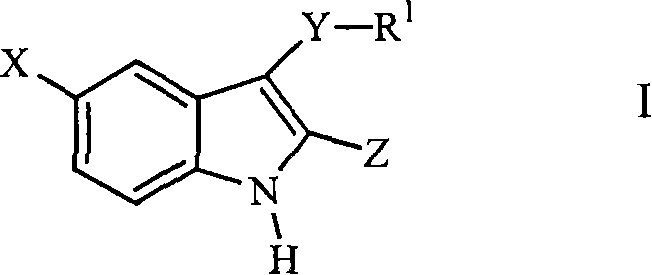
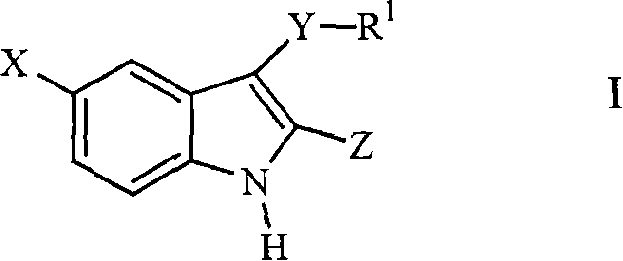
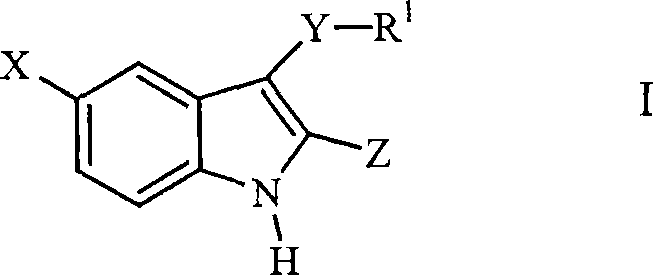
3-arylthioindole-2-carboxamide derivatives and analogs thereof as inhibitors of casein kinase i
一种酪蛋白激酶、甲酰胺的技术,应用在作为酪蛋白激酶Iε抑制剂的3-芳基硫代吲哚-2-甲酰胺衍生物及其类似物领域,能够解决蛋白稳定性降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0173] The following examples are intended to illustrate the present invention in more detail, but do not limit the scope of the present invention in any way.
[0174] General Synthetic Method
[0175] Materials were purchased from suppliers and used without further purification unless otherwise stated. All reactions are performed under an inert atmosphere such as nitrogen or argon using anhydrous reagents and solvents. Flash chromatography was performed using EM Science Silica Gel 60 (40-63 mm) and using the solvent system described. TLC using 0.25 mm silica gel coated 60F-254 plates (EM) and using iodine vapor, UV light or stains such as KMnO 4 The solution develops color.
[0176] Infrared (IR) spectra were recorded on a Nexus 670 FTIR (Nicolet) spectrometer, the samples were prepared according to the instructions, and the results were expressed in wavenumbers (cm -1 )express. 1 H NMR spectra were recorded on Varian Gemini and / or mercury 300, Unity 400 or Unity plus an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com