Orally disintegrating tablet of cariprazine and preparation method thereof
A technology of orally disintegrating tablets and cariprazine, which is applied in the field of cariprazine orally disintegrating tablets and its preparation, can solve the problems of drug efficacy, taste, taking compliance and production cost, and the difficulty of large-scale production. problem, to achieve the effect of cool and slightly sweet taste, low production cost, and improved therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of cariprazine orally disintegrating tablets (specification 1.5 mg, calculated as cariprazine)
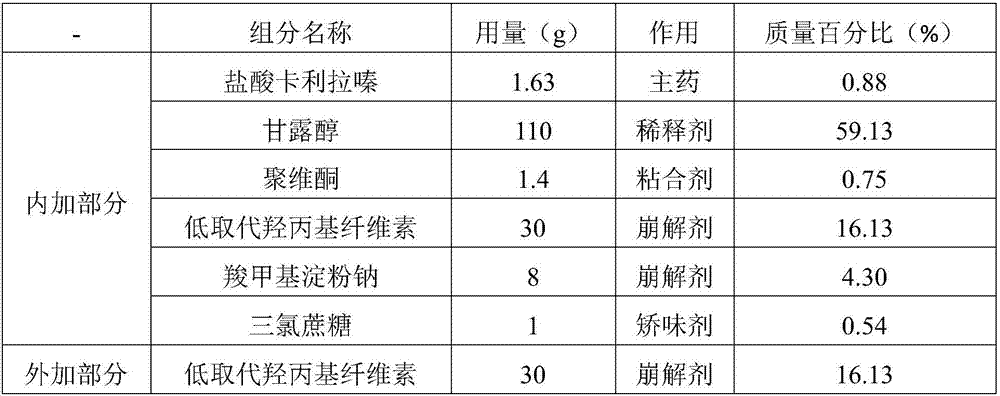
[0051] The formula composition of cariprazine orally disintegrating tablet is shown in Table 1.
[0052] Table 1
[0053]
[0054]
[0055] Cariprazine orally disintegrating tablet preparation method is as follows:
[0056] (1) According to the formula in Table 1, mix cariprazine hydrochloride, diluent, flavoring agent, and internally added disintegrant (that is, the internally added disintegrant) evenly, and then use a multifunctional fluidized bed top spray One-step granulation is carried out in a one-step granulation process, and an aqueous binder solution is added during the one-step granulation process to obtain granules after granulation; wherein, the mass percentage of the binder in the binder solution is 2.5%;
[0057] (2) Sizing the obtained granules, passing through an 80-mesh sieve, adding an additional disintegrating agent (that ...
Embodiment 2
[0058] Embodiment 2: Preparation of cariprazine orally disintegrating tablets (specification 3.0 mg, calculated as cariprazine)
[0059] The formula composition of cariprazine orally disintegrating tablet is shown in Table 2.
[0060] Table 2
[0061]
[0062] Prepare cariprazine orally disintegrating tablets according to the formula in Table 2, and the specific operation steps are the same as in Example 1; wherein, the solvent in the binder solution is an aqueous ethanol solution, and the mass percent of the binder in the binder solution is 1%.
Embodiment 3
[0063] Embodiment 3: Preparation of cariprazine orally disintegrating tablets (specification 6.0 mg, calculated as cariprazine)
[0064] The formula composition of cariprazine orally disintegrating tablet is shown in Table 3.
[0065] table 3
[0066]
[0067] Cariprazine orally disintegrating tablets were prepared according to the formula in Table 3, and the specific operation steps were the same as in Example 1; wherein, the mass percentage of the binder in the binder solution was 5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com