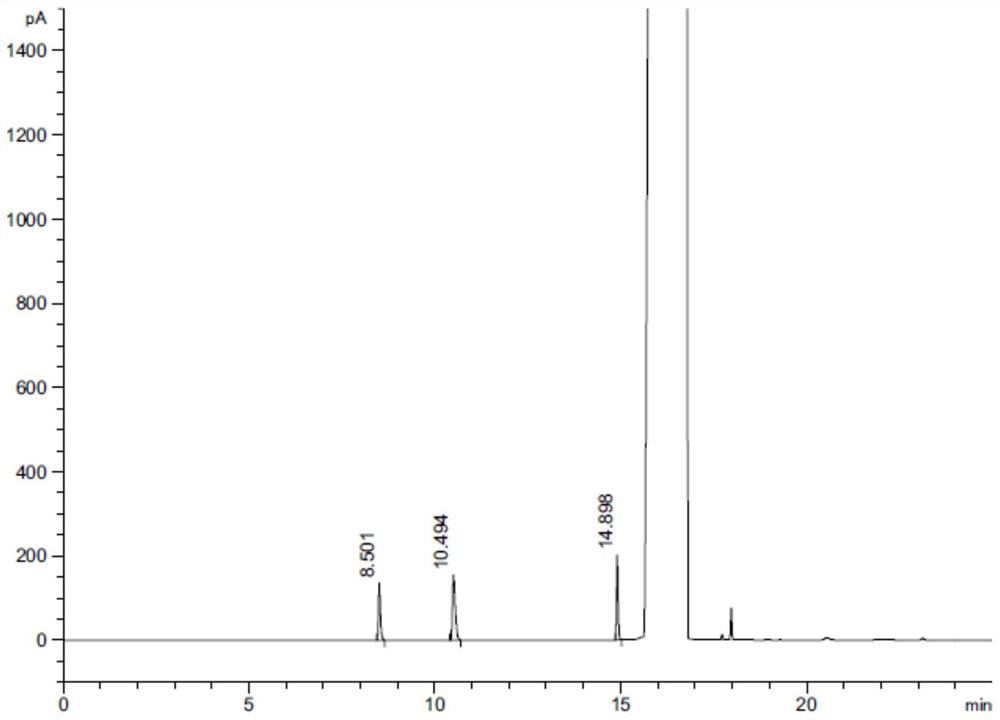
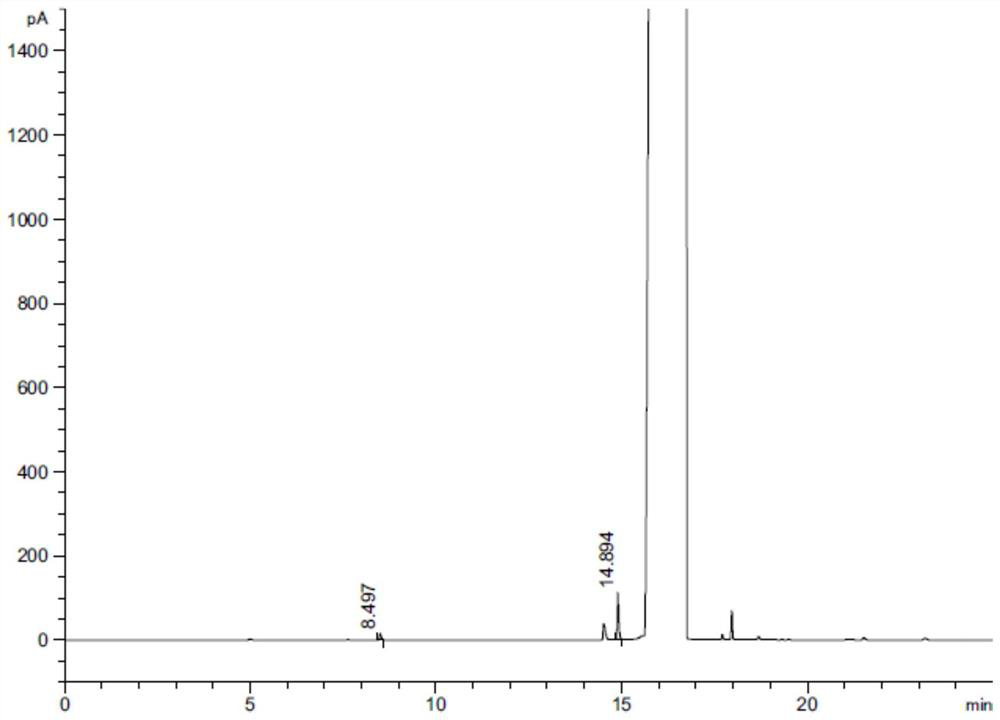
A method for separating and determining lurasidone hydrochloride intermediate related substances by gas chromatography
A technology for lurasidone hydrochloride and related substances, which is applied in the field of separation and determination of lurasidone hydrochloride intermediates and related substances by gas chromatography technology, can solve the problems of incomplete removal of impurities and influence on the purity and quality of drugs, and achieve separation and determination problems, increase yield, and reduce the effect of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Instruments and Conditions
[0035] Chromatograph: Agilent 7890A gas chromatograph;
[0036] Detector: hydrogen flame ionization detector;
[0037] Chromatographic column: DB-624 capillary column (Agilent, 30m´0.32mm, 1.8mm);
[0038] Inlet temperature: 180℃;
[0039] Detector temperature: 250℃;
[0040] Carrier gas (nitrogen) flow rate: 1.0mL / min;
[0041] Split ratio: 10:1;
[0042] Injection volume: 1 μL.
[0043] Oven temperature program:
[0044] Heating rate (℃ / min) Temperature (℃) Hold time (min) / 50 5 10 180 7
[0045] Experimental steps:
[0046] Take an appropriate amount of lurasidone hydrochloride, dissolve it in dimethyl sulfoxide to make a solution containing 100 mg lurasidone hydrochloride per 1ml, as the test solution; take an appropriate amount of piperazine, n-butanol and ethyl acetate, add two Methyl sulfoxide was dissolved to prepare a solution containing about 0.5 mg each of piperazine, n-butanol and ethyl ace...
Embodiment 2
[0048] Instruments and Conditions
[0049] Chromatograph: Agilent 7890A gas chromatograph;
[0050] Detector: hydrogen flame ionization detector;
[0051] Chromatographic column: DB-624 capillary column (Agilent, 30m´0.32mm, 1.8mm);
[0052] Inlet temperature: 190℃;
[0053] Detector temperature: 250℃;
[0054] Carrier gas (nitrogen) flow rate: 1.0mL / min;
[0055] Split ratio: 10:1;
[0056] Injection volume: 1 μL
[0057] Oven temperature program:
[0058] Heating rate (℃ / min) Temperature (℃) Hold time (min) / 50 5 10 180 7
[0059] Experimental steps:
[0060] Take an appropriate amount of piperazine, n-butanol and ethyl acetate, add dimethyl sulfoxide and dissolve it to prepare a solution containing about 0.5 mg each of piperazine, n-butanol and ethyl acetate per 1 ml, as the test solution; Methyl sulfoxide was used as blank solution. The above-mentioned test solution and blank solution were injected into the gas chromatograph, analyz...
Embodiment 3
[0062] Instruments and Conditions
[0063] Chromatograph: Agilent 7890A gas chromatograph;
[0064] Detector: hydrogen flame ionization detector;
[0065] Chromatographic column: DB-624 capillary column (Agilent, 30m´0.32mm, 1.8mm);
[0066] Inlet temperature: 180℃;
[0067] Detector temperature: 250℃;
[0068] Carrier gas (nitrogen) flow rate: 1.1mL / min;
[0069] Split ratio: 10:1;
[0070] Injection volume: 1 μL.
[0071] Oven temperature program:
[0072] Heating rate (℃ / min) Temperature (℃) Hold time (min) / 50 5 10 180 7
[0073] Experimental steps:
[0074] Take an appropriate amount of piperazine, n-butanol and ethyl acetate, add dimethyl sulfoxide and dissolve it to prepare a solution containing about 0.5 mg each of piperazine, n-butanol and ethyl acetate per 1 ml, as the test solution; Methyl sulfoxide was used as blank solution. The above-mentioned test solution and blank solution were injected into the gas chromatograph, analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com