The preparation method of steroid medicine intermediate
A technology of steroidal drugs and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of many by-products and low yield of target products, and achieve the effects of improving production efficiency and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
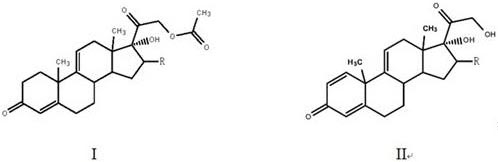
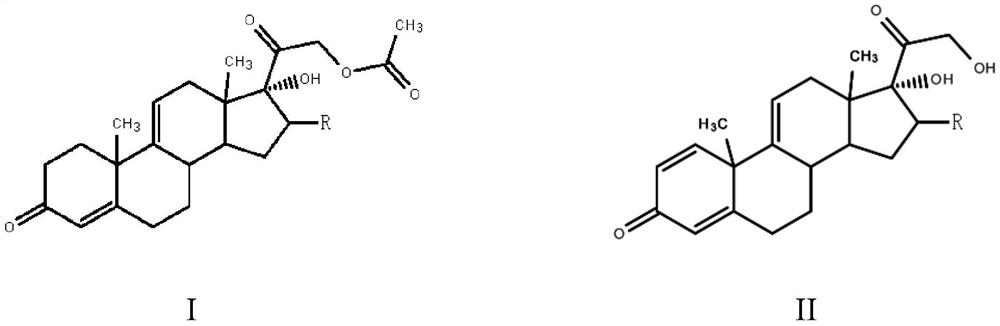
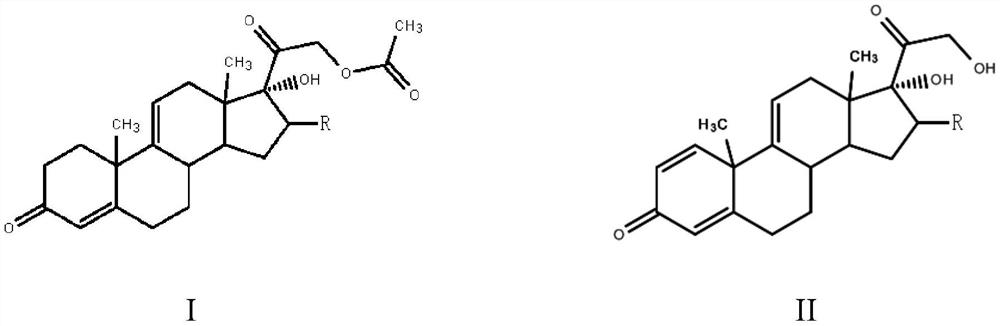
[0023] The preparation method of a steroid drug intermediate according to one embodiment of the present invention comprises the following steps: using Nocardia simplex to carry out microbial transformation of the first compound to obtain a steroid drug intermediate, the first compound is shown in general formula I, steroid The body drug intermediate is shown in general formula II,
[0024]
[0025] In the general formula I and the general formula II, R is H, a halogen atom, an alkyl group, an alkoxy group, a hydroxyl group or a phenyl group.
[0026] In this embodiment, the first compound represented by the above general formula I is selected as the substrate, and only Nocardioides simplex is used for biotransformation, and the substrate is simultaneously dehydrogenated at positions 1 and 2 and acetate at position 21 Hydrolysis to obtain steroid drug intermediates, hydrolysis of 21-position acetate can effectively promote the dehydrogenation of 1 and 2 positions, so that mo...
Embodiment 1
[0048] Pack 12 liters of fermentation medium in a 20 liter fermenter, and inoculate 600 mL of Nocardia simplex seed liquid (thalline concentration: 2.4×10 9 cells / ml), culture was started, and the culture conditions were temperature of 31° C., rotation speed of 160 rpm, and culture time of 24 hours.
[0049] The sample was diluted 30 times, and detected with a spectrophotometer at 580 nm, and the OD value was 0.62. 240 g of 4,9(11)-pregna-17-hydroxy-3,20-dione-21-acetate and 9.6 g of PPE were added to the fermenter to start the fermentative conversion. The culture conditions during fermentation transformation were temperature 31±1°C, rotation speed 180rpm, air flow 0.2-0.3vvm, and samples were taken after 72 hours of transformation for HPLC analysis. The results are shown in Table 1:
[0050] Table 1
[0051]
[0052] After the conversion is completed, the fermented liquid is extinguished and filtered to obtain a filter cake with a wet weight of 390g, which is dried to ob...
Embodiment 2
[0066] Pack 12 liters of fermentation medium in a 20 liter fermenter, and inoculate 600 mL of Nocardia simplex seed liquid (thalline concentration: 3.2×10 9 cells / ml), culture was started, and the culture conditions were temperature of 31° C., rotation speed of 160 rpm, and culture time of 24 hours.
[0067] The sample was diluted 30 times, and detected with a spectrophotometer at 580 nm, and the OD value was 0.56. 240 g of 4,9(11)-pregna-17-hydroxy-3,20-dione-21-acetate and 9.6 g of PPE were added to the fermenter to start the fermentative transformation. The culture conditions during fermentation transformation were temperature 31±1°C, rotation speed 180rpm, air flow rate 0.2~0.3vvm, after 72 hours of transformation, samples were taken and sent to HPLC analysis, the results are shown in Table 5:
[0068] table 5
[0069]
[0070] After the conversion is completed, the fermentation broth is extinguished and filtered to obtain a filter cake with a wet weight of 373g, whic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com