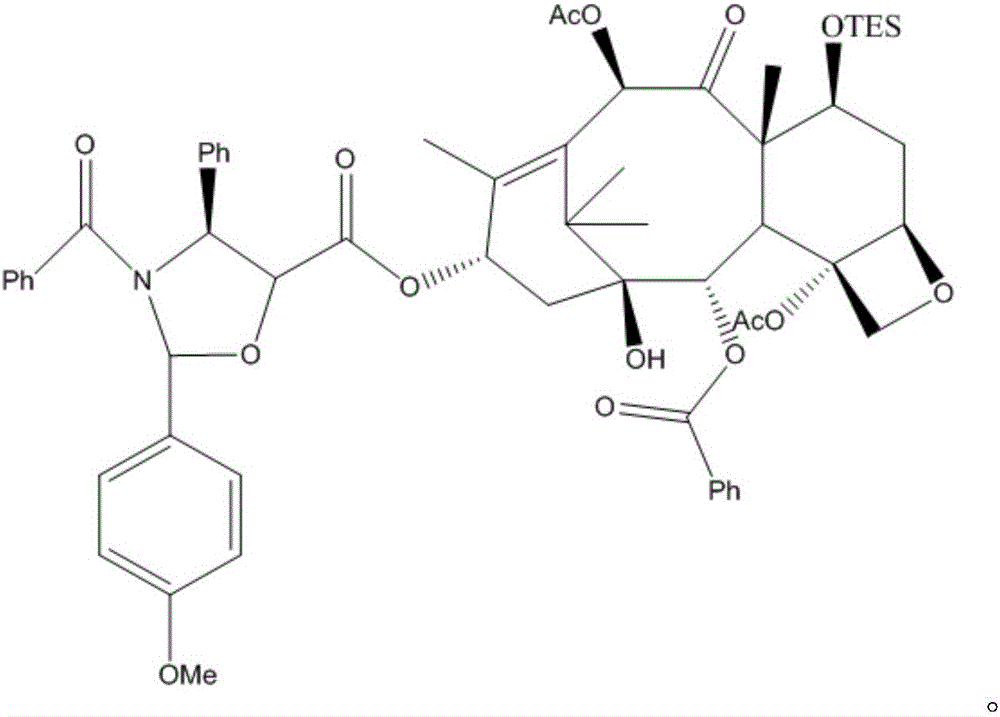
Methods for preparing semi-synthetic paclitaxel and intermediate thereof
A technology for intermediates and paclitaxel, which is applied in the field of semi-synthetic paclitaxel and the preparation of intermediates thereof, can solve the problems of complex system components, generation of impurities, little improvement in the purity of paclitaxel, high requirements for reaction reagents, etc., and achieves yield and purity. Improve, ensure yield, protect effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Weigh the 10-DABⅢ raw material, add a certain weight of dry pyridine and stir to dissolve, then add a certain weight of triethylchlorosilane dropwise under the protection of nitrogen, the dropping time is kept at 30min, and the dropping temperature is controlled at 10°C. After the dropwise addition, control the reaction temperature to 10°C, stop the reaction when 10-DABⅢ remains less than 0.2% in the reaction solution, add water dropwise to quench the reaction, then neutralize pyridine with concentrated hydrochloric acid, extract with dichloromethane, concentrate and dry, Intermediate I is obtained.
[0052] (2) Weigh intermediate I, add a certain weight of dry pyridine and stir to dissolve, then add a certain weight of acetyl chloride dropwise under the protection of nitrogen, the dropping time is kept at 30min, the dropping temperature is controlled at -15°C, and the dropping is completed Finally, control the reaction temperature to -15°C, stop the reaction when t...
Embodiment 2
[0065] (1) Weigh the 10-DABⅢ raw material, add a certain weight of dry pyridine and stir to dissolve, then add a certain weight of triethylchlorosilane dropwise under the protection of nitrogen, the dropping time is kept at 60min, and the dropping temperature is controlled at 20°C. After the dropwise addition, control the reaction temperature to 30°C, stop the reaction when 10-DABⅢ remains less than 0.2% in the reaction solution, add water dropwise to quench the reaction, then neutralize pyridine with concentrated hydrochloric acid, extract with dichloromethane, concentrate and dry, Intermediate I is obtained.
[0066] (2) Weigh intermediate I, add a certain weight of dry pyridine to stir and dissolve, then add a certain weight of acetyl chloride dropwise under the protection of nitrogen, keep the dropping time at 60min, and control the dropping temperature at 5°C. , control the reaction temperature at 5°C, stop the reaction when the residual intermediate I in the reaction sol...
Embodiment 3
[0079] (1) Weigh the 10-DABⅢ raw material, add a certain weight of dry pyridine and stir to dissolve, then add a certain weight of triethylchlorosilane dropwise under the protection of nitrogen, the dropping time is kept at 45min, and the dropping temperature is controlled at 15°C. After the dropwise addition, control the reaction temperature to 20°C, stop the reaction when 10-DABⅢ remains less than 0.2% in the reaction solution, add water dropwise to quench the reaction, then neutralize pyridine with concentrated hydrochloric acid, extract with dichloromethane, concentrate and dry, Intermediate I is obtained.
[0080] (2) Weigh intermediate I, add a certain weight of dry pyridine and stir to dissolve, then add a certain weight of acetyl chloride dropwise under the protection of nitrogen, the dropping time is kept at 45min, the dropping temperature is controlled at -5°C, and the dropping is completed Finally, control the reaction temperature to -5°C, stop the reaction when the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com